Abstract
Fusarium is considered one of the most important fungi that attack plants and cause serious diseases resulting in huge losses to crops, especially wheat. Fungicides have been used to control it, but they have drawbacks, including residues and toxicity to mammals, which encouraged researchers to find alternatives to these methods and materials. This study was conducted to find natural alternatives to the chemicals used as fungicides. The Dodonaea viscosa plant extract was evaluated as an extract (DVE) and nanoparticles (chitosan NPs loaded with DVE) to inhibit the growth of Fusarium spp. strains and production ability of Deoxynivalenol (DON) and Moniliformin (MON) mycotoxins. The wheat samples were taken from storage in eighteen different governorates in Iraq. Fusarium spp. strains were detected phenotypically, and seven strains were identified by using the polymerase chain reaction technique (PCR) as F. oxysporum, F. pseudograminearum and F. chlamydosporum. DVE effectively inhibited the growth of Fusarium spp. strains at three different concentrations (0.5, 1.0, and 1.5%) on PDA. The highest percentage was 68.94% for F. oxysporum strain 5, and the lowest percentage was 22.58% for F. pseudograminearum strain 6 at a concentration of 1.5%. However, applying chitosan NPs loaded with DVE at a concentration of 0.75% effectively increased the inhibition rate. The treatment of chitosan NPs loaded with DVE played a role in inhibiting the percentage of mycotoxins produced. The highest percentage of inhibition of the DON toxin was recorded as 73.75% in Fusarium pseudograminearum strain 2, and the highest percentage of inhibition of the production of the (MON) toxin was 73.62% in isolate Fusarium chlamydosporum strain 8. Overall, this study highlights for the first time the potential of Dodonaea viscosa nano-formulation to suppress both fungal growth and mycotoxin biosynthesis, providing a sustainable and safe strategy for protecting stored grains.
Key Contribution:
This study demonstrates the nano-encapsulated Dodonaea viscosa extract can simultaneously suppress both fungal growth and mycotoxin (DON and MON) biosynthesis in stored wheat. The findings highlight nanotechnology as an effective strategy to enhance the bioactivity and stability of plant-derived antifungal agents.
1. Introduction
Certain fungi produce toxic secondary metabolites, namely mycotoxins, in cereals, nuts, and fruits, that affect humans and animals when grains are used in the human diet and animal feed [1,2]. One of the concerns in wheat production is the possible accumulation of mycotoxins, both as a commercial risk and as a health risk. Among the mycotoxigenic fungi, Fusarium spp. are of particular importance, as they can infect grains both pre- and post-harvest, and the infection may persist during storage, resulting in quality deterioration and grain loss [3]. To date, more than 400 mycotoxins have been identified [4], with deoxynivalenol (DON), nivalenol, zearalenone, T-2 toxin, and fumonisin B1 recognized as the most prevalent and hazardous compounds [5]. DON, also referred to as vomitoxin, is a type B trichothecene mycotoxin frequently detected in wheat and commonly produced by Fusarium pseudograminearum [6]. Moniliformin (MON) is another important mycotoxin produced by various Fusarium species, and, to date, approximately 40 species have been reported as MON producers [7,8].
The use of extracts and natural materials as an alternative to chemical pesticides is the goal of many researchers. Due to the adverse effects of synthetic pesticides on the environment, humans, and animals, the search for safer alternative pest control strategies has become imperative. Natural compounds and extracts of plants have been analyzed in many studies for their antifungal activities and the identification of the ergosterol synthesis inhibition mechanism [9]. Dodonaea viscosa also known as the broadleaf hopbush, is a flowering plant belonging to the Sapindaceae family. It has been used in the treatment of burns, teeth, skin infections, stomach pain, etc., and has an inhibitory effect against microbes and insects as an effective pesticide, and it has also antioxidant and antiparasitic activity [10]. A flavone compound 5,6,8-trihydroxy-7,4′ dimethoxy flavone was isolated from the crude extract of Dodonaea viscosa var. angustifolia Jacq has demonstrated antifungal activity by inhibiting ergosterol synthesis in C. albicans with an MIC value of 0.39 mg/mL and MFC value of 1.56 mg/mL [11]. The antimicrobial (particularly antibacterial) activity of Dodonaea viscosa has been clearly confirmed in the study. The methanol extract alone exhibits a significant effect against both Gram-positive (particularly S. aureus and B. subtilis) and Gram-negative (E. coli, K. pneumoniae) bacteria. MIC values have decreased to levels as low as 500–1000 µg/mL in some pathogens [12]. In the study conducted by Khurram et al. (2009), the plant’s ethanolic extract and various solvent fractions (particularly ethyl acetate and n-butanol) formed strong inhibition zones and showed a marked effect on pathogens such as S. aureus, M. luteus, E. coli and P. aeruginosa [13]. In order to enhance the stability, solubility, and bioefficacy of plant extracts, nanotechnology-based delivery systems have recently gained significant attention—a strategy also adopted in the present study.
Nanotechnology can be defined as the process of producing materials with one or more dimensions on a scale of 100 nm or less [14]. The extracts could be converted into nanoparticles by binding them to nano chitosan, where chitosan has been used in agricultural applications due to its important properties such as biodegradability and environmental friendliness when used in fertilizers and seed coatings [15]. The effectiveness of chitosan nanoparticles loaded with Syzygium aromaticum extract against Klebsiella pneumoniae has been demonstrated in a previous study [16]. In another study, there was a clear effect of the produced CH@CuO nanoparticles on cultivated tomato plants infected with isolates of F. oxysporum [17]. In addition, they are used as nanomaterials due to the combination of fungicide or chemicals with chitosan through ionic or covalent bonds or encapsulating these materials with chitosan matrices due to their homogeneous and stable encapsulation property [18].
Therefore, the main objective of this study was to evaluate the antifungal efficiency of Dodonaea viscosa extract and its nano-formulation against Fusarium species isolated from stored wheat, with particular emphasis on their potential to inhibit fungal growth and the biosynthesis of the mycotoxins deoxynivalenol (DON) and moniliformin (MON). In addition, the study aimed to compare the effectiveness of the conventional extract and its nano-chitosan-loaded form to highlight the role of nanotechnology in enhancing the bioactivity, stability, and efficacy of plant-derived antifungal agents.
2. Results and Discussion
2.1. Wheat Sampling and Identification of Fusarium spp. Strains
A total of 51 stored wheat samples were collected from eighteen governorates in Iraq, from which seven Fusarium strains were successfully isolated, each originating from a different governorate (Nineveh, Maysan, Dohuk, Anbar, Ta’mim, Salah al-Din, and Basra). These strains belonged to three Fusarium species: Fusarium oxysporum, F. pseudograminearum, and F. chlamydosporum.
On PDA medium, the colonies exhibited soft, pale, cottony mycelial growth. Strains 4 and 5 were identified as F. oxysporum, showing colony colors ranging from white to pale purple. Strains 2, 3, 6, and 7 were classified as F. pseudograminearum, with colonies displaying white to cream pigmentation. Strain 8 was identified as F. chlamydosporum, characterized by a light pink to dark red colony color.
The genetic identity of all strains was confirmed by polymerase chain reaction (PCR) analysis, as presented in Table 1.

Table 1.
The results of PCR analysis of seven Fusarium strains.
Three Fusarium strains representing the species previously identified morphologically were further confirmed at the molecular level and deposited in the GenBank database. Their corresponding accession numbers are shown in Figure 1: F. oxysporum (PQ721292.1, Baghdad, Iraq; Figure 1A), F. pseudograminearum (PQ721291.1, Baghdad, Iraq; Figure 1B), and F. chlamydosporum (PQ721294.1, Baghdad, Iraq; Figure 1C). The phylogenetic clustering of these isolates with reference strains from different geographical regions validates their taxonomic identity and demonstrates their genetic relatedness at a global scale. This molecular confirmation not only supports the accuracy of strain identification but also contributes to the enrichment of publicly available Fusarium genomic data from Iraq.
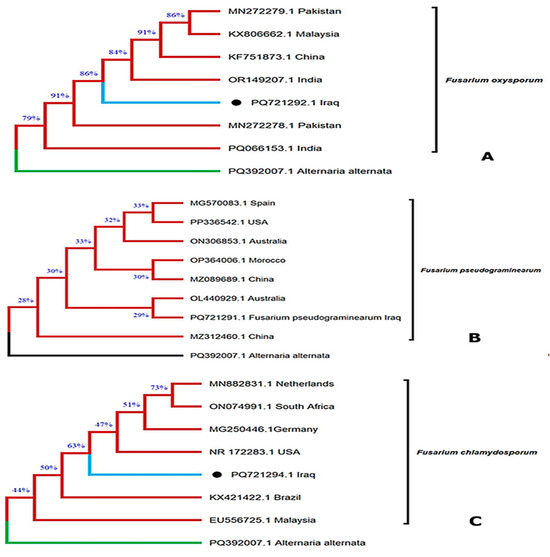
Figure 1.
Phylogenetic tree was constructed for three isolates belonging to three Fusarium species isolated from wheat stored in Iraq. (A): F. oxysporum (PQ721292.1 Iraq); (B): F. pseudograminearum (PQ721291.1 Iraq); (C): F. chlamydosporum (PQ721294.1 Iraq).
The findings of this study are in agreement with previous reports documenting the occurrence of Fusarium pseudograminearum, F. graminearum, F. boothii, and F. culmorum in infected wheat samples across Iraq [19]. Similarly, during the 2020/2021 agricultural season, F. pseudograminearum was isolated from wheat samples collected from fields in the Basra, Maysan, and Dhi Qar governorates. The present detection of F. pseudograminearum is therefore consistent with earlier observations from Basra, where multiple Fusarium species, including F. pseudograminearum, were reported in both wheat and barley crops [20]. Furthermore, F. chlamydosporum was previously reported for the first time in wheat in Iraq in that study, and its occurrence is reaffirmed by the current research.
Importantly, F. pseudograminearum and F. chlamydosporum are recognized as major causal agents of Fusarium crown rot (FCR), Fusarium head blight (FHB), and Fusarium root rot (FRR), posing a serious threat to wheat production systems in Iraq [21].
2.2. Testing the Effect of DVE on the Growth of Fusarium spp. Strains
The inhibitory effect of DVE on the tested Fusarium strains increased progressively with rising extract concentration (Table 2). At 0.5% extract concentration, the highest growth inhibition was observed in F. oxysporum strain 5 (63.37%), whereas the lowest was recorded in F. pseudograminearum strain 6 (15.16%). Increasing the extract concentration to 1.0% further enhanced the inhibitory effect, with F. oxysporum strain 5 again showing the strongest response (67.13%), while F. pseudograminearum strain 6 remained the least affected (20.64%). The maximum inhibition levels were recorded at 1.5% DVE, reaching 68.94% in F. oxysporum strain 5, compared to only 22.58% inhibition in F. pseudograminearum strain 6.

Table 2.
Mean inhibition percentage (%) of Fusarium strains treated with different concentrations of Dodonaea viscosa extract (DVE).
Analysis of variance confirmed that the effect of DVE was statistically significant across all strains and concentrations. Post hoc LSD analysis further revealed that the inhibition at 1.5% was significantly higher than at 0.5% for all strains, while the differences between adjacent concentrations (0.5% vs. 1.0% and 1.0% vs. 1.5%) were comparatively less pronounced.
These findings align with earlier reports indicating potent antifungal activity of DVE against pathogenic fungi such as Aspergillus niger and A. flavus. The variability in strain sensitivity may be attributed to differences in the phytochemical profile of the extract, which depends largely on the solvent used for extraction. Previous studies similarly reported that ethanolic extracts of D. viscosa displayed variable inhibitory effects across fungal species [22].
2.3. Identified Major Components of DVE Using GC-MS System
A total of 27 compounds were detected in the DVE based on gas chromatography–mass spectrometry (GC–MS) analysis (Table 3). The major chemical classes identified were glycosides, saponins, and alkaloids. The most abundant compound was 4-Cyclohexyl-1-(furan-2-ylmethyl)-4H,5H,7H-pyrazolo [3,4-b]pyridin-6-one, followed by 4′,5,7-trihydroxy-3,6-dimethoxyflavone, 6-methoxy-2-[2-(imidazol-1-yl)ethoxy]-8-nitroquinoline, and 3-heptanol (TMS derivative). Dodonaea viscosa is a member of the Sapindaceae family and has been reported to contain antimicrobial, antitoxic, and cytotoxic bioactive compounds [22]. It also contains catechol, a phenolic compound known for its inhibitory effects against certain fungi and bacteria, including Fusarium oxysporum and Penicillium italicum [23]. In addition, neophytadiene, a terpenoid constituent of the leaf extract, has been documented as a strong antioxidant and an active antimicrobial agent against fungal and microbial infections [24].

Table 3.
Identified major components of ethanolic leaf extract of Dodonaea viscosa using GC-MS.
Omosa et al. (2014) further reported the isolation of flavonoids such as 5-hydroxy-3,4′,7-trimethoxyflavone, santin, and pinocembrin, as well as diterpenoids including dodonic acid and hautriwaic acid from the leaf surface exudates of D. angustifolia. These phytochemical classes exhibit strong chemical similarity to the major constituents identified in the present study. Therefore, the current findings strongly confirm that Dodonaea viscosa possesses a phytochemical profile predominantly characterized by flavonoids and diterpenoids [25].
2.4. Testing the Effect of Chitosan NPs Loaded with DVE on Fusarium spp. Strains
The antifungal activity of Chitosan NPs loaded with DVE was evaluated at a concentration of 0.75%, which corresponds to the average concentration used for the alcoholic extract in the standard formulation. As shown in Table 4, the nanoparticles exhibited a clear inhibitory effect against all Fusarium strains tested. The highest inhibition rate (70.58%) was recorded against F. pseudograminearum strain 7, whereas the lowest inhibition rate (61.17%) was observed in F. chlamydosporum strain 8. Statistical analysis confirmed that the treatment exerted a significant inhibitory effect (LSD = 6.429 *), with F. pseudograminearum strain 7 being the most susceptible strain.

Table 4.
Mean inhibition percentage (%) of Fusarium strains treated with the chitosan NPs loaded with DVE.
Encapsulation of plant-derived extracts into nanocarriers is known to overcome limitations such as poor stability, limited bioavailability, and degradation of active compounds during delivery. In this study, nano-chitosan was used as a protective and controlled-release carrier for DVE, enhancing its antifungal efficiency—a finding consistent with previous reports on chitosan-based phytonanocarriers [26].
2.5. Conformational and Morphological Analysis of Chitosan NPs Loaded with DVE
Ultraviolet (UV–Vis) spectrophotometer was used as an initial confirmatory technique to verify the biosynthesis of chitosan NPs loaded with DVE. The UV–Vis spectrum of the DVE (Figure 2A) exhibited two characteristic absorption bands at 266 nm (OD ≈ 2.358) and 233 nm (OD ≈ 0.297), which are typical for aromatic phenolic and flavonoid compounds. In contrast, the spectrum of the DVE-loaded chitosan nanoparticles (Figure 2B) revealed a distinct bathochromic (red) shift in the main peak toward 275 nm (OD ≈ 2.613), accompanied by a secondary absorption band at 341 nm, indicating strong intermolecular interactions between DVE phytochemicals and the protonated amino groups of chitosan. This wavelength shift—along with the notable increase in absorbance intensity—confirms the successful encapsulation and molecular stabilization of DVE within the chitosan.
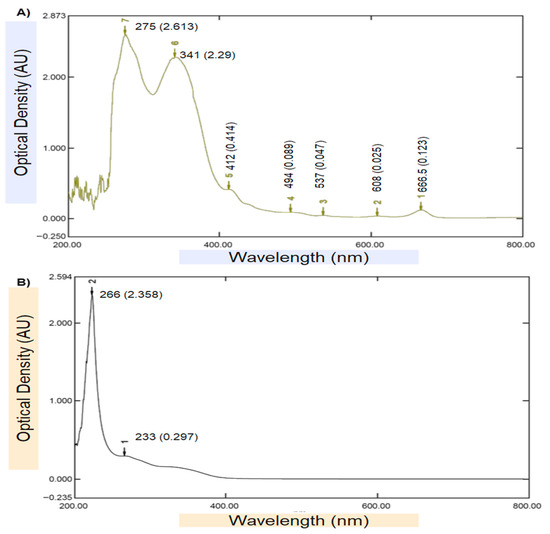
Figure 2.
UV–Visible spectrophotometer test: (A). DVE, (B). Chitosan NPs loaded with DVE.
Furthermore, the broad secondary absorbance region extended across the 400–700 nm range in the nanoparticle spectrum suggests enhanced light scattering due to nanoscale colloidal behavior and surface roughness, which is typical of plant-derived biopolymeric nanocarriers. The absence of additional degradation peaks suggests that no photolytic breakdown occurred during nanoparticle synthesis. Overall, the observed spectral changes strongly affirm the formation of chitosan NPs loaded with DVE and are consistent with previous findings for plant-extract-based biopolymeric nanocarriers [27,28].
Figure 3 exhibits the FTIR spectra of the DVE and chitosan NPs loaded with DVE and shows distinct peaks characteristic of various biomolecules, including phenol and alcohol (3610–3640 cm−1). A key observation is the difference in intensity between the amide I peak (1699.29) cm−1 in DVE spectrum (Figure 3A) and the corresponding peak (1639.94) cm−1 in the DVE-loaded chitosan NPs spectrum (Figure 3B). The higher intensity in the nanoparticle’s spectrum suggests a higher concentration of amides associated with the nanoparticles. This finding could be attributed to the attachment of many biomolecules during the synthesis process, potentially acting as a stabilizing agent. On the other hand, the absence of some peaks in chitosan NPs loaded with DVE (1150–1300, 1685–1710, 2850–3000 cm−1) referred to the attachment between DVE, and chitosan molecules and our results agreed with the result of the previous study [29]. When the C-H stretch was seen at 2927 cm−1, it provided structural information on chitosan.
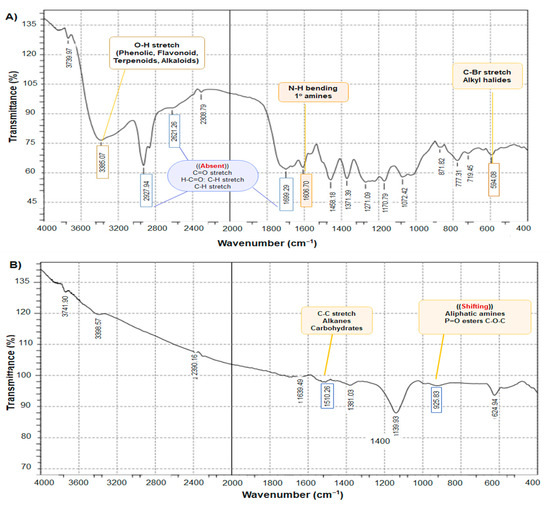
Figure 3.
FTIR spectra (A)—DVE, (B)—chitosan NPs loaded with DVE.
By comparing the DVE/chitosan NPs loaded with DVE, it was noticed that the absence of many peaks due to the cross-linking of TPP may be attributed to the fact that the large molecules were fragmented into smaller units. The interaction of chitosan with TPP was the reason for the increase in the total surface area as shown in Figure 3B, indicating that the repositioned chemical groups facilitated the efficient loading of the extract into the chitosan/TPP matrix and the formation of nano-encapsulation of the extract. As additional supporting evidence, the shifted peaks are consistent with the formation of a new complex. The modifications in the functional groups of bioactive molecules suggest a relationship with the synthesis of DVE/chitosan NPs [30].
The AFM analysis of the biosynthesized chitosan NPs loaded with DVE is presented in Figure 4. A total of 158 individual nanoparticles were characterized, with a particle density of 9.92 × 106 particles/mm2. The particles exhibited a high degree of morphological heterogeneity, showing predominantly elongated and plate-like structures with occasional sharp edges and clustered aggregates.

Figure 4.
AFM surface morphology and particle size distribution of chitosan NPs loaded with DVE.
According to the statistical output, the mean particle diameter was 105.7 nm, which is consistent with the expected nanoscale range. The surface topography displayed a relatively rough and uneven texture, as confirmed by the 3D scan, which enhances the available surface area for potential interactions with bioactive molecules such as plant-derived compounds. The mean Z-height of the particles was 205.4 nm, indicating pronounced vertical protrusions rather than a flat morphology.
The observed nanoparticle aggregation could partially be attributed to sample handling and drying effects during AFM preparation, which is a common observation in chitosan-based nanostructures. These findings are in agreement with [16], who reported similar AFM features for clove-extract-loaded chitosan nanoparticles, showing non-spherical particles and moderate aggregation behavior due to intermolecular interactions between chitosan and phytochemical constituents.
Figure 5A shows that the spherical nanoparticles with diameters of approximately 57–86 nm indicate the successful ionic gelation reaction with TPP. This morphological feature is commonly observed in chitosan-based nanoparticle systems and represents the early-stage state of nanoparticle aggregation following fabrication (Figure 5B). In the 30kX image (Figure 5C), the surface appears more complex and interlaced, where high-density regions composed of tightly packed nanoparticles are interspersed with low-density areas exhibiting pore- or void-like structures. These structural differences may be attributed to the non-homogeneous dispersion of the plant extract within the chitosan matrix or rapid solvent evaporation, which can lead to surface shrinkage. Overall, the majority of the nanoparticles exhibited a spherical morphology with a relatively uniform size distribution ranging between 38 and 79 nm.

Figure 5.
SEM micrographs of chitosan NPs loaded with DVE: (A) primary nanoparticles (100,000×), (B) secondary agglomerates after drying (50,000×), and (C) irregular surface morphology with apparent porosity (30,000×).
2.6. Effect of DVE and Chitosan NPs Loaded with DVE on the Production of DON and MON Mycotoxins
The samples were analyzed using an HPLC system, and the corresponding chromatograms are provided in Supplementary File S1. Statistical analysis revealed clear significant differences between the three treatments (control, DVE, and chitosan NPs loaded with DVE). The average DON concentration decreased after treatment with DVE, and the highest inhibition rate was observed in F. pseudograminearum strain 6 (60.23%), while the lowest was recorded for strain 2 (58.35%). When the strains were treated with chitosan NPs loaded with DVE, DON concentrations were further reduced, resulting in a marked increase in the inhibition rate. Under this treatment, the highest inhibition was recorded for F. pseudograminearum strain 2 (73.75%), whereas the lowest was observed in strain 6 (68.87%) (Table 5).

Table 5.
Deoxynivalenol (µg/L) concentrations of Fusarium strains after treatment with the DVE and chitosan NPs loaded with DVE and inhibition ratios.
It should also be noted that within the same treatment group, the differences between strains were not statistically significant (NS), consistent with the L.S.D. values shown in Table 5.
F. oxysporum strain 4, F. oxysporum strain 5 and F. chlamydosporum strain 8—which are known MON-producing strains—were treated with DVE and NPs loaded with DVE. Statistical analysis confirmed significant differences in MON inhibition among the control, DVE, and NPs loaded with DVE treatments. When free DVE was applied, the highest inhibition rate was observed in F. oxysporum strain 4 (53.67%), while the lowest was recorded in F. oxysporum strain 5 (48.25%). Upon using NPs loaded with DVE, the inhibition rate increased in all strains, reaching the highest level in F. chlamydosporum strain 8 (73.62%), followed by F. oxysporum strain 4 (72.09%) and F. oxysporum strain 5 (68.18%).
Within each treatment group (control, DVE, and NPs loaded with DVE), there were no statistically significant differences (NS) between MON concentrations of the three Fusarium strains. However, numerically, the lowest MON concentration after NPs loaded with DVE treatment was observed in F. oxysporum strain 4 (12.44 μg/L), while the highest was recorded in F. oxysporum strain 5 (13.25 μg/L) (Table 6).

Table 6.
Moniliformin (µg/L) concentrations of Fusarium strains after treatment with the DVE and chitosan NPs loaded with DVE and inhibition ratios.
The presence of F. pseudograminearum is considered a strong indicator of potential mycotoxin contamination, particularly deoxynivalenol (DON), as this species is a well-known DON producer. Its detection may reflect a high toxin load in plant tissues, even in the absence of visible disease symptoms [31]. In the present study, our findings agreed with a survey conducted on seventeen wheat fields in the Basra Governorate, where DON was detected in six fields at concentrations ranging from 8 to 1060 µg/kg [21]. HPLC analysis further confirmed the presence of MON toxin, produced by F. oxysporum strains 4 and 5, and F. chlamydosporum strain 8. To the best of our knowledge, this is the first report to demonstrate MON production by Fusarium spp. isolated from wheat samples stored in Iraq. Research on MON remains limited, and further studies are needed to clarify its toxicological impact, behavior under storage and processing conditions, and the efficacy of control strategies [8].
Mycotoxin biosynthesis by Fusarium spp. is influenced by multiple factors, including microbial interactions within the matrix, agricultural practices, and environmental conditions such as temperature and humidity. For instance, DON production has been shown to be maximized at approximately 28 °C. Therefore, discrepancies observed among different studies are likely attributable to variations in fungal isolates, substrate composition, and experimental conditions [32].
3. Conclusions
In summary, this study demonstrated that Dodonaea viscosa extract (DVE) and its nano-formulation effectively inhibited the growth of several Fusarium species isolated from stored wheat and significantly reduced the production of the major mycotoxins deoxynivalenol (DON) and moniliformin (MON). The nano-formulated extract exhibited a higher inhibitory effect than the conventional extract, confirming that nanotechnology enhances the antifungal and antimycotoxigenic potential of plant-derived compounds. However, the study was limited to in vitro evaluations and a restricted number of Fusarium isolates. Further research should therefore focus on in vivo applications, field-scale validation, and toxicological safety assessments to confirm the practical potential of Dodonaea viscosa nanoparticle in grain protection. Additionally, mechanistic studies are needed to elucidate the molecular interactions between the nano-formulated compounds and fungal cell structures. Overall, these findings highlight Dodonaea viscosa loaded chitosan nanoparticle as promising eco-friendly alternatives to chemical fungicides for the management of Fusarium contamination and mycotoxin accumulation in stored cereals.
4. Materials and Methods
4.1. Wheat Sampling and Isolation of Fusarium spp. Strains from Seeds
During the period from April to May 2021, wheat silos in the eighteen governorates representing the southern, central and northern regions of Iraq: Baghdad (4), Al-Anbar (3), Erbil (3), Babil (3), Basra (2), Dhi Qar (3), Duhok (3), Diyala (2), Karbala (2), Muthanna (2), Nineveh (4), Najaf (3), Qadisiyah (2), Salah al-Din (3), Sulaymaniyah (3), Wasit (4), Ta’mim (3), Maysan (2) were visited for wheat sampling. These regions were characterized by different environmental conditions in terms of temperature, humidity, rainfall and soil type. Samples were collected from the warehouses and silos of the General Company for Grain Trade affiliated with the Iraqi Ministry of Trade. Wheat samples were collected from silos according to the International Standards Organization (ISO) 136,901.2 [33]. The collected material was treated as a composite (bulk) sample, where all subsamples from each batch were combined, homogenized, and subsequently reduced to a 3 kg laboratory sample using a rotary divider.
All samples were placed in labeled plastic bags and stored at 4 °C until analysis. The wheat grains were surface-sterilized using 1% sodium hypochlorite (NaOCl) for 3 min, rinsed twice with sterile distilled water, and then air-dried on sterile filter paper. Ten grains per plate were subsequently cultured on potato dextrose agar (PDA, Merck, Darmstadt, Germany) supplemented with chloramphenicol (50 mg/L) in Petri dishes. The plates were incubated at 25 ± 2 °C for 5 days, after which emerging fungal colonies were subcultured on fresh PDA plates for purification and species identification [34]. Purified isolates were identified at the genus level based on the macroscopic characteristics of the colonies (color, morphology, growth pattern) and microscopic examination using a digital microscope (Olympus, Tokyo, Japan). Microscopic slides were prepared with lactophenol cotton blue stain, and fungal identification was performed according to established taxonomic keys and standard mycological procedures [35].
4.2. Identification of Fusarium Strains
The Fusarium spp. strains were genetically identified by Polymerase Chain Reaction (PCR) technique using the appropriate primers, ITS1 (5′ TCCTCCGCTTATTGATATGC 3′) and ITS4 (5′ TCCGTAGGTGAACCTGCGG 3′). DNAs of the strains were isolated using the Presto Mini gDNA Yeast kit (GBYB100, Geneaid Biotech, New Taipei City, Taiwan) and PCR was performed in a total volume of 20 μL containing 1 μL of each primer pairs, 6.5 μL AccuPower® PCR PreMix, 5 μL of extracted DNA, and 6.5 μL nuclease free water [36]. Amplification was performed in a thermocycler (Bio-Rad, Pleasanton, CA, USA) under the following conditions: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 5 min. Prior to sequencing, PCR amplicons were separated by electrophoresis in a 1X TAE Buffer using agarose gel (1% w/v) stained with ethidium bromide at 0.1 µg/mL. The size of the PCR products was estimated by using a 100 bp size marker (Bioneer, Daejeon, Korea) [37]. Electrophoresis results of Fusarium strains (strains 4, 3, and 6) are exhibited in Figure 6.

Figure 6.
Electrophoresis results of Fusarium strains: (A) strain 4, (B) strain 3 and 6.
Commercially prepared PCR amplicons of each strain were sequenced in both forward and reverse directions by an automated DNA sequencer (ABI3730XL) according to the instruction manuals of Macrogen Company (Daejeon, Republic of Korea). The DNA sequences were edited, aligned, and the consensus sequences were generated with BioEdit Sequence Alignment Editor Software version 7.1 (DNASTAR, Madison, WI, USA). Each of the consensus sequences of the Fusarium strains was subjected to the Basic Local Alignment Search Tool (BLAST, version 2.15.0+) program and compared with those strains available at the National Center for Biotechnology Information (NCBI) and deposited in GenBank. For phylogenetic analysis, the accession numbers of these strains are listed in Table 1, and MEGAX software (Version 10.1) was used to construct the phylogenetic tree using the neighbor-joining method (Figure 1) [38]. The FASTA sequences of the three Fusarium isolates identified in this study were deposited in GenBank (supported by Macrogen, Seoul, Republic of Korea).
4.3. Preparation of Alcoholic Extract for Dodonaea viscosa (DVE)
Dodonaea viscosa leaves were collected from the gardens of Baghdad University, dried and ground into powder. To prepare the Dodonaea viscosa extract (DVE), one liter of ethyl alcohol (96%) was added to 300 g of Dodonaea viscosa leaves powder in a glass jar and stirred for three days at room temperature, 28 °C. The mixture was filtered with a Buchner funnel and then filtered with a glass funnel to remove impurities. A rotary evaporator was used to remove the solvent and dry the solution at 40–60 °C. After three hours, a viscous liquid was obtained and placed in tightly sealed containers in the freezer at −20 °C until used in the experiment [39]. After solvent evaporation, a viscous crude extract was obtained (48.6 g from 300 g of dry leaf powder), corresponding to an extraction yield of 16.2% (w/w).
4.4. Identification of the Major Components of DVE Using GC-MS System
A GC-MS system QP2010 model (Shimadzu, Kyoto, Japan) was used for the identification of components of DVE. Helium was used as a carrier gas, and a micro-syringe was used to inject eight µL of DVE into the GC-MS system. The column temperature program started at 40 °C. The injector temperature was 280 °C while the detector was at 200 °C. The split ratio was 1:30. The data was processed using software to match and identify the organic compounds in the extracts. The results were evaluated by comparing the retention indices and fragmentation patterns of the mass spectra with those stored in a computer library. The compounds were identified by comparing their spectra with those in the NIST08.LIB mass spectra libraries [40].
4.5. Biosynthesis of Chitosan Nanoparticles Loaded DVE
Chitosan (AC00671K, Avonchem Ltd., Macclesfield, UK) was purchased commercially. It was dissolved in acetic acid (200 mg/100 mL acetic acid) and mixed with Dodonaea viscosa extract (1 g DVE/100 mL DW) in equal amounts, and the reaction was carried out using a reflux distillation apparatus. The product was separated using syringe filters, washed three times with pure methanol, hot distilled water to remove polar and irrelevant compounds, and finally dried in an electric oven at 50 °C [41].
The ion coagulation method was used to produce chitosan nanoparticles loaded with DVE. Acetic acid was used as a solvent at 1%, and 5 mg/mL of the product (chitosan NPs loaded with DVE) was dissolved in it. Tripolyphosphate (TPP) was used at a 1:2.5 (w/w) ratio and stirring continued while adjusting the pH to 7 until a clear solution was obtained. This reaction was carried out at 25 °C and continued for 6 h [16].
4.6. Measurement and Morphological and Structural Characterization of Nanoparticles
A dual-beam UV–Vis spectrophotometer (Shimadzu UV-1800, Kyoto, Japan) was used, and all spectra were recorded using a quartz cuvette with a 1 cm optical path length at room temperature. Distilled deionized water was used as the blank. Absorbance was measured in the range of 200–800 nm, and the concentrated samples were diluted 1:10 prior to analysis [42]. Fourier-transform infrared (FT-IR) spectroscopy was performed to detect the vibrational transitions of the functional groups, which occur in the mid-infrared region (4000–400 cm−1). The spectra were recorded using an FT-IR spectrometer (Shimadzu 8400S, Japan), and the characteristic absorption bands were analyzed to identify the functional groups present in the sample [43]. The surface morphology of nanoparticles was examined by atomic force microscopy (AFM, NaioAFM2022, Nanosurf AG, Switzerland), where samples to be examined were dropped and distributed on the microscope platform, and the analysis was carried out according to the procedure followed by [44]. A Scanning Electron Microscope (SEM, TESCAN-MIRA3 Czech Republic) was used to investigate the morphology of chitosan NPs-loaded DVE at different magnifications (100 kX, 50 kX, and 30 kX) [45].
4.7. In Vitro Efficacy of DVE and Chitosan NPs Loaded with DVE in Inhibiting the Growth of Fusarium spp.
The DVE was added at three concentrations (0.5, 1, and 1.5%) to each 100 mL of PDA after autoclave. After the medium solidified, all plates were inoculated with each freshly grown Fusarium spp. strains (1 cm piece in size taken with a cork hole) and placed in the center of the plate. The control group consisted of Petri dishes containing standard PDA (potato dextrose agar) medium (without added DVE/nanoparticles), inoculated with Fusarium strains tested only. The experiment was conducted at a rate of three replicates for each concentration and incubated at 25 °C for seven days in an incubator. The same previous steps were carried out to examine the effect of the DVE-loaded chitosan NPs with the concentration of 0.75% being used, (representing the average concentrations used as extract) [46]. The inhibition rate was calculated using follower formula [47].
where
Inhibition percentage (%) = ((R − R1)/R) × 100
R = growth diameter rate in control dishes (distance measured in mm);
R1 = growth diameter rate in treatment dishes.
4.8. Testing the DON and MON Mycotoxins Production from Fusarium spp. Strains After DVE and Chitosan NPs Loaded with DVE Treatments
For mycotoxin induction, 150 g of wheat was moistened with 100 mL of distilled water to serve as the culture substrate. The glass incubation bottles were sterilized in an autoclave at 121 °C and 1.5 kg/cm2 pressure for 20 min, after which they were inoculated with Fusarium spp. previously grown on PDA. The inoculated wheat was thoroughly mixed and incubated at 25 ± 2 °C for 21 days. The DVE and its nano-chitosan-loaded formulation were applied only during the active toxin production phase at different concentrations. The reduction in DON and MON levels as a direct result of the extract/nanoparticle interference with active mycotoxin biosynthesis was quantified using high-performance liquid chromatography (HPLC). A HPLC system (Syknm S4011, Stuttgart, Germany) was used to measure DON and MON concentrations, following previously reported protocols with minor modifications [48,49].
For the extraction of DON and MON, 20 g of sample was mixed with 100 mL of distilled water in Nalgene bottles and shaken for 1 h using a reciprocating shaker. The mixture was then centrifuged at 3200× g for 5 min and filtered through Whatman No. 1 folded filter paper. A 2 mL aliquot of the filtrate was diluted to 10 mL with distilled water and passed through a 0.45 µm membrane filter using a micro-syringe for clarification. For HPLC confirmation, another 2 mL aliquot was diluted to 10 mL with water and loaded onto an Oasis HLB cartridge preconditioned with 5 mL of methanol followed by 5 mL of water. The cartridge was washed with 2 mL of water, dried under vacuum for 15 min, and eluted with 4 mL of methanol. The eluate was evaporated to dryness under a gentle nitrogen stream and reconstituted in 200 µL of methanol/0.1% aqueous acetic acid + 1 mM ammonium acetate (20:80, v/v). HPLC separation was performed using a Zorbax SB-C18 column (25 cm × 4.6 mm i.d.) with a mobile phase consisting of water and acetonitrile (30:70, v/v) at a flow rate of 1.2 mL/min [50]. Calibration curves were constructed using certified DON and MON standards within the range of 1–5 µg/L. The calibration curves showed linearity with R2 > 0.999 for both toxins. The retention time was 3.9 and 6 min for deoxynivalenol and moniliformin, respectively. LOD and LOQ values were 0.062 and 0.189 µg/L for moniliformin while they were 0.137 and 0.415 µg/L for deoxynivalenol, respectively. The following equation was used to calculate the mycotoxin inhibition ratio:
Inhibition ratio (%) = ((B0 − B1)/B0) × 100
B0, B1 values represent the DON and MON mycotoxin concentration produced before treatment (control group), and after treatment, respectively [51]. (Figures S1–S4 shows the chromatograms of mycotoxin standards, before and after treatments).
4.9. Statistical Analysis
Laboratory experiments were conducted using three replicates for each treatment [52]. The Statistical Analysis System-SAS (Version 9.6) program was used to detect the effect of different factors on study parameters. This study used the least significant difference (LSD) to significantly compare means (ANOVA/One-way).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins17110551/s1, Figure S1. Deoxynivalenol and Moniliformin standard chromatogram by HPLC chromatographic analysis. (A) DON standard results, (B) MON standard. Figure S2. Chromatograms of deoxynivalenol and moniliformin before treatment by HPLC chromatography analysis; (sample 2) F. pseudograminearum strain 2, (sample 3) F. pseudograminearum strain 3, (sample 4) F. oxysporum strain 4, (sample 5) F. oxysporum strain 5, (sample 6) F. pseudograminearum strain 6, (sample 7) F. pseudograminearum strain 7, (sample 8) F. chlamydosporum strain 8. Figure S3. Chromatograms of deoxynivalenol and moniliformin after treatment of Dodoneae viscosa extract (DVE) by HPLC chromatography analysis (sample 2) F. pseudograminearum strain 2, (sample 3) F. pseudograminearum strain 3, (sample 4) F. oxysporum strain 4, (sample 5) F. oxysporum strain 5, (sample 6) F. pseudograminearum strain 6, (sample 7) F. pseudograminearum strain 7, (sample 8) F. chlamydosporum strain 8. Figure S4. Chromatograms of deoxynivalenol and moniliformin after treatment of chitosan NPs loaded DVE by HPLC chromatography analysis (sample 2) F. pseudograminearum strain 2, (sample 3) F. pseudograminearum strain 3, (sample 4) F. oxysporum strain 4, (sample 5) F. oxysporum strain 5, (sample 6) F. pseudograminearum strain 6, (sample 7) F. pseudograminearum strain 7, (sample 8) F. chlamydosporum strain 8.
Author Contributions
H.A.S.A. and S.H. contributed to article supervision, Conceptualization, data interpretation, and assistance and advice in writing the manuscript. H.A.S.A. contributed to wheat sample collection, isolation of Fusarium strains, Methodology and formal analysis and testing. K.K. also contributed to data analysis, tables, and results, and for writing, visualization and editing the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that there are no conflicts of interest related to this article.
References
- Bevan, J.R.; Firth, C.; Neicho, M. Storage of Organically Produced Crops; MAFF Project no: OF0127T; Ministry of Agriculture Fisheries and Food (MAFF) Report; Registered in Cardiff No: 2188402; The Henry Doubleday Research Association Ryton Organic Gardens: Coventry, UK, 1997; 216p, Available online: https://orgprints.org/id/eprint/8241/1/Storage_organic_produce_report.pdf (accessed on 1 August 2025).
- Birck, N.M.M.; Lorini, I.; Scussel, V.M. Fungus and mycotoxins in wheat grain at post harvest. In Proceedings of the 9th International Working Conference on Stored Product Protection PS2-12, São Paulo, Brazil, 15–18 October 2006; Volume 6281, pp. 198–205. [Google Scholar]
- Lacey, J.; Bateman, G.L.; Mirocha, C.J. Effects of infection time and moisture on development of ear blight and deoxynivalenol production by Fusarium spp. in wheat. Ann. Appl. Biol. 1999, 134, 277–283. [Google Scholar] [CrossRef]
- Oadi, M. Mycotoxin: Global risk and silent killer in our food. Int. J. Agric. Sci. Vet. Med. 2020, 8, 26–32. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Cui, H.; Wang, S.; Yang, X.; Zhang, W.; Chen, M.; Wu, Y.; Li, S.; Li, L.; Cai, D.; Guo, B.; et al. Predictive models for assessing the risk of Fusarium pseudograminearum mycotoxin contamination in post-harvest wheat with multi-parameter integrated sensors. Food Chem. X 2022, 16, 100472. [Google Scholar] [CrossRef]
- Ivić, D.; Domijan, A.M.; Peraica, M.; Miličević, T.; Cvjetković, B. Kontaminacija zrna pšenice, kukuruza, soje i graška vrstama Fusariuma u Hrvatskoj. Arhiv za Higijenu Rada i Toksikologiju 2009, 60, 435–441. [Google Scholar] [CrossRef]
- Radić, B.Đ.; Kos, J.J.; Tanackov, S.D.K.; Hajnal, E.P.J.; Mandić, A.I. Occurrence of Moniliformin in cereals. Food Feed Res. 2019, 46, 149–160. [Google Scholar] [CrossRef]
- Vishwakarma, M.; Haider, T.; Soni, V. Update on fungal lipid biosynthesis inhibitors as antifungal agents. Microbiol. Res. 2024, 278, 127517. [Google Scholar] [CrossRef]
- Beshah, F.; Hunde, Y.; Getachew, M.; Bachheti, R.K.; Husen, A.; Bachheti, A. Ethnopharmacological, phytochemistry and other potential applications of Dodonaea genus: A comprehensive review. Curr. Res. Biotechnol. 2020, 2, 103–119. [Google Scholar] [CrossRef]
- Patel, M.; Sristava, V.; Ahmad, A. Dodonaea viscosa var. angustifolia derived 5,6,8-trihydroxy-7,4’dimethoxy flavone inhibits ergosterol synthesis and the production of hyphae and biofilm in Candida albicans. J. Ethnopharmacol. 2020, 259, 112965. [Google Scholar] [CrossRef]
- Zonyane, S.; Van Vuuren, S.F.; Makunga, N.P. Antimicrobial interactions of Khoi-San poly-herbal remedies with emphasis on the combination; Agathosma crenulata, Dodonaea viscosa and Eucalyptus globulus. J. Ethnopharmacol. 2013, 148, 144–151. [Google Scholar] [CrossRef]
- Khurram, M.; Khan, M.A.; Hameed, A.; Abbas, N.; Qayum, A.; Inayat, H. Antibacterial Activities of Dodonaea viscosa using Contact Bioautography Technique. Molecules 2009, 14, 1332–1341. [Google Scholar] [CrossRef]
- Peters, R.; Brandhoff, P.; Weigel, S.; Marvin, H.; Bouwmeester, H.; Aschberger, K.; Rauscher, H.; Amenta, V.; Arena, M.; Botelho Moniz, F.; et al. Inventory of nanotechnology applications in the agricultural, feed and food sector. EFSA Support. Publ. 2014, 11, 621E. [Google Scholar] [CrossRef]
- Ibrahim, I.K.; Thalaj, K.M. Mycotoxins and Their Effects and Its Risks, 1st ed.; Dar Al-Kutub and Documents; Abaa Center for Agricultural Research: Baghdad, Iraq, 1998; 343p. [Google Scholar]
- Shaghati, H.A.; Jassim, E.H. Antibacterial activity of Chitosan nanoparticles loaded with Syzygium aromaticum extract against Klebsiella pneumonia. Iraqi J. Biotechnol. 2023, 22, 31–43. [Google Scholar]
- Mosa, M.A.; El-Abeid, S.E. Chitosan-loaded copper oxide nanoparticles: A promising antifungal nanocomposite against Fusarium wilt disease of tomato plants. Sustainability 2023, 15, 14295. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Al-Dairi, S.S.; Al-Waily, D.S. Isolated and diagnose of some Fusarium species causing head blight in wheat in the Southern Region of Iraq. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023; Volume 1262, p. 092011. [Google Scholar] [CrossRef]
- Hameed, M.A.; Rana, R.M.; Ali, Z. Identification and characterization of a novel Iraqi isolate of Fusarium pseudograminearum causing crown rot in wheat. Genet. Mol. Res. 2012, 11, 1341–1348. [Google Scholar] [CrossRef]
- Minati, M.H.; Mohammed-Ameen, M.K. Novel report on six Fusarium species associated with head blight and crown rot of wheat in Basra province, Iraq. Bull. Natl. Res. Cent. 2019, 43, 139. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. A review on Dodonaea viscosa: A potential medicinal plant. IOSR J. Pharm. 2017, 7, 10–21. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforschung C 2006, 61, 639–642. [Google Scholar] [CrossRef]
- Pratama, O.A.; Tunjung, W.A.S.; Sutikno, S.; Daryono, B.S. Bioactive compound profile of melon leaf extract (Cucumis melo L. ‘Hikapel’) infected by downy mildew. Biodiversitas 2019, 20, 3448–3453. [Google Scholar] [CrossRef]
- Omosa, L.K.; Amugune, B.; Ndunda, B.; Milugo, T.K.; Heydenreich, M.; Yenesew, A.; Midiwo, J.O. Antimicrobial flavonoids and diterpenoids from Dodonaea angustifolia. S. Afr. J. Bot. 2014, 91, 58–62. [Google Scholar] [CrossRef]
- Farmoudeh, A.; Shokoohi, A.; Ebrahimnejad, P. Preparation and evaluation of the antibacterial effect of chitosan nanoparticles containing ginger extract tailored by central composite design. Adv. Pharm. Bull. 2021, 11, 643–650. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.A.; Eltarahony, M.; Hafez, E.E.; Bashir, S.I. Green fabrication of chitosan nanoparticles using Lavendula angustifolia, optimization, characterization and in vitro antibiofilm activity. Sci. Rep. 2023, 13, 11127. [Google Scholar] [CrossRef]
- Duraisamy, N.; Dhayalan, S.; Shaik, M.R.; Shaik, A.H.; Shaik, J.P.; Shaik, B. Green synthesis of chitosan nanoparticles using of Martynia annua L. ethanol leaf extract and their antibacterial activity. Crystals 2022, 12, 1550. [Google Scholar] [CrossRef]
- Athiyabama, M.S.; Boomija, R.V.; Muthukumar, S.; Gandhi, M.; Salma, S.; Kokila Prinsha, T.; Rengasamy, B. Green synthesis of chitosan nanoparticles using tea extract and its antimicrobial activity against economically important phytopathogens of rice. Sci. Rep. 2024, 14, 7381–7391. [Google Scholar] [CrossRef]
- Almukhtar, J.G.J.; Karam, F.F. Preparation characterization and application of Chitosan nanoparticles as drug carrier. J. Phys. Conf. Ser. 2020, 1664, 012071. [Google Scholar] [CrossRef]
- Xu, F.; Shi, R.; Liu, L.; Li, S.; Wang, J.; Han, Z.; Liu, W.; Wang, H.; Liu, J.; Fan, J.; et al. Fusarium pseudograminearum biomass and toxin accumulation in wheat tissues with and without Fusarium crown rot symptoms. Front. Plant Sci. 2024, 15, 1356723. [Google Scholar] [CrossRef]
- Mahmoud, A.L.; Kilany, A.H.; Hassan, E.A. Antifungal activity of Lysinibacillus macroides against toxigenic Aspergillus flavus and Fusarium proliferatum and analysis of its mycotoxin minimization potential. BMC Microbiol. 2023, 23, 269. [Google Scholar] [CrossRef]
- Armitage, D. Grain Sampling Methods to Achieve Consumer Confidence and Food Safety; Report, HGCA (Project No 2912); AHDB: Warwickshire, UK, 2003; pp. 1–34. Available online: https://projectblue.blob.core.windows.net/media/Default/Research%20Papers/Cereals%20and%20Oilseed/rr50_complete_final_report.pdf (accessed on 10 August 2025).
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Blackie Academic and Professional: London, UK; Academic Press: New York, NY, USA, 2009; p. 520. [Google Scholar] [CrossRef]
- Alsohaili, S.A.; Bani-Hasan, B.M. Morphological and molecular identification of fungi isolated from different environmental sources in the Northern Eastern desert of Jordan. Jordan J. Biol. Sci. 2018, 11, 329–337. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Zhang, K.; McClure, J.A.; Conly, J.M. Enhanced multiplex PCR assay for typing of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. Mol. Cell. Probes 2012, 26, 218–221. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Photochemical Methods; Halsted Press: Ultimo, Australia; John Wiely and Sons: New York, NY, USA, 1973; 278p. [Google Scholar]
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography-mass spectrometry (GC-MS). J. Pharmacogn. Phytother. 2015, 7, 107–125. [Google Scholar] [CrossRef]
- Abd El-Ghaffar, M.A.; Hashem, M.S. Chitosan and its amino acids condensation adducts as reactive natural polymer supports for cellulase immobilization. Carbohydr. Polym. 2010, 81, 507–516. [Google Scholar] [CrossRef]
- Agarwal, M.; Agarwal, M.K.; Shrivastav, N.; Pandey, S.; Das, R.; Gaur, P. Preparation of chitosan nanoparticles and their in-vitro characterization. Int. J. Life-Sci. Sci. Res. 2018, 4, 1713–1720. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Jaafar, J.; Ismail, A.F.; Othman, M.H.; Rahman, M.A. Fourier transform infrared (FTIR) spectroscopy. In Membrane Characterization; Nidal, H., Ahmad, F.I., Takeshi, M., Darren, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–29. [Google Scholar] [CrossRef]
- Du, W.L.; Xu, Z.R.; Han, X.Y.; Xu, Y.L.; Miao, Z.G. Preparation, characterization and adsorption properties of chitosan nanoparticles for eosin Y as a model anionic dye. J. Hazard. Mater. 2008, 153, 152–156. [Google Scholar] [CrossRef]
- Shaghati, H.A. Characterization of Chitosan Nanoparticles Coated with Syzygium aromaticum Extract and Evaluation Their Inhibitory Activity Against Klebsiella pneumoniae. Master’s Thesis, University of Baghdad, Institute of Genetic Engineering and Biotechnology, Baghdad, Iraq, 2022. [Google Scholar]
- Nwankiti, A.O.; Gwa, V.I. Evaluation of antagonistic effect of Trichoderma harzianum against Fusarium oxysporum causal agent of white yam (Dioscorea rotundata Poir) tuber rot. Trends Tech. Sci. Res. 2018, 1, 12–18. [Google Scholar] [CrossRef]
- Korsten, L.; De Jager, E.S. Mode of Action of Bacillus subtilis for Control of Avocado Postharvest Pathogens; South African Avocado Growers’ Association Yearbook: Tzaneen, South Africa, 1995; Volume 18, pp. 124–130. Available online: http://avocadosource.com/Journals/SAAGA/SAAGA_1995/SAAGA_1995_PG_124-130.pdf (accessed on 17 October 2025).
- Shotwell, O.L.; Hesseltine, C.W.; Stubblefield, R.D.; Sorenson, W.G. Production of aflatoxin on rice. Appl. Microbiol. 1966, 14, 425–428. [Google Scholar] [CrossRef]
- Lai, X.; Zhang, H.; Liu, R.; Liu, C. Potential for Aflatoxin B1 and B2 production by Aspergillus flavus strains isolated from rice samples. Saudi J. Biol. Sci. 2015, 22, 176–180. [Google Scholar] [CrossRef]
- Gonçalves, C.; Mischke, C.; Stroka, J. Determination of Deoxynivalenol and its major conjugates in cereals using an organic solvent-free extraction and IAC clean-up coupled in-line with HPLC-PCD-FLD. Food Addit. Contam. Part A 2020, 37, 1765–1776. [Google Scholar] [CrossRef]
- Yazid, S.N.E.; Tajudin, N.I.; Razman, N.A.A.; Selamat, J.; Ismail, S.I.; Sanny, M.; Samsudin, N.I.P. Mycotoxigenic fungal growth inhibition and multi-mycotoxin reduction of potential biological control agents indigenous to grain maize. Mycotoxin Res. 2023, 39, 177–192. [Google Scholar] [CrossRef] [PubMed]
- SAS Statistical Analysis System, User’s Guide. Statistical, Version 9.6; SAS Institute Inc.: Cary, NC, USA, 2018.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).