Nixtamalization of Maize to Reduce Mycotoxin Exposure: A Human Biomonitoring Intervention Study in Soweto, South Africa
Abstract
1. Introduction
2. Results and Discussion
2.1. Participant Demographics and Knowledge of Mycotoxins
2.2. Mycotoxin Levels in Maize Porridge
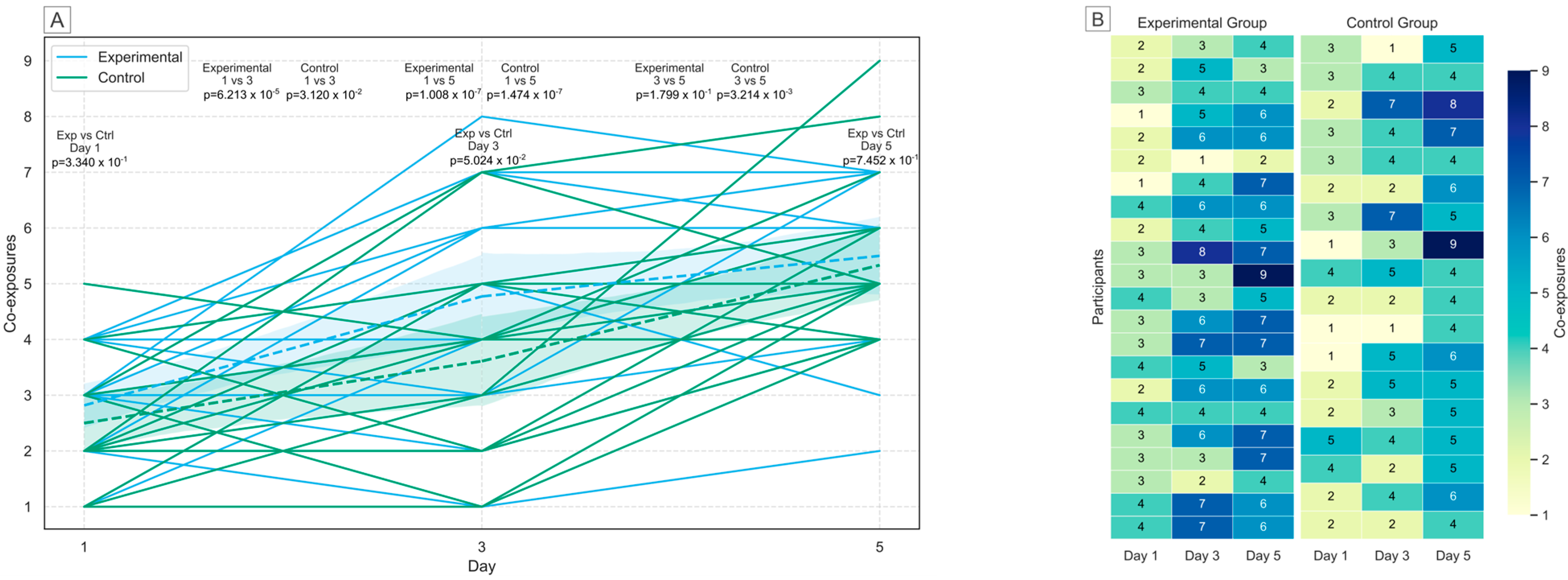
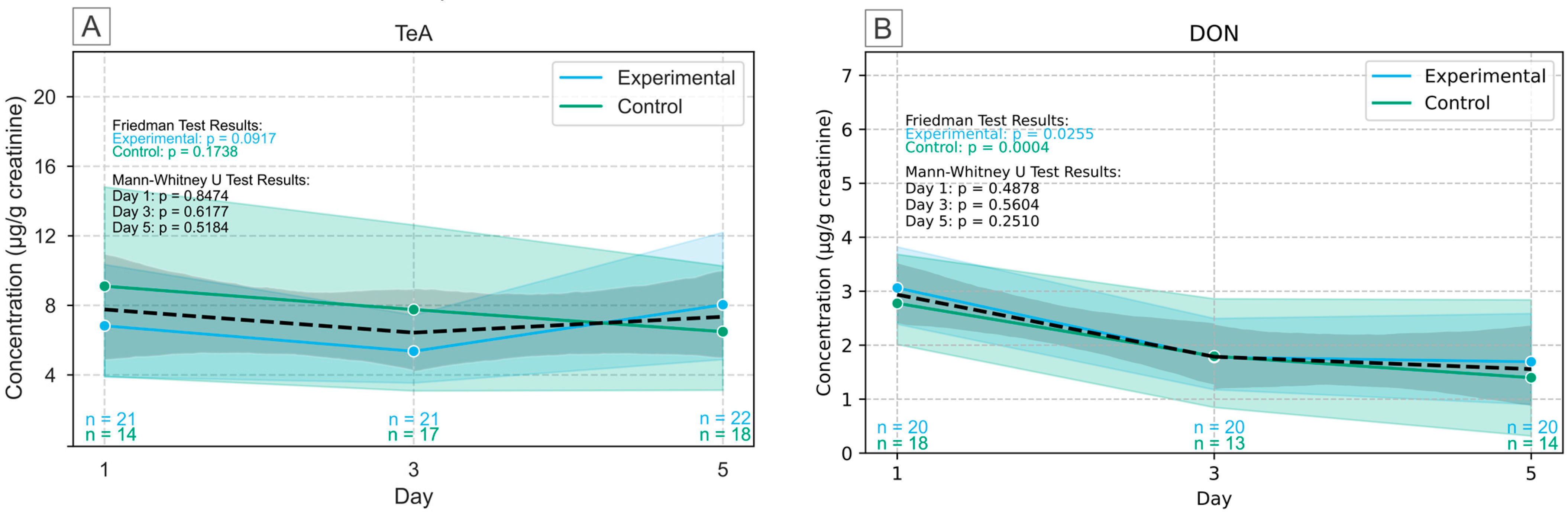
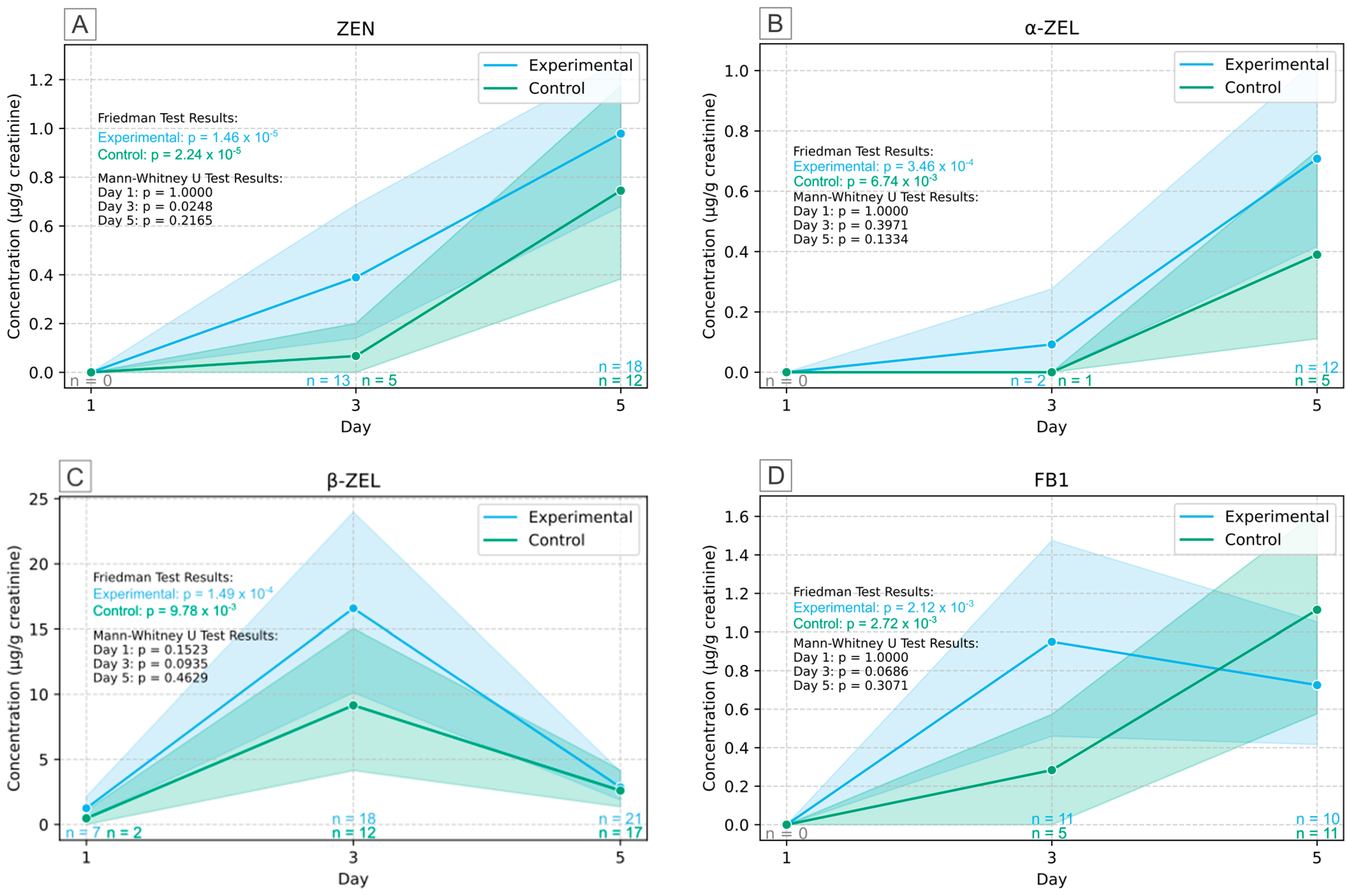
2.3. Human Biomonitoring of Multiple Mycotoxins in Urine
2.4. Implications and Limitations
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
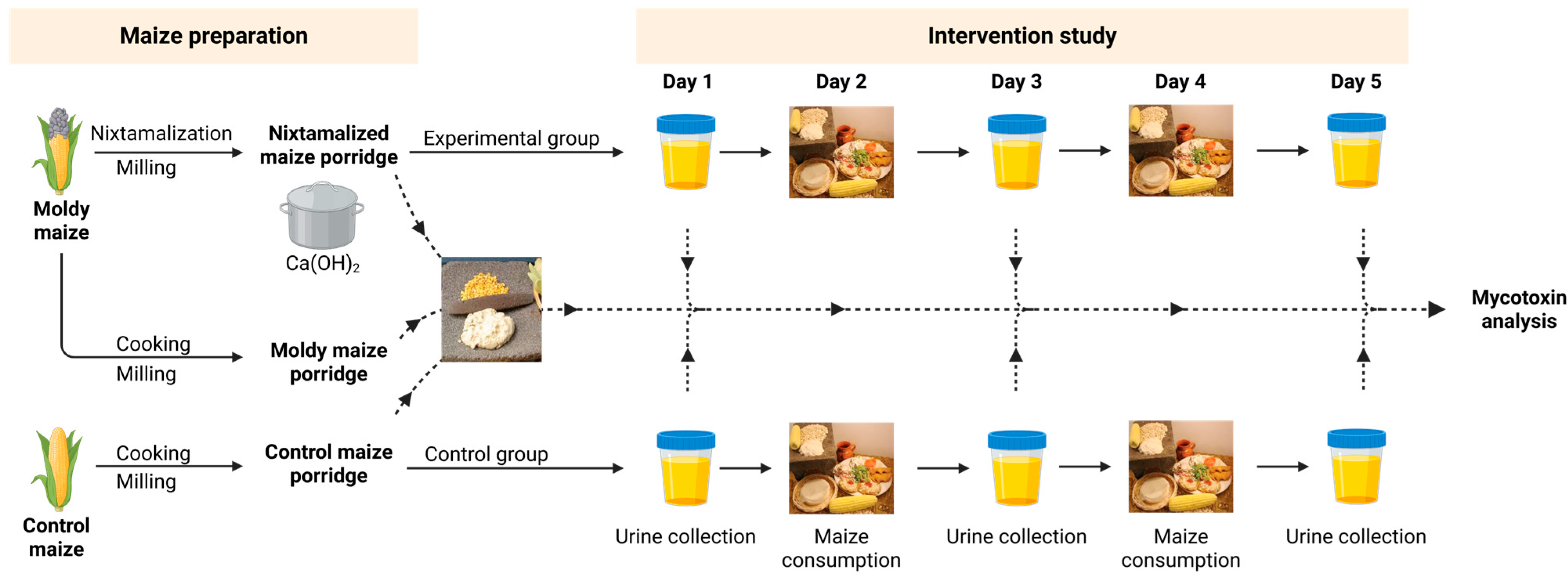
4.2. Study Population, Design and Ethics Approval
4.3. Processing of Maize and Nixtamalization
4.4. Multiple Mycotoxins Analysis of Maize Porridges
4.5. Human Biomonitoring of Multiple Mycotoxins in Urine
4.5.1. Instrumentation
4.5.2. Urine Sample Pretreatment and UHPLC-MS/MS Method Validation
4.5.3. Creatinine-Adjustment in Urine
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 13C | Isotopically labeled carbon |
| 3-ADON | 3-acetyl-deoxynivalenol |
| 15-ADON | 15-acetyl-deoxynivalenol |
| ACN | Acetonitrile |
| AFB1 | Aflatoxin B1 |
| AFB2 | Aflatoxin B2 |
| AFG1 | Aflatoxin G1 |
| AFG2 | Aflatoxin G2 |
| AFM1 | Aflatoxin M1 |
| AFs | Aflatoxins |
| AOH | Alternariol |
| AME | Alternariol monomethyl ether |
| BEAU | Beauvericin |
| Ca(OH)2 | Calcium hydroxide |
| CEMPH | Centre of Excellence in Mycotoxicology and Public Health |
| CIT | Citrinin |
| CPA | Cyclopiazonic acid |
| DAS | Diacetoxyscirpenol |
| DOM | Deepoxy-deoxynivalenol |
| DON | Deoxynivalenol |
| DON-3G | Deoxynivalenol-3-glucoside |
| EC | European Commission |
| ENNA | Enniatin A |
| ENNA1 | Enniatin A1 |
| ENNB | Enniatin B |
| ENNB1 | Enniatin B1 |
| ESI | Electrospray ionization |
| FA | Formic acid |
| FB1 | Fumonisin B1 |
| FB2 | Fumonisin B2 |
| FB3 | Fumonisin B3 |
| FUS-X | Fusarenon-X |
| HFB1 | Hydrolyzed fumonisin B1 |
| HAc | Glacial acetic acid |
| HT-2 | HT-2 toxin |
| INSA-UB | Institute for Research on Nutrition and Food Safety, University of Barcelona |
| LC-HRMS | Liquid chromatography–high-resolution mass spectrometry |
| LLOQ | Lower limit of quantification |
| LOD | Limit of detection |
| MeOH | Methanol |
| MRM | Multiple reaction monitoring |
| NaOH | Sodium hydroxide |
| NEO | Neosolaniol |
| NIV | Nivalenol |
| OTA | Ochratoxin A |
| OTα | Ochratoxin-alpha |
| PACA | Partnership for Aflatoxin Control in Africa |
| ROQ-C | Roquefortin C |
| SAGL | South African Grain Laboratory |
| STERIG | Sterigmatocystin |
| T-2 | T-2 toxin |
| TeA | Tenuazonic acid |
| UHPLC-MS/MS | Ultra-high performance liquid chromatography–tandem mass spectrometry |
| ULOQ | Upper limit of quantification |
| ZAL | Zearalanol |
| ZAN | Zearalanone |
| ZEL | Zearalenol |
| ZEN | Zearalenone |
References
- Adaku, A.A.; Egyir, I.S.; Gadegbeku, C.; Kunadu, A.P.H.; Amanor-Boadu, V.; Laar, A. Barriers to Ensuring and Sustaining Street Food Safety in a Developing Economy. Heliyon 2024, 10, e32190. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Global Strategy for Food Safety 2022–2030: Towards Stronger Food Safety Systems and Global Cooperation; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Mphaga, K.V.; Moyo, D.; Rathebe, P.C. Unlocking Food Safety: A Comprehensive Review of South Africa’s Food Control and Safety Landscape from an Environmental Health Perspective. BMC Public Health 2024, 24, 2040. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An Overview on the Major Mycotoxins in Food Products: Characteristics, Toxicity, and Analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- da Rocha, M.E.B.; da Chagas Oliveira Freire, F.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and Their Effects on Human and Animal Health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Bai, X.; Ran, Y. Recent Development of Methods and Techniques in the Detection of Mycotoxins in Agricultural Products. J. Agric. Food Chem. 2025, 73, 20530–20546. [Google Scholar] [CrossRef]
- Maphaisa, T.C.; Akinmoladun, O.F.; Adelusi, O.A.; Mwanza, M.; Fon, F.; Tangni, E.; Njobeh, P.B. Advances in Mycotoxin Detection Techniques and the Crucial Role of Reference Material in Ensuring Food Safety. A Review. Food Chem. Toxicol. 2025, 200, 115387. [Google Scholar] [CrossRef] [PubMed]
- Misihairabgwi, J.M.; Ezekiel, C.N.; Sulyok, M.; Shephard, G.S.; Krska, R. Mycotoxin Contamination of Foods in Southern Africa: A 10-Year Review (2007–2016). Crit. Rev. Food Sci. Nutr. 2019, 59, 43–58. [Google Scholar] [CrossRef]
- Visagie, C.M.; Meyer, H.; Yilmaz, N. Maize–Fusarium Associations and Their Mycotoxins: Insights from South Africa. Fungal Biol. 2024, 128, 2408–2421. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Åberg, A.T.; Haggblom, P. Estimation of Multi-Mycotoxin Contamination in South African Compound Feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef]
- Ngum, N.Q.; Babalola, O.O.; Ekwomadu, T.I.; Nleya, N.; Mulunda, M. Six Main Contributing Factors to High Levels of Mycotoxin Contamination in African Foods. Toxins 2022, 14, 318. [Google Scholar] [CrossRef]
- Gbashi, S.; Madala, N.E.; Saeger, S.D.; De Boevre, M.; Adekoya, I.; Adebo, O.A.; Njobeh, P.B.; Gbashi, S.; Madala, N.E.; De Saeger, S.; et al. The Socio-Economic Impact of Mycotoxin Contamination in Africa. In Mycotoxins-Impact and Management Strategies; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Chilaka, C.A.; Obidiegwu, J.E.; Chilaka, A.C.; Atanda, O.O.; Mally, A. Mycotoxin Regulatory Status in Africa: A Decade of Weak Institutional Efforts. Toxins 2022, 14, 442. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Delzeit, R.; Ignaciuk, A.; Levers, C.; Braich, G.; Bajaj, K.; Amo-Aidoo, A.; Anderson, W.; Balgah, R.A.; Benton, T.G.; et al. Research Priorities for Global Food Security under Extreme Events. One Earth 2022, 5, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Ncube, E.; Flett, B.C.; Waalwijk, C.; Viljoen, A. Fusarium spp. and Levels of Fumonisins in Maize Produced by Subsistence Farmers in South Africa. S. Afr. J. Sci. 2011, 107, 1–7. [Google Scholar] [CrossRef]
- Phoku, J.Z.; Dutton, M.F.; Njobeh, P.B.; Mwanza, M.; Egbuta, M.A.; Chilaka, C.A. Fusarium Infection of Maize and Maize-Based Products and Exposure of a Rural Population to Fumonisin B1 in Limpopo Province, South Africa. Food Addit. Contam. Part A 2012, 29, 1743–1751. [Google Scholar] [CrossRef]
- Mngqawa, P.; Shephard, G.S.; Green, I.R.; Ngobeni, S.H.; de Rijk, T.C.; Katerere, D.R. Mycotoxin Contamination of Home-Grown Maize in Rural Northern South Africa (Limpopo and Mpumalanga Provinces). Food Addit. Contam Part B Surveill. 2016, 9, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Burger, H.M.; Lombard, M.J.; Shephard, G.S.; Rheeder, J.R.; van der Westhuizen, L.; Gelderblom, W.C.A. Dietary Fumonisin Exposure in a Rural Population of South Africa. Food Chem. Toxicol. 2010, 48, 2103–2108. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Sundheim, L. Fumonisins in African Countries. Toxins 2022, 14, 419. [Google Scholar] [CrossRef]
- Molnár, K.; Rácz, C.; Dövényi-Nagy, T.; Bakó, K.; Pusztahelyi, T.; Kovács, S.; Adácsi, C.; Pócsi, I.; Dobos, A. The Effect of Environmental Factors on Mould Counts and AFB1 Toxin Production by Aspergillus Flavus in Maize. Toxins 2023, 15, 227. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Ayanlade, A.; Oluwaranti, A.; Ayanlade, O.S.; Borderon, M.; Sterly, H.; Sakdapolrak, P.; Jegede, M.O.; Weldemariam, L.F.; Ayinde, A.F.O. Extreme Climate Events in Sub-Saharan Africa: A Call for Improving Agricultural Technology Transfer to Enhance Adaptive Capacity. Clim. Serv. 2022, 27, 100311. [Google Scholar] [CrossRef]
- Ampofo, J.A.; Arfasa, G.F.; Mantey, I.; Aniah, E. Causes of Informal Settlement in Africa: A Systematic Review. ADRRI J. Contemp. Afr. Dev. 2024, 1, 1–18. [Google Scholar]
- UN-Habitat. The Challenge of Slums: Global Report on Human Settlements 2003. Manag. Environ. Qual. Int. J. 2004, 15, 337–338. [Google Scholar] [CrossRef]
- Manga, M.; Bartram, J.; Evans, B.E. Economic Cost Analysis of Low-Cost Sanitation Technology Options in Informal Settlement Areas (Case Study: Soweto, Johannesburg). Int. J. Hyg Environ. Health 2020, 223, 289–298. [Google Scholar] [CrossRef]
- Mgqibandaba, P.Z.; Madilo, F.K.; Du-Preez, C.J.; Mjoka, J.; Unathi, K. Evaluating Food Safety and Hygiene Knowledge and Practices among Foodservice Staff of Feeding Scheme in the Primary Schools in Soweto, South Africa. J. Food Saf. 2020, 40, e12792. [Google Scholar] [CrossRef]
- Murtaza, B.; Wang, L.; Li, X.; Saleemi, M.K.; Nawaz, M.Y.; Li, M.; Xu, Y. Cold Plasma: A Success Road to Mycotoxins Mitigation and Food Value Edition. Food Chem. 2024, 445, 138378. [Google Scholar] [CrossRef]
- Ngure, F.M.; Makule, E.; Mgongo, W.; Phillips, E.; Kassim, N.; Stoltzfus, R.; Nelson, R. Processing Complementary Foods to Reduce Mycotoxins in a Medium Scale Tanzanian Mill: A Hazard Analysis Critical Control Point (HACCP) Approach. Food Control 2024, 162, 110463. [Google Scholar] [CrossRef]
- Liu, Y.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. A Review of Postharvest Approaches to Reduce Fungal and Mycotoxin Contamination of Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Chen, B.; Rao, J. Occurrence and Preventive Strategies to Control Mycotoxins in Cereal-Based Food. Compr. Rev. Food Sci. Food Saf. 2020, 19, 928–953. [Google Scholar] [CrossRef]
- Schaarschmidt, S.; Fauhl-Hassek, C. Mycotoxins during the Processes of Nixtamalization and Tortilla Production. Toxins 2019, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Odukoya, J.O.; De Saeger, S.; De Boevre, M.; Adegoke, G.O.; Audenaert, K.; Croubels, S.; Antonissen, G.; Vermeulen, K.; Gbashi, S.; Njobeh, P.B. Effect of Selected Cooking Ingredients for Nixtamalization on the Reduction of Fusarium Mycotoxins in Maize and Sorghum. Toxins 2021, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Franco, A.; Canelo-Álvarez, F.; García-Salinas, F.; Alcántara-Zavala, A.; Figueroa-Cárdenas, J.d.D.; Méndez-Albores, A. Evaluation of the Nixtamalized Cornbread-Making Process as a Method of Aflatoxin Detoxification. J. Consum. Prot. Food Saf. 2024, 19, 71–80. [Google Scholar] [CrossRef]
- Galinat, W.C. The History and Evolution of Maize. CRC Crit. Rev. Plant Sci. 1988, 7, 197–220. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O. Understanding the Functionality and Manufacturing of Nixtamalized Maize Products. J. Cereal Sci. 2021, 99, 103205. [Google Scholar] [CrossRef]
- Sefa-Dedeh, S.; Cornelius, B.; Sakyi-Dawson, E.; Afoakwa, E.O. Effect of Nixtamalization on the Chemical and Functional Properties of Maize. Food Chem. 2004, 86, 317–324. [Google Scholar] [CrossRef]
- Villada, J.A.; Sánchez-Sinencio, F.; Zelaya-Ángel, O.; Gutiérrez-Cortez, E.; Rodríguez-García, M.E. Study of the Morphological, Structural, Thermal, and Pasting Corn Transformation during the Traditional Nixtamalization Process: From Corn to Tortilla. J. Food Eng. 2017, 212, 242–251. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Nutritional Assessment of Nixtamalized Maize Tortillas Produced from Dry Masa Flour, Landraces, and High Yield Hybrids and Varieties. Front. Nutr. 2023, 10, 1183935. [Google Scholar] [CrossRef]
- Hassan, S.M.; Forsido, S.F.; Tola, Y.B.; Bikila, A.M.; Ahmed, Z. Effect of Nixtamalization on the Nutritional, Anti-Nutritional, Functional, Physicochemical and Mineral Properties of Maize (Zea Mays) Tortillas. J. Food Chem. Nanotechnol. 2023, 9, 132–140. [Google Scholar] [CrossRef]
- Adekoya, I.; Njobeh, P.; Obadina, A.; Chilaka, C.; Okoth, S.; De Boevre, M.; De Saeger, S. Awareness and Prevalence of Mycotoxin Contamination in Selected Nigerian Fermented Foods. Toxins 2017, 9, 363. [Google Scholar] [CrossRef]
- Foodstuffs, Cosmetics and Disinfectants Act 54 of 1972, South African Government. Government Gazette No. 3530. Available online: https://www.gov.za/documents/foodstuffs-cosmetics-and-disinfectants-act-2-jun-1972-0000 (accessed on 21 October 2025).
- Regulations Relating to Maximum Levels of Mycotoxins in Foodstuffs (2024), Issued under Sec. 15 of the Foodstuffs, Cosmetics and Disinfectants Act, 1972 (Act No. 54 of 1972). South African Government. Government Gazette No. 51499. Available online: https://www.health.gov.za/wp-content/uploads/2025/02/51499-01-Nov-2024-Regulations-relating-to-Maximum-levels-of-Mycotoxins-in-Foodstuffs.pdf (accessed on 21 October 2025).
- Shephard, G.S.; Burger, H.M.; Gambacorta, L.; Krska, R.; Powers, S.P.; Rheeder, J.P.; Solfrizzo, M.; Sulyok, M.; Visconti, A.; Warth, B.; et al. Mycological Analysis and Multimycotoxins in Maize from Rural Subsistence Farmers in the Former Transkei, South Africa. J. Agric. Food Chem. 2013, 61, 8232–8240. [Google Scholar] [CrossRef]
- Shephard, G.S.; Marasas, W.F.O.; Burger, H.M.; Somdyala, N.I.M.; Rheeder, J.P.; Van der Westhuizen, L.; Gatyeni, P.; Van Schalkwyk, D.J. Exposure Assessment for Fumonisins in the Former Transkei Region of South Africa. Food Addit. Contam. 2007, 24, 621–629. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Multi-Mycotoxin Screening of Feed and Feed Raw Materials from Africa. World Mycotoxin J. 2018, 11, 369–384. [Google Scholar] [CrossRef]
- Tebele, S.M.; Gbashi, S.; Adebo, O.; Changwa, R.; Naidu, K.; Njobeh, P.B. Quantification of Multi-Mycotoxin in Cereals (Maize, Maize Porridge, Sorghum and Wheat) from Limpopo Province of South Africa. Food Addit. Contam. Part A 2020, 37, 1922–1938. [Google Scholar] [CrossRef]
- Nji, Q.N.; Mwanza, M. Three-Year Multi-Mycotoxin Analysis of South African Commercial Maize from Three Provinces. Front. Fungal Biol. 2024, 5, 1426782. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; et al. Scientific Opinion on the Risks for Public Health Related to the Presence of Zearalenone in Food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- Shephard, G.S.; Burger, H.M.; Gambacorta, L.; Gong, Y.Y.; Krska, R.; Rheeder, J.P.; Solfrizzo, M.; Srey, C.; Sulyok, M.; Visconti, A.; et al. Multiple Mycotoxin Exposure Determined by Urinary Biomarkers in Rural Subsistence Farmers in the Former Transkei, South Africa. Food Chem. Toxicol. 2013, 62, 217–225. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of Food Processing and Detoxification Treatments on Mycotoxin Contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Mary, A.O.; Basson, A.K.; Osunsanmi, F.O.; Ge, Z. Nutritional Composition and Organoleptic Properties of Composite Maize Porridge. J. Food Process. Technol. 2019, 10, 798. [Google Scholar]
- Acosta-Estrada, B.A.; Serna-Saldívar, S.O.; Chuck-Hernández, C. Quality Assessment of Maize Tortillas Produced from Landraces and High Yield Hybrids and Varieties. Front. Nutr. 2023, 10, 1105619. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Skhosana, Z.D.; Motlanthe, M.; Louw, W.; Rohwer, E. Long Term Monitoring (2014–2018) of Multi-Mycotoxins in South African Commercial Maize and Wheat with a Locally Developed and Validated LC-MS/MS Method. Toxins 2019, 11, 271. [Google Scholar] [CrossRef]
- Southern African Grain Laboratory SANAS Certificate of Accreditation. Available online: https://sagl.co.za/wp-content/uploads/CQD-T0116-SANAS-2025-04-10.pdf (accessed on 8 May 2025).
- Delgado-Povedano, M.d.M.; Maris, E.; Kellner, N.; Mulisa, G.; Gámiz-Gracia, L.; García-Campaña, A.M.; De Boevre, M.; De Saeger, S.; Pero-Gascon, R. Liquid Chromatography-Tandem Mass Spectrometry for the Determination of Multiple Mycotoxins in Serum through Suspect Screening and Targeted Approaches: Advancing Human Mycotoxin Biomonitoring. Microchem. J. 2025, 208, 112562. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; De Saeger, S.; Nunes, C.; Torres, D.; Goios, A.; Lopes, C.; Assunção, R.; Alvito, P. Exposure Assessment of Portuguese Population to Multiple Mycotoxins: The Human Biomonitoring Approach. Int. J. Hyg. Environ. Health 2019, 222, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans Significantly Metabolize and Excrete the Mycotoxin Deoxynivalenol and Its Modified Form Deoxynivalenol-3-Glucoside within 24 Hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Regulation No 401/2006 of 23 February 2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs. Off. J. Eur. Union 2006, L70, 12–24. [Google Scholar]
- European Commission (EC). Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Off. J. Eur. Union 2002, 221, 8–36. [Google Scholar]
- Turner, P.C.; Hopton, R.P.; Lecluse, Y.; White, K.L.M.; Fisher, J.; Lebailly, P. Determinants of Urinary Deoxynivalenol and De-Epoxy Deoxynivalenol in Male Farmers from Normandy, France. J. Agric. Food Chem. 2010, 58, 5206–5212. [Google Scholar] [CrossRef]
| Characteristic | Category | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Sex | Male | 13 | 32.5 |
| Female | 27 | 67.5 | |
| Education Background | Secondary | 35 | 87.5 |
| Tertiary | 5 | 12.5 | |
| Race | Black | 28 | 70 |
| Colored | 12 | 30 | |
| Marital status | Married | 5 | 12.5 |
| Single | 35 | 87.5 | |
| Employment Status | Retired | 4 | 10 |
| Unemployed | 36 | 90 | |
| Age Group (Years) | 18–27 | 18 | 46.2 |
| 27–36 | 9 | 23.1 | |
| 36–46 | 7 | 17.9 | |
| 46–55 | 2 | 5.1 | |
| 55–65 | 3 | 7.7 | |
| Maize Consumption per Week | 1 day/week | 11 | 27.5 |
| 2–3 days/week | 6 | 15 | |
| 3–5 days/week | 4 | 10 | |
| 5–6 days/week | 5 | 12.5 | |
| Daily | 14 | 35 | |
| Suffering from Any Disease or Sickness | No | 40 | 100 |
| Awareness of Mycotoxins | No | 40 | 100 |
| Food Contamination with Fungi | No | 20 | 50 |
| Yes | 20 | 50 | |
| Frequency of Contamination within a 6-month period (if Yes) | Not sure | 1 | 5 |
| 2–3 times | 8 | 40 | |
| 4–6 times | 9 | 45 | |
| >6 times | 2 | 10 |
| Mycotoxin | Control Maize (µg/kg) | Moldy Maize (µg/kg) | Nixtamalized Maize (µg/kg) | Reduction (%) |
|---|---|---|---|---|
| AFB1 | <LOD | <LLOQ | <LOD | - |
| DON | <LOD | 229 | <LOD | 100 |
| FB1 | 69 | 2336 | 114 | 95.1 |
| FB2 | <LLOQ | 996 | 52 | 94.8 |
| FB3 | <LOD | 340 | <LLOQ | 100 |
| ZEN | 552 | 7661 | 843 | 89.0 |
| Group | Day Comparison | t-Statistic | p-Value |
|---|---|---|---|
| Control | Day 1 vs. Day 3 | −2.24767 | 3.120 × 10−2 |
| Control | Day 1 vs. Day 5 | −6.59378 | 1.474 × 10−7 |
| Control | Day 3 vs. Day 5 | −3.17073 | 3.214 × 10−3 |
| Experimental | Day 1 vs. Day 3 | −4.44979 | 6.213 × 10−5 |
| Experimental | Day 1 vs. Day 5 | −6.41384 | 1.008 × 10−7 |
| Experimental | Day 3 vs. Day 5 | −1.36389 | 1.799 × 10−1 |
| Control vs. Experimental | Day 1 | 0.97857 | 3.340 × 10−1 |
| Control vs. Experimental | Day 3 | 2.022152 | 5.024 × 10−2 |
| Control vs. Experimental | Day 5 | 0.327383 | 7.452 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maris, E.; Ndlangamandla, P.; Adelusi, O.A.; Akinmoladun, O.F.; Odukoya, J.O.; Fagbohun, R.T.; Oyeyinka, S.A.; Sekhejane, P.; Pero-Gascon, R.; De Boevre, M.; et al. Nixtamalization of Maize to Reduce Mycotoxin Exposure: A Human Biomonitoring Intervention Study in Soweto, South Africa. Toxins 2025, 17, 527. https://doi.org/10.3390/toxins17110527
Maris E, Ndlangamandla P, Adelusi OA, Akinmoladun OF, Odukoya JO, Fagbohun RT, Oyeyinka SA, Sekhejane P, Pero-Gascon R, De Boevre M, et al. Nixtamalization of Maize to Reduce Mycotoxin Exposure: A Human Biomonitoring Intervention Study in Soweto, South Africa. Toxins. 2025; 17(11):527. https://doi.org/10.3390/toxins17110527
Chicago/Turabian StyleMaris, Elias, Palesa Ndlangamandla, Oluwasola A. Adelusi, Oluwakamisi F. Akinmoladun, Julianah O. Odukoya, Richard T. Fagbohun, Samson A. Oyeyinka, Palesa Sekhejane, Roger Pero-Gascon, Marthe De Boevre, and et al. 2025. "Nixtamalization of Maize to Reduce Mycotoxin Exposure: A Human Biomonitoring Intervention Study in Soweto, South Africa" Toxins 17, no. 11: 527. https://doi.org/10.3390/toxins17110527
APA StyleMaris, E., Ndlangamandla, P., Adelusi, O. A., Akinmoladun, O. F., Odukoya, J. O., Fagbohun, R. T., Oyeyinka, S. A., Sekhejane, P., Pero-Gascon, R., De Boevre, M., Croubels, S., Njobeh, P. B., & De Saeger, S. (2025). Nixtamalization of Maize to Reduce Mycotoxin Exposure: A Human Biomonitoring Intervention Study in Soweto, South Africa. Toxins, 17(11), 527. https://doi.org/10.3390/toxins17110527










