A General Food Chain Model for Bioaccumulation of Ciguatoxin into Herbivorous Fish in the Pacific Ocean Suggests Few Gambierdiscus Species Can Produce Poisonous Herbivores, and Even Fewer Can Produce Poisonous Higher Trophic Level Fish
Abstract
1. Introduction
2. Results and Discussion
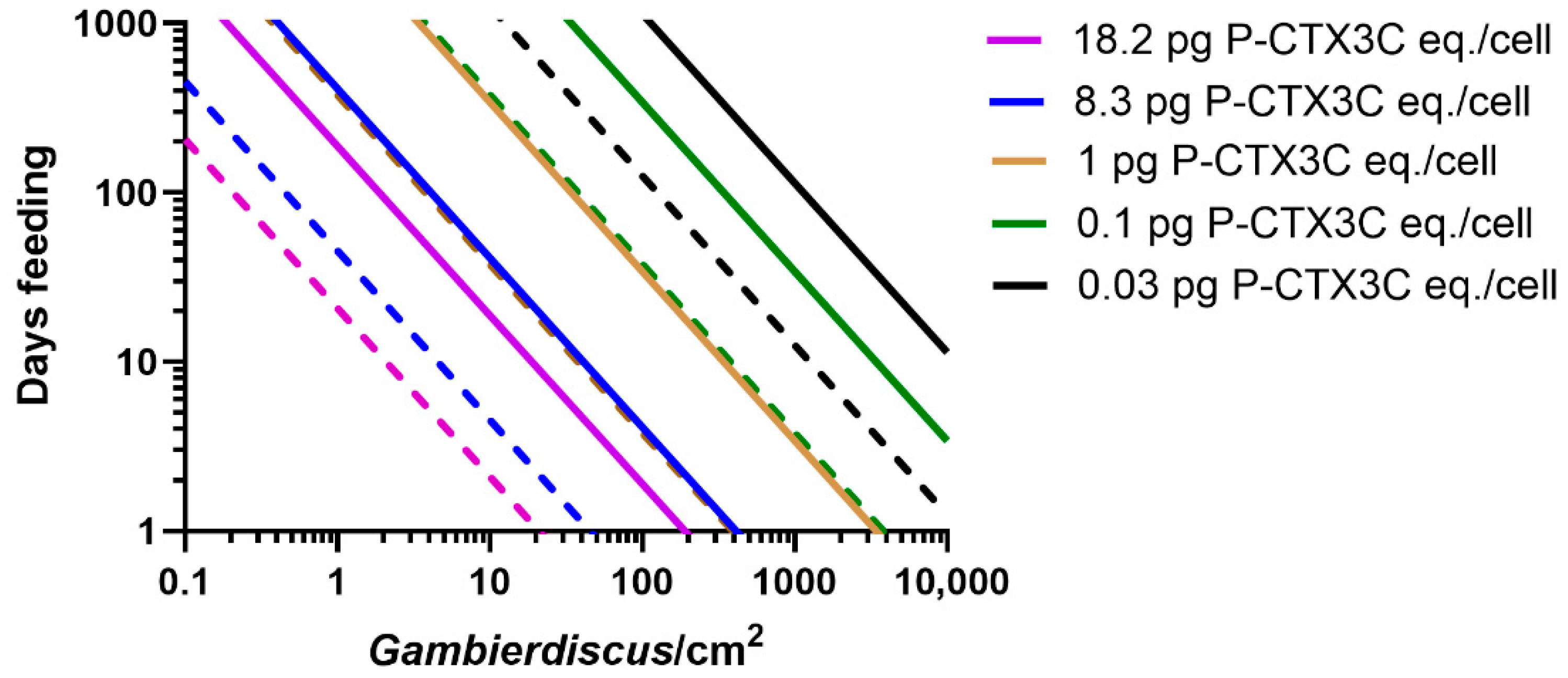
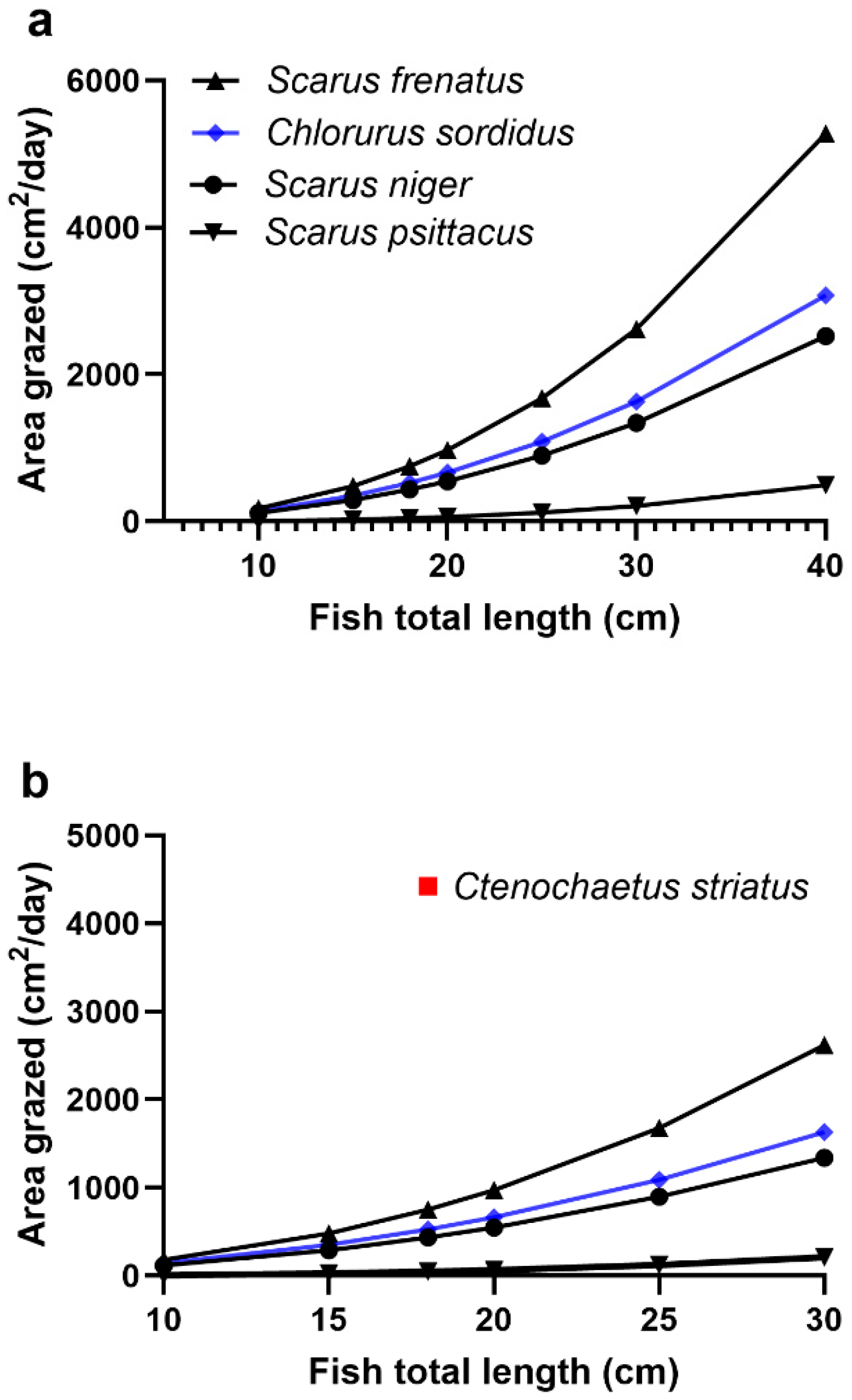
2.1. Modelling the Bioaccumulation of P-CTX3C into the Flesh of Parrotfish
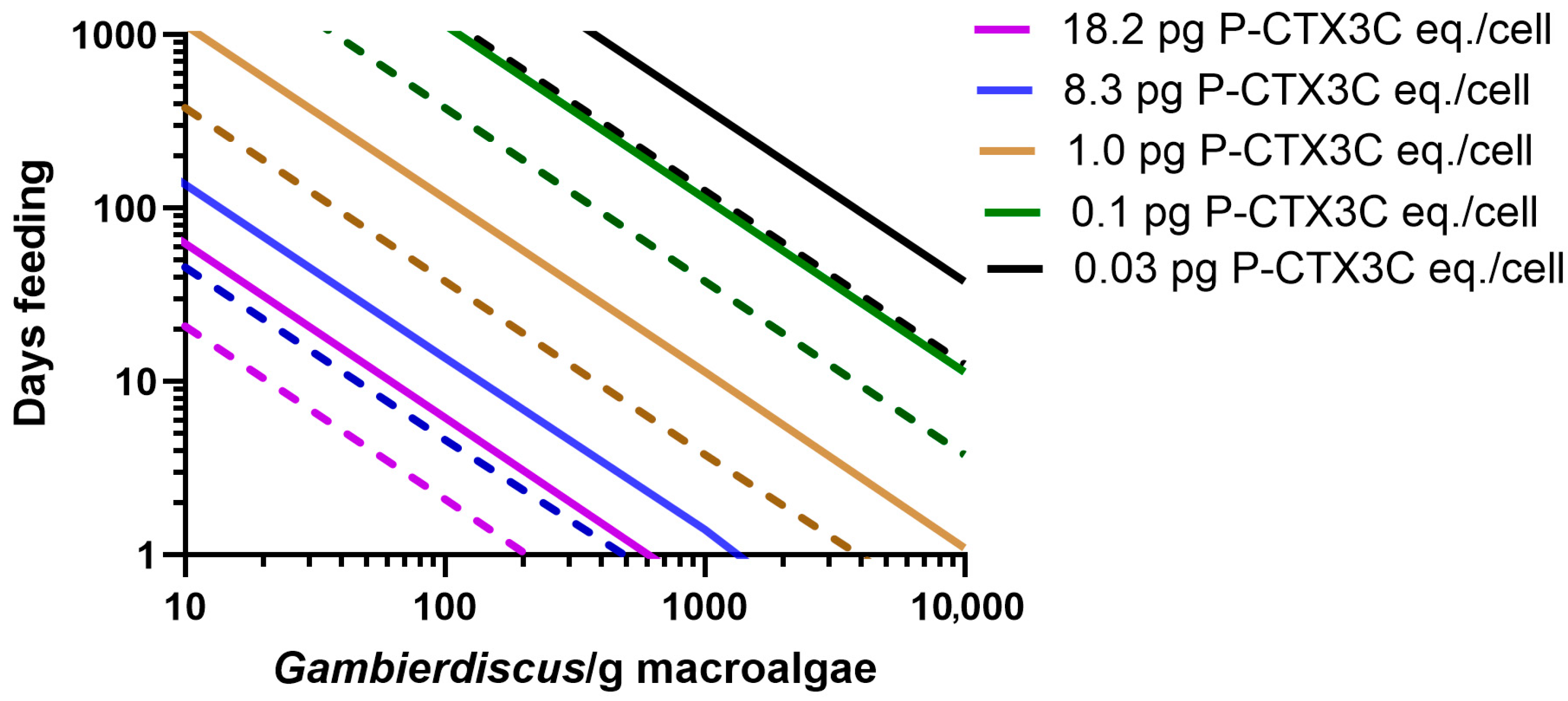
2.2. Modelling the Bioaccumulation of P-CTX3C into the Flesh of Naso Unicornis
2.3. The Species of Herbivore Feeding on Gambierdiscus Affects the Risk of Ciguateric Fishes Being Produced in Food Chains
2.4. More than One Food Chain Can Produce Poisonous Herbivores but Only High CTX-Producing Benthic Dinoflagellates Likely Cause Poisonous Carnivores
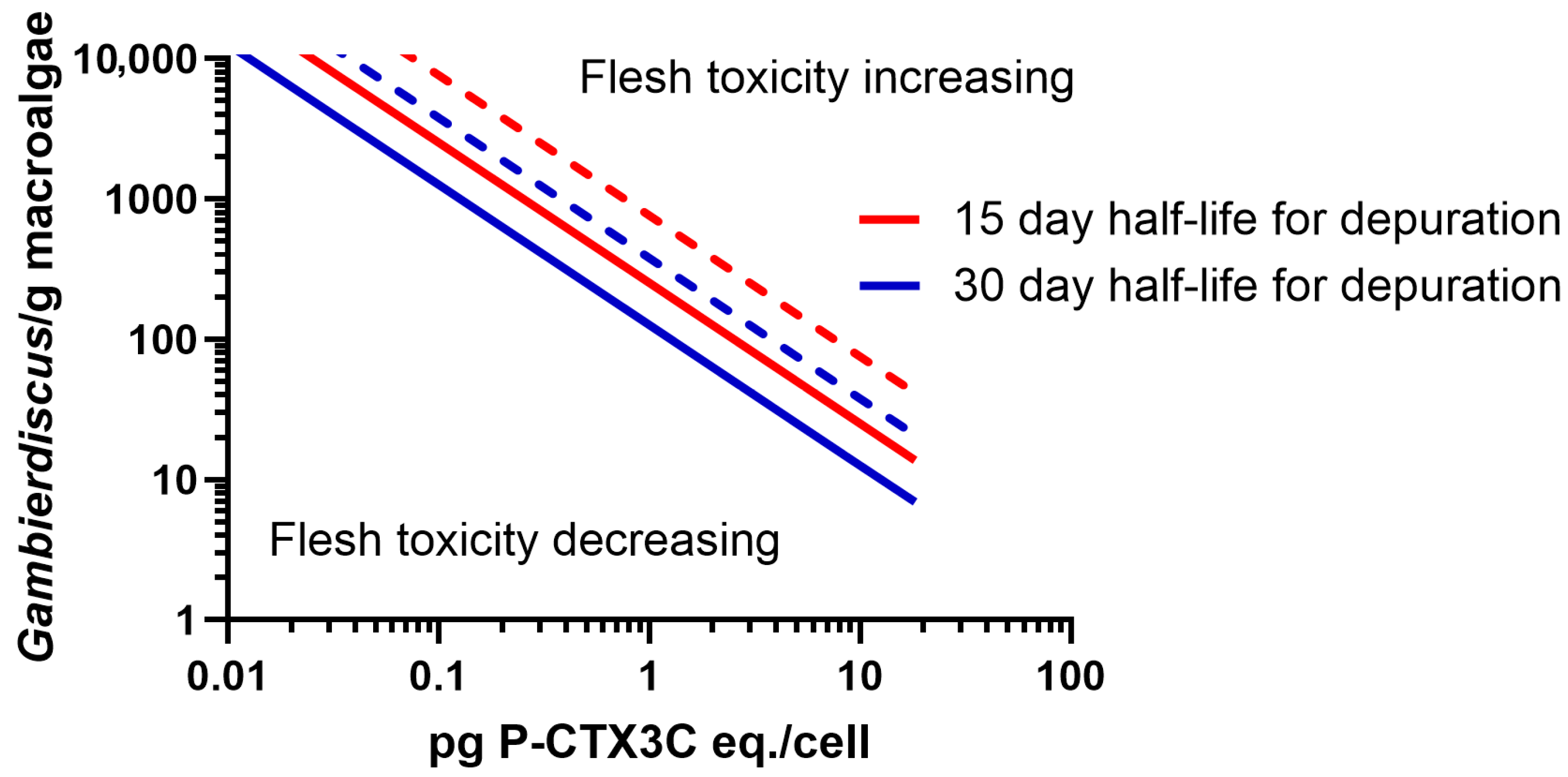
2.5. Depuration: The Missing Link of Food Chain Models
3. Conclusions
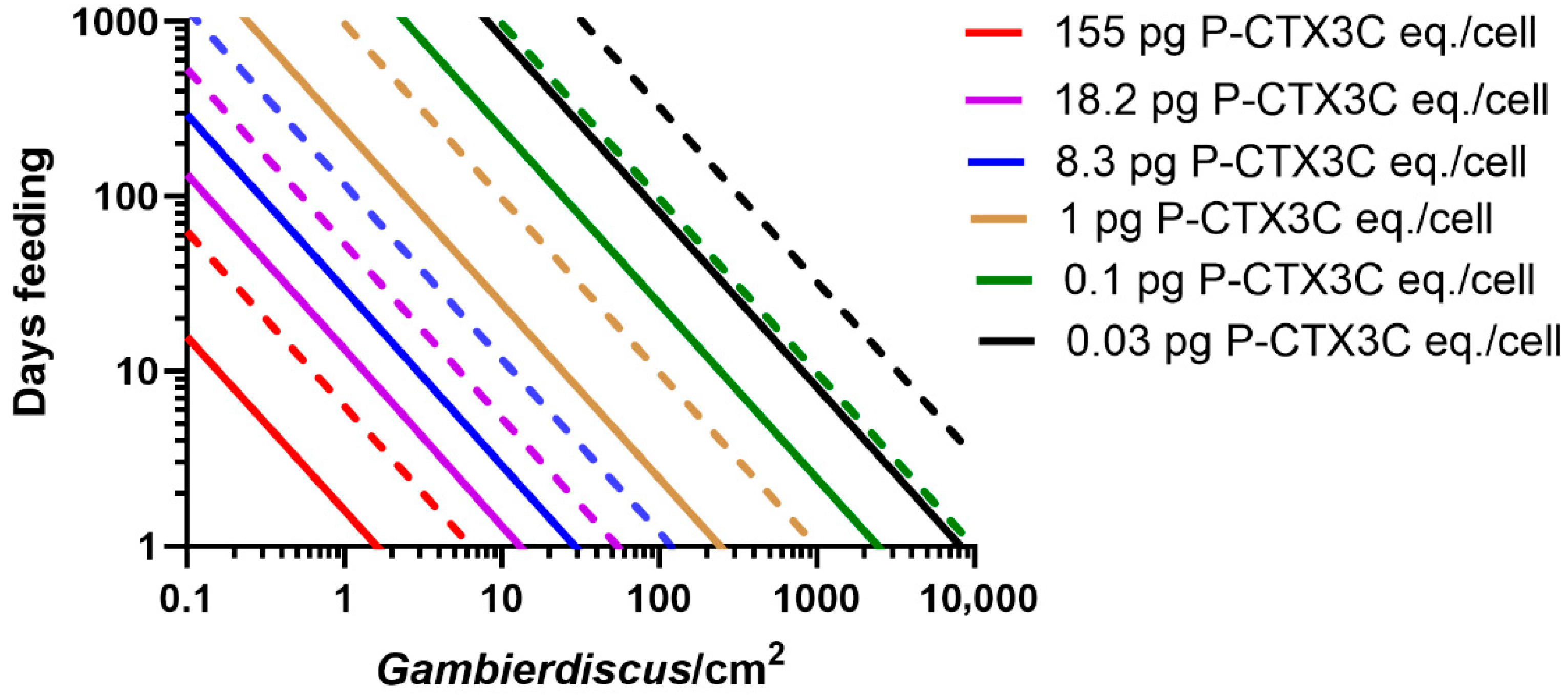
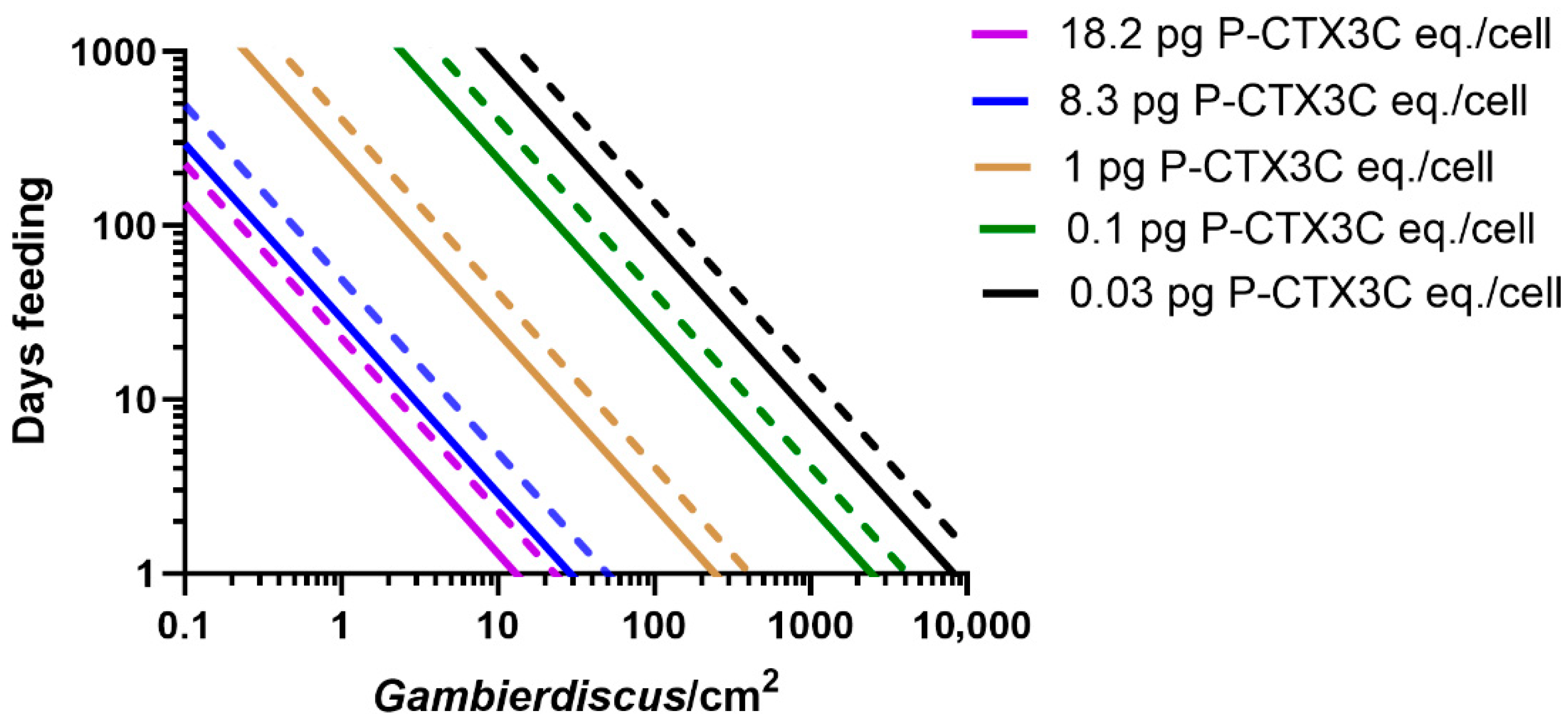
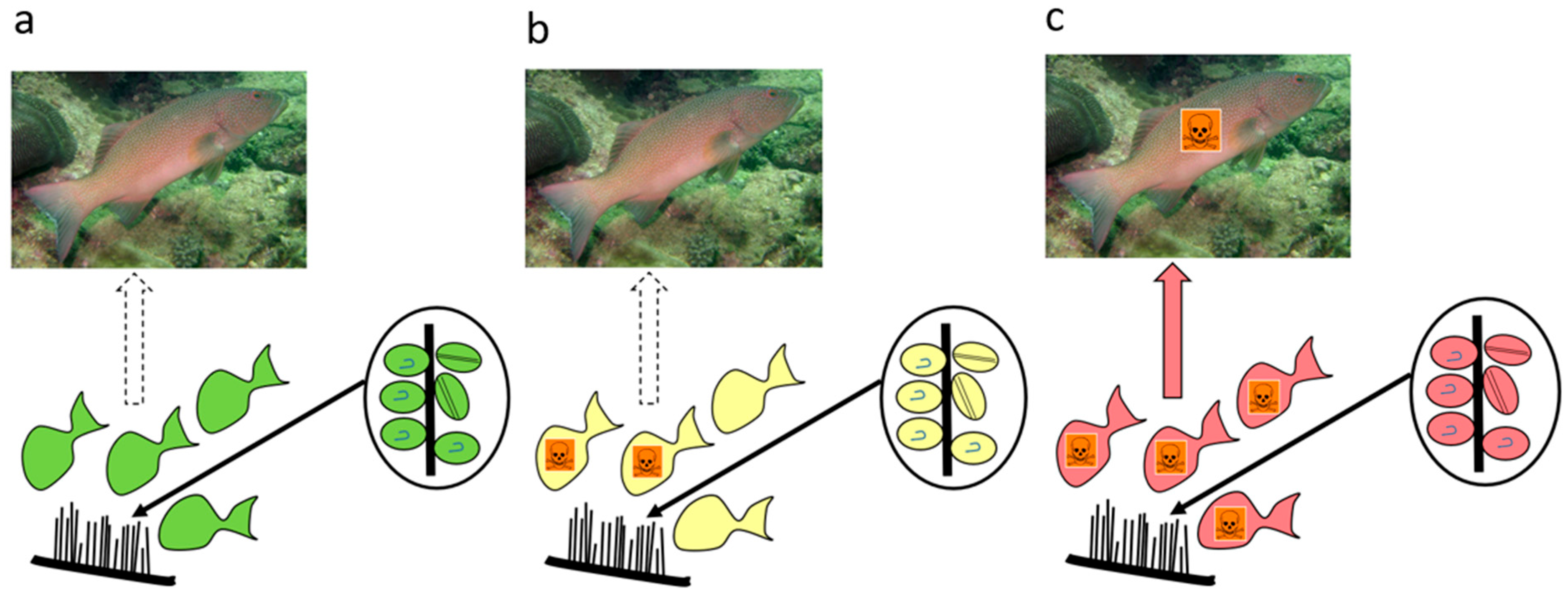
- Based upon known concentrations of CTX produced by Gambierdiscus species (Table 1), most do not produce sufficient CTX to cause ciguatera (Figure 8a). Based upon our model for the Pacific, we suggest that species that produce CTX concentrations ≤0.02 pg P-CTX3C eq./cell have a minimal role in ciguatera.
- Some Gambierdiscus species produce sufficient CTX to potentially accumulate in herbivorous fishes to produce mildly poisonous flesh (Figure 8b). However, it is possible that this scenario is limited to herbivore species that graze large areas/amounts of algae relative to their size. Such mildly poisonous herbivores are unlikely to carry sufficient CTX load that, if preyed upon, would produce poisonous ≥third trophic level fishes (Table S1). Based upon our model for the Pacific, we suggest production of mildly poisonous herbivorous fishes is mostly limited to Gambierdiscus species that produce CTX concentrations >0.03 P-CTX3C eq./cell. Apart from G. polynesiensis, only G. belizeanus and possibly G. silvae and G. australes are thought to produce >0.03 pg P-CTX3C eq./cell in the Pacific (G. excentricus and G. caribaeus in the Atlantic).
- Only high CTX-producing Gambierdiscus (>0.1 pg P-CTX3C eq./cell) likely produce sufficient CTX to accumulate in food chains to produce highly toxic ciguateric second trophic level fishes, and weakly to highly toxic ≥third trophic level fishes (Figure 8c). To date, only G. polynesiensis in the Pacific (and G. excentricus in the Atlantic) is known to be capable of producing >0.1 pg P-CTX3C eq./cell.
4. Material and Methods
4.1. Models for Bioaccumulation of P-CTX3C into Herbivorous Fish
4.2. Modelling Depuration Rates That Balance Ingestion of CTX to Keep Fish Flesh Poisonous
4.2.1. Parrotfish Depuration of CTX
4.2.2. Naso unicornis Depuration of CTX
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gillespie, N.C.; Lewis, R.J.; Pearn, J.H.; Bourke, A.T.C.; Holmes, M.J.; Bourke, J.B.; Shields, W.J. Ciguatera in Australia: Occurrence, clinical features, pathophysiology and management. Med. J. Aust. 1986, 145, 584–590. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Report of the Expert Meeting on Ciguatera Poisoning: Rome, 19–23 November 2018; Food Safety and Quality No. 9; FAO: Rome, Italy, 2020. Available online: https://books.google.com.sg/books?hl=zh-TW&lr=&id=HhvtDwAAQBAJ&oi=fnd&pg=PR6&dq=10.4060/ca8817en&ots=UUfascmP-s&sig=UbOO36DHmBc8aCkBNQpXUxfuHdk#v=onepage&q=10.4060%2Fca8817en&f=false (accessed on 1 April 2025).
- Kretzschmar, A.L.; Larsson, M.E.; Hoppenrath, M.; Doblin, M.A.; Murray, S.A. Characterisation of Two Toxic Gambierdiscus spp. (Gonyaulacales, Dinophyceae) from the Great Barrier Reef (Australia): G. lewisii sp. nov. and G. holmesii sp. nov. Protist 2019, 170, 125699. [Google Scholar] [CrossRef]
- Li, Z.; Park, J.S.; Kang, N.S.; Chomérat, N.; Mertens, K.; Gu, H.; Lee, K.-W.; Kim, K.H.; Baek, S.H.; Shin, K.; et al. A new potentially toxic dinoflagellate Fukuyoa koreansis sp. nov. (Gonyaulacales, Dinophyceae) from Korean coastal waters: Morphology, phylogeny, and effects of temperature and salinity on growth. Harmful Algae 2021, 109, 102107. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, L.; Larsen, J.; Doan-Nhu, H.; Nguyen, X.-V.; Chomerat, N.; Lundholm, N.; Phan-Tan, L.; Dao, H.V.; Nguyen, L.-N.; Nguyen, H.-H.; et al. Gambierdiscus (gonyaulacales, dinophyceae) diversity in Vietnamese waters with description of G. vietnamensis sp. nov. J. Phycol. 2023, 59, 496–517. [Google Scholar] [CrossRef]
- Murray, S.A.; Verma, A.; Hoppenrath, M.; Harwood, D.T.; Murray, J.S.; Smith, K.F.; Lewis, R.; Finch, S.C.; Islam, S.S.; Ashfaq, A.; et al. High ciguatoxin-producing Gambierdiscus clade (Gonyaulacales, Dinophyceae) as a source of toxins causing ciguatera poisoning. Sci. Total Environ. 2025, 994, 179990. [Google Scholar] [CrossRef]
- Holmes, M.J.; Venables, B.; Lewis, R.J. Critical review and conceptual and quantitative models for the transfer and depuration of ciguatoxins in fishes. Toxins 2021, 13, 515. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Legrand, A.-M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and Configurations of Ciguatoxin from the Moray Eel Gymnothorax javanicus and Its Likely Precursor from the Dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Satake, M.; Murata, M.; Yasumoto, T. The structure of CTX3C, a ciguatoxin congener isolated from Gambierdiscus toxicus. Tetrahedron Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Mudge, E.M.; Miles, C.O.; Ivanova, L.; Uhlig, S.; James, K.S.; Erdner, D.L.; Fæste, C.K.; McCarron, P.; Robertson, A. Algal ciguatoxin identified as source of ciguatera poisoning in the Caribbean. Chemosphere 2023, 330, 138659. [Google Scholar] [CrossRef]
- Mudge, E.M.; Robertson, A.; Uhlig, S.; McCarron, P.; Miles, C.O. 3-epimers of 1 Caribbean ciguatoxins in fish and algae. Toxicon 2023, 237, 107536. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Hurbungs, M.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef]
- Lewis, R.J.; Holmes, M.J. Origin and transfer of toxins involved in ciguatera. Comp. Biochem. Physiol. 1993, 106C, 615–628. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.-M.; Yasumoto, T. Isolation and Structure of Ciguatoxin-4A, a New Ciguatoxin Precursor, from Cultures of Dinoflagellate Gambierdiscus toxicus and Parrotfish Scarus gibbus. Biosci. Biotech. Biochem. 1997, 60, 2103–2105. [Google Scholar] [CrossRef]
- Yasumoto, T.; Igarashi, T.; Legrand, A.-M.; Cruchet, P.; Chinain, M.; Fujita, T.; Naoki, H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectrometry. J. Am. Chem. Soc. 2000, 122, 4988–4989. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of Ciguatoxins Leads to Species-Specific Toxin Profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef]
- Loeffler, C.R.; Spielmeyer, A.; Blaschke, V.; Bodi, D.; Kappenstein, O. Ciguatera poisoning in Europe: A traceback to Indian Ocean sourced snapper fish (Lutjanus bohar). Food Control 2023, 151, 109799. [Google Scholar] [CrossRef]
- Barreiro-Crespo, L.; Sanchez-Henao, A.; Gimeno-Monforte, S.; Reverté, J.; Campàs, M.; Tsumuraya, T.; Diogène, J.; Tunin-Ley, A.; Maillot, F.; Flores, C.; et al. Toxicity and toxin profile of La Réunion (Indian Ocean) fish containing CTX-like compounds. Harmful Algae 2025, 147, 102882. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lew, K.; Leyau, Y.L.; Shen, P.; Chua, J.; Lin, K.J.; Wu, Y.; Chan, S.H. Application of high-resolution mass spectrometry for ciguatoxin detection in fish from the Asia–Pacific Region. Toxins 2025, 17, 100. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Reviewing Evidence for Disturbance to Coral Reefs Increasing the Risk of Ciguatera. Toxins 2025, 17, 195. [Google Scholar] [CrossRef]
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750. [Google Scholar] [CrossRef]
- Chinain, M.; Howell, C.G.; Roué, M.; Ung, A.; Henry, K.; Revel, T.; Cruchet, P.; Viallon, J.; Darius, H.T. Ciguatera poisoning in French Polynesia: A review of the distribution and toxicity of Gambierdiscus spp., and related impacts on food web components and human health. Harmful Algae 2023, 129, 102525. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Viallon, J.; Darius, H.T.; Hess, P.; Chinain, M. Intraspecific Variability in the Toxin Production and Toxin Profiles of In Vitro Cultures of Gambierdiscus polynesiensis (Dinophyceae) from French Polynesia. Toxins 2019, 11, 735. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Darius, H.T.; Hess, P.; Chinain, M. Effects of pH and Nutrients (Nitrogen) on Growth and Toxin Profile of the Ciguatera-Causing Dinoflagellate Gambierdiscus polynesiensis (Dinophyceae). Toxins 2020, 12, 767. [Google Scholar] [CrossRef]
- Darius, H.T.; Revel, T.; Viallon, J.; Sibat, M.; Cruchet, P.; Longo, S.; Hardison, D.R.; Holland, W.C.; Tester, P.A.; Litaker, R.W.; et al. Comparative study on the performance of three detection methods for the quantification of Pacific ciguatoxins in French Polynesian strains of Gambierdiscus polynesiensis. Toxins 2022, 20, 348. [Google Scholar] [CrossRef]
- Murray, J.S.; Passfield, E.M.F.; Rhodes, L.L.; Puddick, J.; Finch, S.C.; Smith, K.F.; van Ginkel, R.; Mudge, E.M.; Nishimura, T.; Funaki, H.; et al. Targeted Metabolite Fingerprints of Thirteen Gambierdiscus, Five Coolia and Two Fukuyoa Species. Mar. Drugs 2024, 22, 119. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.L.; Harwood, T.; Smith, K.; Argyle, P.; Munday, R. Production of ciguatoxin and maitotoxin by strains of Gambierdiscus australes, G. pacificus and G. polynesiensis (Dinophyceae) isolated from Rarotonga, Cook Islands. Harmful Algae 2014, 39, 185–190. [Google Scholar] [CrossRef]
- Rhodes, L.L.; Smith, K.F.; Murray, J.S.; Nishimura, T.; Finch, S.C. Ciguatera Fish Poisoning: The Risk from an Aotearoa/New Zealand Perspective. Toxins 2020, 12, 50. [Google Scholar] [CrossRef] [PubMed]
- Raposo-Garcia, S.; Louzao, M.C.; Fuwa, H.; Sasaki, M.; Vale, C.; Botana, L.M. Determination of the toxicity equivalency factors for ciguatoxins using human sodium channels. Food Chem. Toxicol. 2022, 160, 112812. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Origin of ciguateric fish: Quantitative modelling of the flow of ciguatoxin through a marine food chain. Toxins 2022, 14, 534. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.J.; Lewis, R.J. Model of the origin of a ciguatoxic grouper (Plectropomus leopardus). Toxins 2023, 15, 230. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J. Modelling the Bioaccumulation of Ciguatoxins in Parrotfish on the Great Barrier Reef Reveals Why Biomagnification Is Not a Property of Ciguatoxin Food Chains. Toxins 2025, 17, 380. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- USFDA Natural Toxins. Fish and Fishery Products Hazards and Control Guidance, 4th ed.; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition: White Oak, MD, USA, 2021. Available online: https://www.fda.gov/media/80400/download (accessed on 30 August 2025).
- Parsons, M.L.; Richlen, M.L.; Smith, T.B.; Anderson, D.M.; Abram, A.L.; Erdner, D.L.; Robertson, A. CiguaMOD I: A conceptual model of ciguatoxin loading in the Greater Caribbean Region. Harmful Algae 2024, 131, 102561. [Google Scholar] [CrossRef]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Tchou Fouc, M.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Darius, H.T.; Ung, A.; Fouc, M.T.; Revel, T.; Cruchet, P.; Paullac, S.; Laurent, D. Ciguatera risk management in French Polynesia: The case study of Raivavae Island (Australes Archipelago). Toxicon 2010, 56, 674–690. [Google Scholar] [CrossRef]
- Rongo, T.; van Woesik, R. Ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2011, 10, 345–355. [Google Scholar] [CrossRef]
- Rongo, T.; van Woesik, R. Socioeconomic consequences of ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae 2012, 20, 92–100. [Google Scholar] [CrossRef]
- Gaboriau, M.; Ponton, D.; Darius, H.T.; Chinain, M. Ciguatera fish toxicity in French Polynesia: Size does not always matter. Toxicon 2014, 84, 41–50. [Google Scholar] [CrossRef]
- Morin, E.; Gatti, C.; Bambridge, T.; Chinain, M. Ciguatera fish poisoning: Incidence, health costs and risk perception on Moorea Island (Society archipelago, French Polynesia). Harmful Algae 2016, 60, 1–10. [Google Scholar] [CrossRef]
- Nassiri, A.; Thébaud, O.; Holbrook, S.J.; Lauer, M.; Rassweiler, A.; Schmitt, R.J.; Claudet, J. Hedonic evaluation of coral reef fish prices on a direct sale market. Mar. Policy 2021, 129, 104525. [Google Scholar] [CrossRef]
- Bravo, I.; Rodríguez, F.; Ramilo, I.; Afonso-Carrillo, J. Epibenthic Harmful Marine Dinoflagellates from Fuerteventura (Canary Islands), with Special Reference to the Ciguatoxin-Producing Gambierdiscus. J. Mar. Sci. Eng. 2020, 8, 909. [Google Scholar] [CrossRef]
- Richlen, M.L.; Horn, K.; Uva, V.; Fachon, E.; Heidmann, S.L.; Smith, T.B.; Parsons, M.L.; Anderson, D.M. Gambierdiscus species diversity and community structure in St. Thomas, USVI and the Florida Keys, USA. Harmful Algae 2024, 131, 102562. [Google Scholar] [CrossRef] [PubMed]
- Tester, P.A.; Litaker, R.W.; Soler-Onís, E.; Fernández-Zabala, J.; Berdalet, E. Using artificial substrates to quantify Gambierdiscus and other toxic benthic dinoflagellates for monitoring purposes. Harmful Algae 2022, 120, 102351. [Google Scholar] [CrossRef]
- Tudó, A.; Gaiani, G.; Varela, M.R.; Tsumuraya, T.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Diogène, J. Further advance of Gambierdiscus Species in the Canary Islands, with the First Report of Gambierdiscus belizeanus. Toxins 2020, 12, 692. [Google Scholar] [CrossRef]
- Pisapiaa, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoce, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef]
- Dai, X.; Mak, Y.L.; Lu, C.-K.; Mei, H.-H.; Wu, J.J.; Lee, W.H.; Chan, L.L.; Lim, P.T.; Mustapa, N.I.; Lim, H.C.; et al. Taxonomic assignment of the benthic toxigenic dinoflagellate Gambierdiscus sp. type 6 as Gambierdiscus balechii (Dinophyceae), including its distribution and ciguatoxicity. Harmful Algae 2017, 67, 107–118. [Google Scholar] [CrossRef]
- Larsson, M.E.; Laczka, O.F.; Harwood, D.T.; Lewis, R.J.; Himaya, S.W.A.; Murray, S.A.; Doblin, M.A. Toxicology of Gambierdiscus spp. (Dinophyceae) from Tropical and Temperate Australian Waters. Mar. Drugs 2018, 16, 7. [Google Scholar] [CrossRef]
- Munday, R.; Murray, S.; Rhodes, L.L.; Larsson, M.E.; Harwood, D.T. Ciguatoxins and Maitotoxins in Extracts of Sixteen Gambierdiscus Isolates and One Fukuyoa Isolate from the South Pacific and Their Toxicity to Mice by Intraperitoneal and Oral Administration. Mar. Drugs 2017, 15, 208. [Google Scholar] [CrossRef]
- Luo, Z.; Lin, X.; Liu, X.; Hii, K.S.; Li, H.; Li, Y.; Xu, X.; Xiao, J.; Mohamed, H.F.; Zheng, X.; et al. Morphology, phylogeny, and toxicity of three Gambierdiscus species from the South China Sea, including a coral-killing bloom of G. carpenteri in reef tanks. Mar. Environ. Res. 2025, 206, 107031. [Google Scholar] [CrossRef]
- Funaki, H.; Nishimura, T.; Yoshioka, T.; Ataka, T.; Tanii, Y.; Hashimoto, K.; Yamaguchi, H.; Adachi, M. Toxicity and growth characteristics of epiphytic dinoflagellate Gambierdiscus silvae in Japan. Harmful Algae 2022, 115, 102230. [Google Scholar] [CrossRef]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Tudó, A.; Bravo, I.; Díaz, P.A.; Diogène, J.; Riobó, P. Toxicity Characterisation of Gambierdiscus Species from the Canary Islands. Toxins 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, W.; Yan, Z.; Zhou, S.; Guo, R.; Zheng, J.; Dai, X.; Lu, D.; Mu, Q.; Zeng, J.; et al. Discovery of Fukuyoa yasumotoi (Dinophyceae) from the Xisha Islands, South China Sea: A comprehensive study on morphology, molecular phylogeny and toxicity. Harmful Algae 2025, 149, 102938. [Google Scholar] [CrossRef]
- Laza-Martínez, A.; David, H.; Riobó, P.; Miguel, I.; Orive, E. Characterization of a Strain of Fukuyoa paulensis (Dinophyceae) from the Western Mediterranean Sea. J. Eukaryot. Microbiol. 2016, 63, 481–497. [Google Scholar] [CrossRef]
- Gaiani, G.; Leonardo, S.; Tudó, Á.; Toldrà, A.; Rey, M.; Andree, K.B.; Tsumuraya, T.; Hirama, M.; Diogène, J.; O’Sullivan, C.K.; et al. Rapid detection of ciguatoxins in Gambierdiscus and Fukuyoa with immunosensing tools. Ecotoxicol. Environ. Saf. 2020, 204, 111004. [Google Scholar] [CrossRef]
- Leung, P.T.Y.; Yan, M.; Lam, V.T.T.; Yiu, S.K.F.; Chen, C.-Y.; Murray, J.S.; Harwood, D.T.; Rhodes, L.L.; Lam, P.K.S.; Wai, T.-C. Phylogeny, morphology and toxicity of benthic dinoflagellates of the genus Fukuyoa (Goniodomataceae, Dinophyceae) from a subtropical reef ecosystem in the South China Sea. Harmful Algae 2018, 74, 78–97. [Google Scholar] [CrossRef]
- Lange, I.D.; Perry, C.T.; Morgan, K.M.; Roche, R.; Benkwitt, C.; Graham, N.A.J. Site-Level Variation in Parrotfish Grazing and Bioerosion as a Function of Species-Specific Feeding Metrics. Diversity 2020, 12, 379. [Google Scholar] [CrossRef]
- van der Reis, A.L.; Clements, K.D. DNA, databases and diet: A case study on the parrotfish Scarus rivulatus. Coral Reefs 2024, 43, 1189–1206. [Google Scholar] [CrossRef]
- Sibat, M.; Mai, T.; Chomérat, N.; Bilien, G.; Lhaute, K.; Hess, P.; Séchet, V.; Jauffraus, T. Gambierdiscus polynesiensis from New Caledonia (South West Pacific Ocean): Morpho-molecular characterization, toxin profile and response to light intensity. Harmful Algae 2025, 145, 102859. [Google Scholar] [CrossRef]
- Yasumoto, T.; Nakajima, I.; Chungue, E.; Bagnis, R. Toxins in the gut contents of parrotfish. Bull. Jap. Soc. Sci. Fish. 1977, 43, 69–74. [Google Scholar] [CrossRef]
- Campbell, B.; Nakagawa, L.K.; Kobayashi, M.N.; Hokama, Y. Gambierdiscus toxicus in the gut content of the surgeonfish Ctenochaetus strigosus (herbivore) and its relationship to toxicity. Toxicon 1987, 25, 1125–1127. [Google Scholar] [CrossRef]
- Yasumoto, T. The Chemistry and Biological Function of Natural Marine Toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS Analysis of Ciguatoxins Revealing Distinct Regional and Species Characteristics in Fish and Causative Alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, R.J. Multiple ciguatoxins in the flesh of fish. Toxicon 1992, 30, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.M.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Haslauer, K.; Sarowar, C.; Kretzschmar, A.L.; Boulter, M.; Harwood, D.T.; Laczka, O.; Murray, S.A. Qualitative and quantitative assessment of the presence of ciguatoxin, P-CTX-1B, in Spanish Mackerel (Scomberomorus commerson) from waters in New South Wales (Australia). Toxicol. Rep. 2017, 4, 328–334. [Google Scholar] [CrossRef]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef]
- Zhu, J.; Lee, W.-H.; Wu, J.; Zhou, S.; Yip, K.-C.; Liu, X.; Kirata, T.; Chan, L.-L. The Occurrence, Distribution, and Toxicity of High-Risk Ciguatera Fish Species (Grouper and Snapper) in Kiritimati Island and Marakei Island of the Republic of Kiribati. Toxins 2022, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, J.; Wu, J.; Liu, X.; Lin, Y.; Deng, H.; Qin, X.; Wong, M.H.; Chan, L.L. The prevalence of marine lipophilic phycotoxins causes potential risks in a tropical small island developing State. Environ. Sci. Technol. 2024, 58, 9815–9827. [Google Scholar] [CrossRef]
- Oshiro, N.; Tomikawa, T.; Kuniyoshi, K.; Ishikawa, A.; Toyofuku, H.; Kojima, T.; Asakura, H. LC–MS/MS Analysis of Ciguatoxins Revealing the Regional and Species Distinction of Fish in the Tropical Western Pacific. J. Mar. Sci. Eng. 2021, 9, 299. [Google Scholar] [CrossRef]
- Oshiro, N.; Yogi, Y.; Asato, S.; Sasaki, T.; Tamanaha, K.; Hirama, M.; Yasumoto, T.; Inafuku, Y. Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon 2010, 56, 656–661. [Google Scholar] [CrossRef]
- Oshiro, N.; Nagasawa, H.; Watanabe, M.; Nishimura, M.; Kuniyoshi, K.; Kobayashi, N.; Sugita-Konishi, Y.; Asakura, H.; Tachihara, K.; Yasumoto, Y. An Extensive Survey of Ciguatoxins on Grouper Variola louti from the Ryukyu Islands, Japan, Using Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS). J. Mar. Sci. Eng. 2022, 10, 423. [Google Scholar] [CrossRef]
- Oshiro, N.; Nagasawa, H.; Nishimura, M.; Kuniyoshi, K.; Kobayashi, N.; Sugita-Konishi, Y.; Ikehara, T.; Tachihara, K.; Yasumoto, T. Analytical Studies on Ciguateric Fish in Okinawa, Japan (II): The Grouper Variola albimarginata. J. Mar. Sci. Eng. 2023, 11, 242. [Google Scholar] [CrossRef]
- Kindinger, T.L.; Adam, T.C.; Baum, J.K.; Dimoff, S.A.; Hoey, A.S.; Williams, I.D. Herbivory through the lens of ecological processes across Pacific coral reefs. Ecosphere 2024, 15, e4791. [Google Scholar] [CrossRef]
- Gillespie, N.C.; Holmes, M.J.; Burke, J.B.; Doley, J. Distribution and Periodicity of Gambierdiscus toxicus in Queensland, Australia. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: Oxford, UK, 1985; pp. 183–188. [Google Scholar]
- Kohli, G.S.; Murray, S.A.; Neilan, B.A.; Rhodes, L.L.; Harwood, T.; Smith, K.F.; Meyer, L.; Capper, A.; Brett, S.; Hallegraeff, G.M. High abundance of the potentially maitotoxic dinoflagellate Gambierdiscus carpenteri in temperate waters of New South Wales, Australia. Harmful Algae 2014, 39, 134–145. [Google Scholar] [CrossRef]
- Rasher, D.B.; Hoey, A.S.; Hay, M.E. Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 2013, 94, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Tebbett, S.B.; Hoey, A.S.; Depczynski, M.; Wismer, S.; Bellwood, D.R. Macroalgae removal on coral reefs: Realised ecosystem functions transcend biogeographic locations. Coral Reefs 2020, 39, 203–214. [Google Scholar] [CrossRef]
- Sura, A.A.; Molina, N.E.; Blumstein, D.T.; Fong, P. Selective consumption of macroalgal species by herbivorous fishes suggests reduced functional complementarity on a fringing reef in Moorea, French Polynesia. J. Exp. Mar. Biol. Ecol. 2021, 536, 151508. [Google Scholar] [CrossRef]
- Wu, P.; Wang, T.; Liu, Y.; Li, C.; Xiao, Y.; Xu, S.; Han, T.; Lin, L.; Quan, Q. Differences of Macroalgal Consumption by Eight Herbivorous Coral Reef Fishes from the Xisha Islands, China. Front. Mar. Sci. 2022, 9, 882196. [Google Scholar] [CrossRef]
- Cook, D.T.; Schmitt, R.J.; Holbrook, S.J.; Moeller, H.V. Modeling the effects of selectively fishing key functional groups of herbivores on coral resilience. Ecosphere 2024, 15, e4749. [Google Scholar] [CrossRef]
- Leenhardt, P.; Lauer, M.; Madi Moussa, R.; Holbrook, S.J.; Rassweiler, A.; Schmitt, R.J.; Claudet, J. Complexities and Uncertainties in Transitioning Small-Scale Coral Reef Fisheries. Front. Mar. Sci. 2016, 3, 70. [Google Scholar] [CrossRef]
- Rassweiler, A.; Lauer, M.; Lester, S.E.; Holbrook, S.J.; Schmitt, R.J.; Moussa, R.M.; Munsterman, K.S.; Lenihan, H.S.; Brooks, A.J.; Wencelius, J.; et al. Perceptions and responses of Pacific Island fishers to changing coral reefs. Ambio 2020, 49, 130–143. [Google Scholar] [CrossRef]
- Rassweiler, A.; Miller, S.D.; Holbrook, S.J.; Lauer, M.; Strother, M.A.; Lester, S.E.; Adam, T.C.; Wencélius, J.; Schmitt, R.J. How do fisher responses to macroalgal overgrowth influence the resilience of coral reefs? Limnol. Oceanogr. 2022, 67, S365–S377. [Google Scholar] [CrossRef]
- Lefèvre, C.D.; Bellwood, D.R. Temporal variation in coral reef ecosystem processes: Herbivory of macroalgae by fishes. Mar. Ecol. Prog. Ser. 2011, 422, 239–251. [Google Scholar] [CrossRef]
- Tester, P.A.; Kibler, S.R.; Holland, W.C.; Usup, G.; Vandersea, M.W.; Leaw, C.P.; Teen, L.P.; Larsen, J.; Mohammad-Noor, N.; Faust, M.A.; et al. Sampling harmful benthic dinoflagellates: Comparison of artificial and natural substrate methods. Harmful Algae 2014, 39, 8–25. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef]
- Clausing, R.J.; Ben Gharbia, H.; Sdiri, K.; Sibat, M.; Ranada-Mestizo, M.L.; Lavenu, L.; Hess, P.; Chinain, M.; Bottein, M.-Y.D. Tissue Distribution and Metabolization of Ciguatoxins in an Herbivorous Fish following Experimental Dietary Exposure to Gambierdiscus polynesiensis. Mar. Drugs 2024, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.L.; Graham, N.A.J.; Januchowski-Hartley, F.A.; Bellwood, D.R. Influence of habitat condition and competition on foraging behaviour of parrotfishes. Mar. Ecol. Prog. Ser. 2012, 457, 113–124. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Tebbett, S.B.; Bellwood, O.; Mihalatis, M.; Morais, R.A.; Streit, R.P.; Fulton, C.J. The role of the reef flat in coral reef trophodynamics: Past, present, and future. Ecol. Evol. 2018, 8, 4108–4119. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Ecomorphological divergence and trophic resource partitioning in 15 syntopic Indo-Pacific parrotfishes (Labridae: Scarini). Biol. J. Linn. Soc. 2021, 132, 590–611. [Google Scholar] [CrossRef]
- García-Seoane, R.; White, W.L.; Taylor, B.M.; Clements, K.D. Characterizing the trophic ecology of herbivorous coral reef fishes using stable isotope and fatty acid biomarkers. PLoS ONE 2025, 20, e0327594. [Google Scholar] [CrossRef] [PubMed]
- Tebbett, S.B.; Goatley, C.H.R.; Bellwood, D.R. Clarifying functional roles: Algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus. Coral Reefs 2017, 36, 803–813. [Google Scholar] [CrossRef]
- Annandale, S.F.; Turner, J.P.; Lippi, D.L.; Dance, M.; Wells, R.J.D.; Rooker, J.R. Habitat use and movements of parrotfishes in a Hawaiian coral reef seascape. Front. Fish Sci. 2024, 2, 1448809. [Google Scholar] [CrossRef]
- Bagnis, R.; Bennett, J.; Prieur, C.; Legrand, A.M. The Dynamics of Three Benthic Dinoflagellates and the Toxicity of Ciguateric Surgeonfish in French Polynesia. In Toxic Dinoflagellates; Anderson, D.M., White, A.W., Baden, D.G., Eds.; Elsevier: Oxford, UK, 1985; pp. 177–182. [Google Scholar]
- Gillespie, N.C.; Lewis, R.J.; Burke, J.; Holmes, M. The Significance of the Absence of Ciguatoxin in a Wild Population of G. toxicus. In Proceedings of the Fifth International Coral Reef Congress, Tahiti, France, 27 May–1 June 1985; Gabrie, C., Salvat, B., Eds.; Antenne Museum-Ephe: Moorea, France, 1985; pp. 437–441. [Google Scholar]
- Chinain, M.; Germain, M.; Deparis, X.; Pauillac, S.; Legrand, A.-M. Seasonal abundance and toxicity of the dinoflagellate Gambierdiscus spp. (Dinophyceae), the causative agent of ciguatera in Tahiti, French Polynesia. Mar. Biol. 1999, 135, 259–267. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Roue, M.; Darius, H.T. Ciguatera-Causing Dinoflagellates in the Genera Gambierdiscus and Fukuyoa: Distribution, Ecophysiology and Toxicology. In Dinoflagellates; Subba Rao, D.V., Ed.; Nova Science: New York, NY, USA, 2020; pp. 405–457. [Google Scholar]
- Funaki, H.; Gaonkar, C.C.; Kataoka, T.; Nishimura, T.; Tanaka, K.; Yanagida, I.; Abe, S.; Yamaguchi, H.; Nagasaki, K.; Adachi, M. Horizontal and vertical distribution of Gambierdiscus spp. (Dinophyceae) including novel phylotypes in Japan identified by 18S rDNA metabarcoding. Harmful Algae 2022, 111, 102163. [Google Scholar] [CrossRef]
- Yasumoto, T. Chemistry, etiology, and food chain dynamics of marine toxins. Proc. Jpn. Acad. 2005, 81, 43–51. [Google Scholar] [CrossRef]
- Amin, A.M.; El-Ganainy, A.A.; Sabrah, M.M. Length-Weight Relationships of Thirteen Species of Parrotfish (Family Scaridae) inhabiting the Egyptian coasts of the Red Sea. Egypt. J. Aquat. Biol. Fish. 2019, 23, 357–366. [Google Scholar] [CrossRef]
- Longenecker, K.; Langston, G.; Bolick, H.; Crane, M.; Donaldson, T.J.; Franklin, E.C.; Kelokelo, M.; Kondio, U.; Potuku, T. Rapid reproductive analysis and length–weight relations for five species of coral-reef fishes (Actinopterygii) from Papua New Guinea: Nemipterus isacanthus, Parupeneus barberinus, Kyphosus cinerascens, Ctenchaetus striatus (Perciformes), and Balistapus undulatus (Tetraodontiformes). Acta Ichthyol. Piscat. 2017, 47, 107–124. [Google Scholar]
- Froese, R.; Pauly, D. (Eds.) World Wide Web Electronic Publication; Version (02/2025); FishBase; 2025; Available online: www.fishbase.org (accessed on 14 August 2025).
- Polunin, N.V.C.; Harmelin-Vivien, M.; Galzin, R. Contrasts in algal food processing among five herbivorous coral-reef fishes. J. Fish Biol. 1995, 47, 455–465. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Huertas, V.; Mihalitsis, M.; Bellwood, D.R. A functional evaluation of feeding in the surgeonfish Ctenochaetus striatus: The role of soft tissues. R. Soc. Open Sci. 2018, 5, 17111. [Google Scholar] [CrossRef]
- Cheal, A.; Emslie, M.; Miller, I.; Sweatman, H. The distribution of herbivorous fishes on the Great Barrier Reef. Mar. Biol. 2012, 159, 1143–1154. [Google Scholar] [CrossRef]
- Goatley, C.H.R.; Bellwood, D.R. Biologically mediated sediment fluxes on coral reefs: Sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar. Ecol. Prog. Ser. 2010, 415, 237–245. [Google Scholar] [CrossRef]
- Marshell, A.; Mumby, P.J. The role of surgeonfish (Acanthuridae) in maintaining algal turf biomass on coral reefs. J. Exp. Mar. Biol. Ecol. 2015, 473, 152–160. [Google Scholar] [CrossRef]
- Bogorodsky, S.V.; Thieme, P.; Senou, H.; Mahmoud, Z.N.; Alpermann, T.J.; Durand, J.-D. Contributions to the Taxonomy of the Mugilid Genus Moolgarda Whitley (Teleostei: Mugilidae), with Redescriptions of M. crenilabis, M. seheli and M. tade from the Red Sea. Diversity 2024, 16, 325. [Google Scholar] [CrossRef]
- Hiatt, R.W.; Strasburg, D.W. Ecological relationships of the fish fauna on coral reefs of the Marshall Islands. Ecol. Monogr. 1960, 30, 65–127. [Google Scholar] [CrossRef]
- Ledreux, A.; Brand, H.; Chinain, M.; Dechraoui Bottein, M.-Y.; Ramsdell, J.S. Dynamics of ciguatoxins from Gambierdiscus polynesiensis in the benthic herbivore Mugil cephalus: Trophic transfer implications. Harmful Algae 2014, 39, 165–174. [Google Scholar] [CrossRef]
- Cooper, M.J. Ciguatera and other marine poisoning in the Gilbert Islands. Pac. Sci. 1964, 4, 411–440. [Google Scholar]
- Houk, P.; Rhodes, K.; Cuetos-Bueno, J.; Lindfield, S.; Fread, V.; McIlwain, J.L. Commercial coral-reef fisheries across Micronesia: A need for improving management. Coral Reefs 2012, 31, 13–26. [Google Scholar] [CrossRef]
- Taylor, B.M.; Duenas, A.E.K.; Lange, I.D. Decadal changes in parrotfish assemblages around reefs of Guam, Micronesia. Coral Reefs 2022, 41, 1693–1703. [Google Scholar] [CrossRef]
- Taylor, B.M.; Prince, J.; Mutz, S.; Pardee, C.; Wiley, J.; Robertson, D.R.; Choat, J.H. A widespread, consistent, and perplexing biphasic pattern in log catch-at-age data from a widely harvested family of tropical reef fishes. Fish Fish. 2024, 25, 910–917. [Google Scholar] [CrossRef]
- Johansen, J.L.; Prachett, M.S.; Messmer, V.; Coker, D.J.; Tobin, A.J.; Hoey, A.S. Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci. Rep. 2015, 5, 13830. [Google Scholar] [CrossRef]
- Kingsford, M.J. Spatial and temporal variation in predation on reef fishes by coral trout (Plectropomus leopardus, Serranidae). Coral Reefs 1992, 11, 193–198. [Google Scholar] [CrossRef]
- Matley, J.K.; Maes, G.E.; Devloo-Delva, F.; Huerlimann, R.; Chua, G.; Tobin, A.J.; Fisk, A.T.; Simpfendorfer, C.A.; Heupel, M.R. Integrating complementary methods to improve diet analysis in fishery-targeted species. Ecol. Evol. 2018, 8, 9503–9515. [Google Scholar] [CrossRef]
- Lartigue, J.; Jester, E.L.E.; Dickey, R.W.; Villareal, T.A. Nitrogen source effects on the growth and toxicity of two strains of the ciguatera-causing dinoflagellate Gambierdiscus toxicus. Harmful Algae 2009, 8, 781–791. [Google Scholar] [CrossRef]
- Vacarizas, J.; Benico, G.; Austero, N.; Azanza, R. Taxonomy and toxin production of Gambierdiscus carpenteri (Dinophyceae) in a tropical marine ecosystem: The first record from the Philippines. Mar. Pol. Bull. 2018, 137, 430–443. [Google Scholar] [CrossRef]
- Tosteson, T.R.; Ballantine, D.L.; Durst, H.D. Seasonal frequency of ciguatoxic barracuda in southwest Puerto Rico. Toxicon 1988, 26, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Sellin, M.; Street, R.; Holmes, M.J.; Gillespie, N.C. Excretion of ciguatoxin from Moray eels (Muraenidae) of the central Pacific. In Proceedings of the Third International Conference on Ciguatera Fish Poisoning, La Parguera, Puerto Rico, 30 April–5 May 1990; Tosteson, T.R., Ed.; Polyscience Publications: Quebec City, QC, Canada, 1992; pp. 131–143. [Google Scholar]
- Clausing, R.J.; Losen, B.; Oberhaensli, F.R.; Taiana Darius, H.; Sibat, M.; Hess, P.; Swarzenski, P.A.; Chinain, M.; Dechraoui Bottein, M.-Y. Experimental evidence of dietary ciguatoxin accumulation in an herbivorous coral reef fish. Aquat. Toxicol. 2018, 200, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.T.; Robertson, A. Depuration Kinetics and Growth Dilution of Caribbean Ciguatoxin in the Omnivore Lagodon rhomboides: Implications for Trophic Transfer and Ciguatera Risk. Toxins 2021, 13, 774. [Google Scholar] [CrossRef]
- Li, J.; Mak, Y.L.; Chang, Y.-H.; Xiao, C.; Chen, Y.-M.; Shen, J.; Wang, Q.; Ruan, Y.; Lam, P.K.S. Uptake and Depuration Kinetics of Pacific Ciguatoxins in Orange-Spotted Grouper (Epinephelus coioides). Environ. Sci. Technol. 2020, 54, 4475–4483. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Henao, A.; García-Àlvarez, N.; Padilla, D.; Ramos-Sosa, M.; Sergent, F.S.; Fernández, A.; Estévez, P.; Gago-Martínez, A.; Diogène, J.; Real, F. Accumulation of C-CTX1 in muscle tissue of goldfish (Carassius auratus) by dietary exposure. Animals 2021, 11, 242. [Google Scholar] [CrossRef] [PubMed]
- Leite, I.D.P.; Sdiri, K.; Taylor, A.; Viallon, J.; Gharbia, H.B.; Mafra Júnior, L.L.; Swarzenski, P.; Oberhaensli, F.; Darius, H.T.; Chinain, M.; et al. Experimental evidence of ciguatoxin accumulation and depuration in carnivorous lionfish. Toxins 2021, 13, 564. [Google Scholar] [CrossRef]
- Darius, H.T.; Revel, T.; Cruchet, P.; Viallon, J.; Gatti, C.M.i.; Sibat, M.; Hess, P.; Chinain, M. Deep-Water Fish Are Potential Vectors of Ciguatera Poisoning in the Gambier Islands, French Polynesia. Mar. Drugs 2021, 19, 644. [Google Scholar] [CrossRef]
- Chan, W.H.; Mak, Y.L.; Wu, J.J.; Jin, L.; Sit, W.H.; Lam, J.C.W.; de Mitcheson, Y.S.; Chan, L.L.; Lam, P.K.S.; Murphy, M.B. Spatial distribution of ciguateric fish in the Republic of Kiribati. Chemosphere 2011, 84, 117–123. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Regional variations in the risk and severity of ciguatera caused by eating moray eels. Toxins 2017, 9, 201. [Google Scholar] [CrossRef]
- Holmes, M.J.; Lewis, R.J.; Poli, M.A.; Gillespie, N.C. Strain dependent production of ciguatoxin precursors (gambiertoxins) by Gambierdiscus toxicus (Dinophyceae) in culture. Toxicon 1991, 29, 761–775. [Google Scholar] [CrossRef]
- Holmes, M.; Lewis, R.J.; Sellin, M.; Street, R. The origin of ciguatera in Platypus Bay, Australia. Mem. Qld. Mus. 1994, 34, 505–512. [Google Scholar]
- Roff, G.; Mumby, P.J. Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 2012, 27, 7. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Connolly, S.R.; Bellwood, D.R. Benthic composition changes on coral reefs at global scales. Nat. Ecol. Evol. 2023, 7, 71–81. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Brandl, S.J.; McWilliam, M.; Streit, R.P.; Yan, H.F.; Tebbett, S.B. Studying functions on coral reefs: Past perspectives, current conundrums, and future potential. Coral Reefs 2024, 43, 281–297. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nystrom, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Hemingson, C.R.; Bellwood, D.R. Greater multihabitat use in Caribbean fishes when compared to their Great Barrier Reef counterparts. Est. Coast. Shelf Sci. 2020, 239, 106748. [Google Scholar] [CrossRef]
- Lokrantz, J.; Nyström, M.; Thyresson, M.; Johansson, C. The non-linear relationship between body size and function in parrotfishes. Coral Reefs 2008, 27, 967–974. [Google Scholar] [CrossRef]
- Mill, A.C.; Pinnegar, J.K.; Polunin, N.V.C. Explaining isotope trophic-step fractionation: Why herbivorous fish are different. Funct. Ecol. 2007, 21, 1137–1145. [Google Scholar] [CrossRef]
- Bellwood, D.R. Direct estimate of bioerosion by two parrotfish species, Chlorurus gibbus and C. sordidus, on the Great Barrier Reef, Australia. Mar. Biol. 1995, 121, 421–429. [Google Scholar] [CrossRef]
- Bonaldo, R.M.; Bellwood, D.R. Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar. Ecol. Prog. Ser. 2008, 360, 237–244. [Google Scholar] [CrossRef]
- Welsh, J.Q.; Bellwood, D.R. How far do roving schools of herbivores rove? A case study using Scarus rivulatus. Coral Reefs 2012, 31, 991–1003. [Google Scholar] [CrossRef]
- Brijs, J.; Tran, L.L.; Moore, C.; Souza, T.; Schakmann, M.; Grellman, K.; Johansen, J.L. Outlasting the Heat: Collapse of Herbivorous Fish Control of Invasive Algae During Marine Heatwaves. Glob. Change Biol. 2025, 31, e70438. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Resolving resource partitioning in parrotfishes (Scarini) using microhistology of feeding substrata. Coral Reefs 2020, 39, 1313–1327. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Fine-scale analysis of substrata grazed by parrotfishes (Labridae:Scarini) on the outer-shelf of the Great Barrier Reef, Australia. Mar. Biol. 2023, 170, 121. [Google Scholar]
- Clements, K.D.; German, D.P.; Piche, J.; Tribollet, A.; Choat, J.H. Integrating ecological roles and trophic diversification on coral reefs: Multiple lines of evidence identify parrotfishes as microphages. Biol. J. Linn. Soc. 2017, 120, 729–751. [Google Scholar] [CrossRef]
- Bray, D.J.; Fishes of Australia. Introduction to Australia’s Fishes. In Fishes of Australia; Bray, D.J., Gomon, M.F., Eds.; Museums Victoria and OzFishNet: Melbourne, Australia, 2018; Available online: https://fishesofaustralia.net.au/ (accessed on 14 August 2025).

| Number | Species | Maximum Reported CTX Concentration (pg P-CTX3C eq./cell) | Reference |
|---|---|---|---|
| G. polynesiensis (Cook Islands, Polynesia) | 155 pg CTX (attributed only as CTX/cell) | [30] | |
| 1 | G. polynesiensis (Cook Islands, Polynesia) | 18.2 | [29] |
| G. polynesiensis (French Polynesia) | 8.3 | [27] | |
| 2 | G. belizeanus | 0.1 | [23] |
| 3 | G. australes | 0.03 from Pacific isolate, but 0.5 pg P-CTX-1 eq./cell from Atlantic | [23,48] |
| 4 | G. toxicus | 0.03 | [23] |
| 5 | G. carpenteri | 0.03 | [24] |
| 6 | G. scabrosus | 0.03 | [49] |
| 7 | G. balechii | 0.02 | [50] |
| 8 | G. caribaeus | 0.02 from Pacific isolate but up to 0.17 from Atlantic | [11,24] |
| 9 | G. pacificus | 0.008 | [24] |
| 10 | G. honu | 0.001 | [24] |
| 11 | G. lapillus | -/trace | [28,51] |
| 12 | G. lewisii | -/trace | [28,51] |
| 13 | G. holmesii | -/trace | [6,51] |
| 14 | G. cheloniae | CTX not yet detected | [28,52] |
| 15 | G. vietnamensis | CTX not yet detected | [53] |
| 16 | G. silvae | CTX not quantified from Pacific isolates. Concentrations from outside Pacific suggest ~0.02 pg P-CTX3C eq./cell, 0.08 pg P-CTX-1 eq./cell | [54,55,56] |
| 17 | G. jejeunsis | CTX not yet detected | |
| 18 | G. bagnisii | CTX not yet detected | |
| 1 | F. yasumotoi | CTX not yet detected | [57] |
| 2 | F. paulensis | CTX not detected from Pacific isolates. Concentrations outside Pacific suggest fg/cell | [58,59] |
| 3 | F. ruetzleri | CTX not detected from Pacific isolates. Concentrations outside Pacific suggest ~0.03 pg P-CTX3C eq./cell | [55,60] |
| 4 | F. koreansis | CTX not yet detected |
| Variable | Model Values | Calculations, Assumptions, and Comments |
|---|---|---|
| Model target for P-CTX3C concentration in flesh of herbivorous fish | 0.5 μg P-CTX3C/kg | 0.5 µg P-CTX3C/kg fish is based upon the five-fold lower potency of P-CTX3C relative to P-CTX-1 [2] and is assumed to likely cause mild poisoning in 2 out of 10 people [35]. This CTX concentration is 10-fold more than the US FDA recommended limit of 0.01 μg P-CTX-1 equivalents (eq.)/kg but transformed to P-CTX3C eq., i.e., 0.5 μg P-CTX3C eq./kg. |
| Flesh (fillet) recovery | Parrotfish: 42% N. unicornis: 43% | Parrotfish (42%): Median value of a range of meat recoveries for fillets (40–49%) taken from internet fishing sites for 5 species of Scarus spp. [34]. N. unicornis (43%): We can find no data for filet recovery from fishers, so we have estimated a meat recovery of 43% based upon the average weight of muscle recovered from juvenile N. brevirostris (40%, 47%) [92]. This may be an overestimate for meat recovery from filets. |
| Flesh (fillet) CTX burden | Parrotfish: A range is calculated between 10 and 40% of the CTX load ingested by parrotfish. N. unicornis: 44% | Parrotfish: Flesh estimated to accumulate between 10–40% of the toxin load of the fish based upon Caribbean pinfish [128]. Clausing et al. [92] recently reported a slightly higher relative proportion of CTX retained in the muscle of the unicornfish N. brevirostris (44%). N. unicornis: Based on the 44% retention of CTX reported for N. brevirostris [92]. |
| Fish CTX load (μg) | Calculated depending upon fish weight (Table 4) | Based upon a 43% transfer rate [128] |
| Daily grazing rates. Parrotfish: m2/d on turf algae. N. unicornis: g macroalgae/day. | Parrotfish: Calculated from annual grazing rates (m2/y) depending upon species and fish total length (TL, cm). N. unicornis: Estimated grazing rate as g macroalgae/day based upon the fish consuming a percentage of its body weight. | Parrotfish: Annual grazing rates (m2/y) calculated using equations derived by Lange et al. [61]: S. niger = 0.0367(TL2.2), S. frenatus = 0.0138(TL2.439), S. psittacus = 0.0004(TL2.986), Chlorurus sordidus = 0.0433(TL2.209). Lokrantz et al. [142] also derived equations for the area grazed by S. niger and Ch. sordidus for fish observed feeding at 3 sites near Zanzibar. The equations for the Chumbe site produced the largest area grazed (cm2/min): S. niger = 0.00002(TL3.66), Ch. sordidus = 0.0001(TL3.09). Lokrantz et al. [142] suggest that their equations likely overestimate the areas grazed. N. unicornis: 10–30% body weight of macroalgae [92,127,143] |
| The time parrotfish and N. unicornis spend grazing on turf algae each day | 9 h | Algae is a low-energy food source requiring many of the herbivores that rely on it for nutrition to feed almost continuously during daylight hours [108,144,145,146,147] and 9 h is consistent with the daily feeding times we used previously for parrotfish and surgeonfish on the Great Barrier Reef [33,34]. We have modified the daily feeding from 12 h used by Lange et al. [61] for parrotfish feeding close to the equator in the Maldives and Chagos Archipelago. However, feeding duration likely varies throughout the day, between seasons and with latitude |
| The efficacy of the fish bite to remove and ingest Gambierdiscus from algae. | Parrotfish: 90% N. unicornis: 100% | Parrotfish: This rate is an assumption as there are no data available but is unlikely to be 100% as the bite is acting on a surface covered with turf algae. However, as scraping and excavator parrotfish are targeting microorganisms for nutrition [95,148,149,150] we assume the efficiency to be high. N. unicornis: The model assumes that the fish bite removes pieces of algae with attached epiphytic dinoflagellates. We have assumed 100% bite efficiency but recognize that this is likely an overestimation. |
| Variable | Model Values | Calculations, Assumptions, and Comments |
|---|---|---|
| The transfer rate for CTX between trophic level 1 and 2 | 43% | Based upon an average net CTX assimilation of 43% in pinfish [128], also see Holmes and Lewis [32,33,34]. This term accounts for CTX losses between trophic levels. This transfer efficiency is similar to that reported for CTX from G. polynesiensis into mullet (42%, [115]). The actual transfer rates for the modelled species are not known |
| P-CTX3C concentrations produced by Gambierdiscus and consumed by herbivorous fish. These concentrations are varied depending upon the scenario being explored | 0.01–155 pg P-CTX3C eq./cell | Scenarios for parrotfish and N. unicornis explore a range of potential toxin concentrations based upon concentrations determined experimentally. The maximum concentration is assumed to be 18.2 pg P-CTX3C eq./cell from G. polynesiensis isolated from Rarotonga in the Cook Islands, Polynesia [29] although Rhodes et al. [30] suggest this isolate had earlier produced 155 pg CTX/cell. The maximum known concentration from French Polynesian G. polynesiensis is 8.3 pg P-CTX3C eq./cell [27]. Depuration scenarios for parrotfish include comparisons with Gambierdiscus producing hypothetical P-CTX-1 concentrations between 0.03–1.6 pg P-CTX-1 eq./cell. 1.6 pg P-CTX-1 eq./cell is a hypothetical concentration based upon mouse bioassay of Gambierdiscus strains isolated from Platypus Bay, and the Great Barrier Reef, Australia [32,33]. All calculations for these P-CTX-1 scenarios were as per Holmes and Lewis [34]. |
| Gambierdiscus densities on turf algae or macroalgae | Turf algae: 0.1–10,000 cells/cm2 Macroalgae: 1–10,000 cells/g | Hypothetical range of cell densities of CTX-producing Gambierdiscus epiphytic on turf algae or macroalgae. Parrotfish: Cell densities on turf algae (cells/cm2) are compared with ranges reported from 24 h benthic screen assays ([47] and references therein). We are not aware of any reports of cell densities ≥1000 cells/cm2. ~1 cell/cm2 is the median of a global range on screen assays [47]. N. unicornis: Cell densities (cells/g macroalgae) are compared with ranges from the literature [2,91]. |
| Scraping Parrotfish Species | Common/Local Name | Maximum Total Length (cm) [107,151] | Weight (g)–Total Length (TL, cm) Relationships | Reference for Weight–Length Relationships |
|---|---|---|---|---|
| Scarus frenatus | Sixband parrotfish | 47 | Weight (g) = 0.0366∙TL2.816 | [105] |
| S. niger | Swarthy parrotfish | 40 | Weight = 0.041∙TL2.75 | [105] |
| S. psittacus | Palenose parrotfish | 43 | Weight (g) = 0.0189∙TL3.03 FishBase calculator for American Samoa | [107] |
| Excavator parrotfish species | ||||
| Chlorurus sordidus Note: From Indian Ocean. The fish previously labelled Ch. (Scarus) sordidus from the Pacific is likely Ch. spilurus [151] | Bullethead parrotfish | 40 | Weight (g) = 0.109∙TL2.48 | [105] |
| Surgeonfish species | ||||
| Ctenochaetus striatus | Lined surgeonfish | 26 | Weight (g) = 0.0137∙TL3.083 FishBase calculator for Réunion Is. | [107] |
| Naso unicornis | Bluespine unicornfish | 74 | Weight (g) = 0.0329∙FL2.85 FishBase calculator for American Samoa. Fork Length (FL) = 0.857∙TL | [107] |
| Predator species | ||||
| Plectropomus leopardus | Common coral trout (grouper) | 120 (23.6 kg) | Model a 2 kg fish |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmes, M.J.; Lewis, R.J. A General Food Chain Model for Bioaccumulation of Ciguatoxin into Herbivorous Fish in the Pacific Ocean Suggests Few Gambierdiscus Species Can Produce Poisonous Herbivores, and Even Fewer Can Produce Poisonous Higher Trophic Level Fish. Toxins 2025, 17, 526. https://doi.org/10.3390/toxins17110526
Holmes MJ, Lewis RJ. A General Food Chain Model for Bioaccumulation of Ciguatoxin into Herbivorous Fish in the Pacific Ocean Suggests Few Gambierdiscus Species Can Produce Poisonous Herbivores, and Even Fewer Can Produce Poisonous Higher Trophic Level Fish. Toxins. 2025; 17(11):526. https://doi.org/10.3390/toxins17110526
Chicago/Turabian StyleHolmes, Michael J., and Richard J. Lewis. 2025. "A General Food Chain Model for Bioaccumulation of Ciguatoxin into Herbivorous Fish in the Pacific Ocean Suggests Few Gambierdiscus Species Can Produce Poisonous Herbivores, and Even Fewer Can Produce Poisonous Higher Trophic Level Fish" Toxins 17, no. 11: 526. https://doi.org/10.3390/toxins17110526
APA StyleHolmes, M. J., & Lewis, R. J. (2025). A General Food Chain Model for Bioaccumulation of Ciguatoxin into Herbivorous Fish in the Pacific Ocean Suggests Few Gambierdiscus Species Can Produce Poisonous Herbivores, and Even Fewer Can Produce Poisonous Higher Trophic Level Fish. Toxins, 17(11), 526. https://doi.org/10.3390/toxins17110526





