Leptin and Adiponectin as Uremic Adipokines: Associations with Survival in a Prospective Hemodialysis Cohort
Abstract
1. Introduction
2. Results
2.1. Cohort Description
2.2. Clinical Characteristics Associated with Adipokine Levels
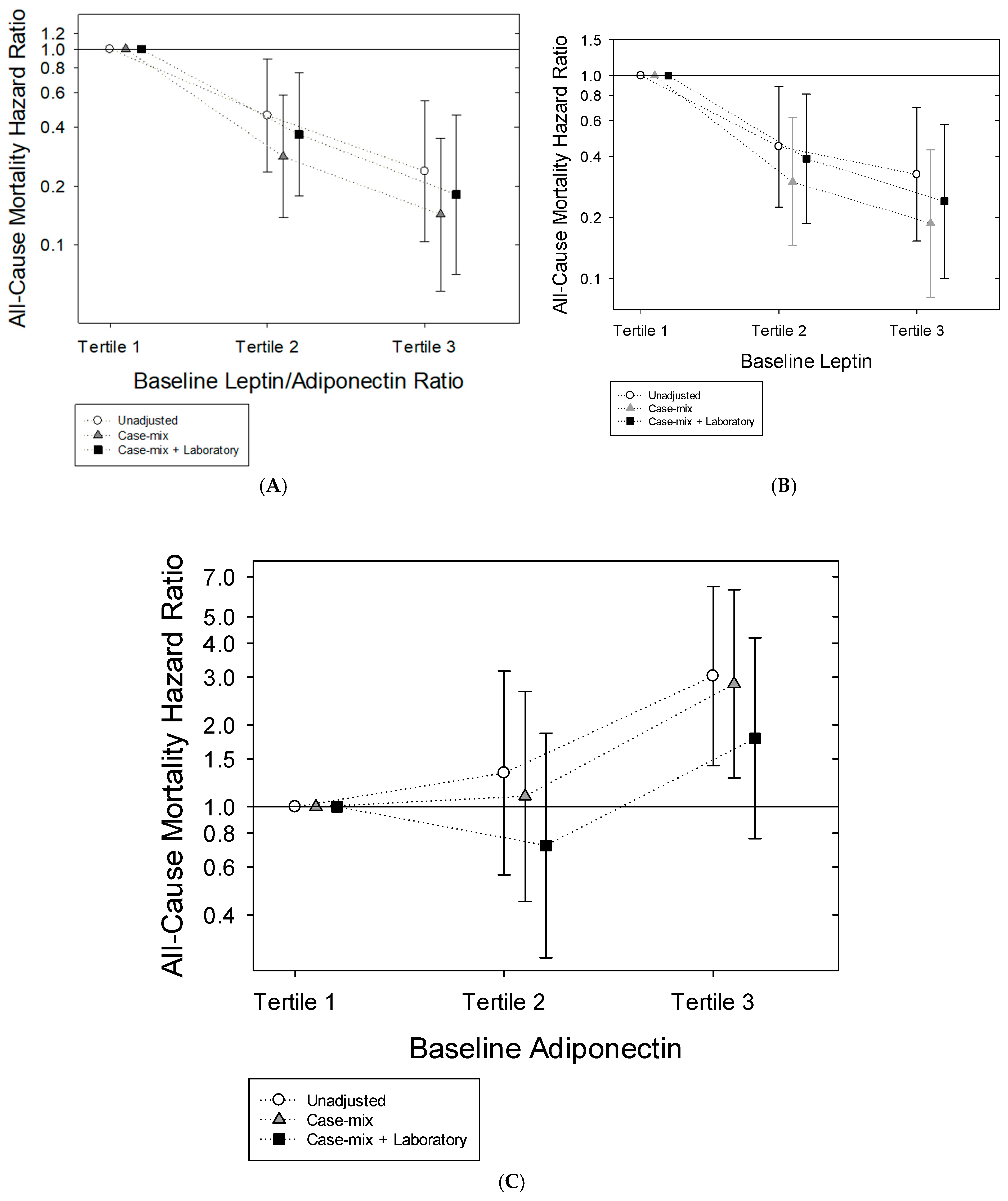
2.3. Leptin-to-Adiponectin Ratio and Mortality Risk
2.4. Leptin and Mortality Risk
2.5. Adiponectin and Mortality Risk
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Exposure Ascertainment
4.3. Socio-Demographic, Comorbidity, and Laboratory Test Measures
4.4. Outcome Ascertainment
4.5. Statistical Methods
- Unadjusted model: No adjustment for covariates;
- Case-mix adjusted model: Adjusted for age, sex, race (Black vs. Non-Black race), ethnicity (Hispanic vs. Non-Hispanic ethnicity), diabetes status, dialysis vintage, and vascular access;
- Case-mix + laboratory adjusted model: Adjusted for covariates in the case-mix model as well as serum albumin, interleukin-6 (IL-6), serum creatinine, and normalized protein catabolic rate (nPCR);
- Expanded case-mix + laboratory adjusted model: Adjusted for covariates in the “case-mix + laboratory” model as well as calcium, phosphorus, parathyroid hormone (PTH), hemoglobin, and ferritin levels.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, J.Z.; Kalantar-Zadeh, K.; Rhee, C.M. Adiponectin and Leptin in Kidney Disease Patients; Rhee, C., Kalantar-Zadeh, K., Brent, G., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Kojta, I.; Chacinska, M.; Blachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef]
- Cumin, F.; Baum, H.P.; Levens, N. Mechanism of leptin removal from the circulation by the kidney. J. Endocrinol. 1997, 155, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Yin, Y.; Steffen, L.M.; Lutsey, P.L.; Grams, M.E.; Walker, K.A.; Ugoji, C.; Matsushita, K.; Rebholz, C.M. Novel Dietary Inflammatory Score and Risk of Incident CKD. Clin. J. Am. Soc. Nephrol. 2025, 20, 485–494. [Google Scholar] [CrossRef]
- Tacke, F.; Wustefeld, T.; Horn, R.; Luedde, T.; Srinivas Rao, A.; Manns, M.P.; Trautwein, C.; Brabant, G. High adiponectin in chronic liver disease and cholestasis suggests biliary route of adiponectin excretion in vivo. J. Hepatol. 2005, 42, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Kusminski, C.M.; Scherer, P.E. Adiponectin, Leptin and Cardiovascular Disorders. Circ. Res. 2021, 128, 136–149. [Google Scholar] [CrossRef]
- Heimburger, O.; Lonnqvist, F.; Danielsson, A.; Nordenstrom, J.; Stenvinkel, P. Serum immunoreactive leptin concentration and its relation to the body fat content in chronic renal failure. J. Am. Soc. Nephrol. 1997, 8, 1423–1430. [Google Scholar] [CrossRef]
- Johansen, K.L.; Mulligan, K.; Tai, V.; Schambelan, M. Leptin, body composition, and indices of malnutrition in patients on dialysis. J. Am. Soc. Nephrol. 1998, 9, 1080–1084. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Shoji, T.; Tanaka, S.; Yamashita, M.; Morita, A.; Emoto, M.; Tabata, T.; Inoue, T.; Morii, H. Plasma leptin level and its relationship with body composition in hemodialysis patients. Am. J. Kidney Dis. 1998, 31, 655–661. [Google Scholar] [CrossRef]
- Lieb, W.; Sullivan, L.M.; Harris, T.B.; Roubenoff, R.; Benjamin, E.J.; Levy, D.; Fox, C.S.; Wang, T.J.; Wilson, P.W.; Kannel, W.B.; et al. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care 2009, 32, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Vavruch, C.; Lanne, T.; Fredrikson, M.; Lindstrom, T.; Ostgren, C.J.; Nystrom, F.H. Serum leptin levels are independently related to the incidence of ischemic heart disease in a prospective study of patients with type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 62. [Google Scholar] [CrossRef]
- Batsis, J.A.; Sahakyan, K.R.; Singh, P.; Bartels, S.J.; Somers, V.K.; Lopez-Jimenez, F. Leptin, adiposity, and mortality: Results from the National Health and Nutrition Examination Survey III, 1988 to 1994. Mayo Clin. Proc. 2015, 90, 481–491. [Google Scholar] [CrossRef]
- Adya, R.; Tan, B.K.; Randeva, H.S. Differential effects of leptin and adiponectin in endothelial angiogenesis. J. Diabetes Res. 2015, 2015, 648239. [Google Scholar] [CrossRef]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef]
- Kumada, M.; Kihara, S.; Sumitsuji, S.; Kawamoto, T.; Matsumoto, S.; Ouchi, N.; Arita, Y.; Okamoto, Y.; Shimomura, I.; Hiraoka, H.; et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 85–89. [Google Scholar] [CrossRef]
- Korczynska, J.; Czumaj, A.; Chmielewski, M.; Swierczynski, J.; Sledzinski, T. The Causes and Potential Injurious Effects of Elevated Serum Leptin Levels in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2021, 22, 4685. [Google Scholar] [CrossRef]
- Molnar, M.Z.; Nagy, K.; Remport, A.; Gaipov, A.; Fulop, T.; Czira, M.E.; Kovesdy, C.P.; Mucsi, I.; Mathe, Z. Association Between Serum Leptin Level and Mortality in Kidney Transplant Recipients. J. Ren. Nutr. 2017, 27, 53–61. [Google Scholar] [CrossRef] [PubMed]
- D’Marco, L.; Puchades, M.J.; Gorriz, J.L.; Romero-Parra, M.; Lima-Martinez, M.; Soto, C.; Bermudez, V.; Raggi, P. Epicardial Adipose Tissue, Adiponectin and Leptin: A Potential Source of Cardiovascular Risk in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 978. [Google Scholar] [CrossRef] [PubMed]
- Scholze, A.; Rattensperger, D.; Zidek, W.; Tepel, M. Low serum leptin predicts mortality in patients with chronic kidney disease stage 5. Obesity 2007, 15, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.W.; Chi, P.J.; Lin, Y.L.; Wang, C.H.; Hsu, B.G. Serum leptin levels are positively associated with aortic stiffness in patients with chronic kidney disease stage 3–5. Adipocyte 2020, 9, 206–211. [Google Scholar] [CrossRef]
- Beberashvili, I.; Sinuani, I.; Azar, A.; Yasur, H.; Feldman, L.; Averbukh, Z.; Weissgarten, J. Longitudinal study of leptin levels in chronic hemodialysis patients. Nutr. J. 2011, 10, 68. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lee, C.T.; Huang, T.L.; Cheng, B.C.; Kuo, C.C.; Su, Y.; Ng, H.Y.; Yang, C.C.; Chuang, F.R.; Liao, S.C. Inflammatory marker but not adipokine predicts mortality among long-term hemodialysis patients. Mediat. Inflamm. 2007, 2007, 19891. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guebre-Egziabher, F.; Bernhard, J.; Funahashi, T.; Hadj-Aissa, A.; Fouque, D. Adiponectin in chronic kidney disease is related more to metabolic disturbances than to decline in renal function. Nephrol. Dial. Transplant. 2005, 20, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.M.; Nguyen, D.V.; Moradi, H.; Brunelli, S.M.; Dukkipati, R.; Jing, J.; Nakata, T.; Kovesdy, C.P.; Brent, G.A.; Kalantar-Zadeh, K. Association of Adiponectin With Body Composition and Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 2015, 66, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.; Molnar, M.Z.; Czira, M.E.; Rudas, A.; Ujszaszi, A.; Kalantar-Zadeh, K.; Rosivall, L.; Mucsi, I. Serum adiponectin levels and mortality after kidney transplantation. Clin. J. Am. Soc. Nephrol. 2013, 8, 460–467. [Google Scholar] [CrossRef][Green Version]
- Przybycinski, J.; Dziedziejko, V.; Puchalowicz, K.; Domanski, L.; Pawlik, A. Adiponectin in Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 9375. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Stankiewicz, M.; Kaska, L.; Malgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity Contributes to Pathogenesis, Nutritional Disorders, and Cardiovascular Risk in Chronic Kidney Disease. Nutrients 2022, 14, 1457. [Google Scholar] [CrossRef]
- Teta, D.; Maillard, M.; Halabi, G.; Burnier, M. The leptin/adiponectin ratio: Potential implications for peritoneal dialysis. Kidney Int. Suppl. 2008, 73, S112–S118. [Google Scholar] [CrossRef]
- Park, J.T.; Yoo, T.H.; Kim, J.K.; Oh, H.J.; Kim, S.J.; Yoo, D.E.; Lee, M.J.; Shin, D.H.; Han, S.H.; Han, D.S.; et al. Leptin/adiponectin ratio is an independent predictor of mortality in nondiabetic peritoneal dialysis patients. Perit. Dial. Int. 2013, 33, 67–74. [Google Scholar] [CrossRef]
- Machiba, Y.; Inaba, M.; Mori, K.; Kurajoh, M.; Nishide, K.; Norimine, K.; Yamakawa, T.; Shoji, S.; Okuno, S. Paradoxical positive association of serum adiponectin with all-cause mortality based on body composition in Japanese haemodialysis patients. Sci. Rep. 2018, 8, 14699. [Google Scholar] [CrossRef]
- Abdallah, E.; Waked, E.; Nabil, M.; El-Bendary, O. Adiponectin and cardiovascular outcomes among hemodialysis patients. Kidney Blood Press. Res. 2012, 35, 247–253. [Google Scholar] [CrossRef]
- Pischon, T.; Girman, C.J.; Hotamisligil, G.S.; Rifai, N.; Hu, F.B.; Rimm, E.B. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004, 291, 1730–1737. [Google Scholar] [CrossRef]
- Zoccali, C.; Postorino, M.; Marino, C.; Pizzini, P.; Cutrupi, S.; Tripepi, G.; Group, C.W. Waist circumference modifies the relationship between the adipose tissue cytokines leptin and adiponectin and all-cause and cardiovascular mortality in haemodialysis patients. J. Intern. Med. 2011, 269, 172–181. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Kirwan, J.P.; Remer, E.M.; Schneider, E.; Addeman, B.; Arrigain, S.; Horwitz, E.; Fink, J.C.; Lash, J.P.; McKenzie, C.A.; et al. Adiposity, Physical Function, and Their Associations With Insulin Resistance, Inflammation, and Adipokines in CKD. Am. J. Kidney Dis. 2021, 77, 44–55. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Rhee, C.M.; Chou, J.; Ahmadi, S.F.; Park, J.; Chen, J.L.; Amin, A.N. The Obesity Paradox in Kidney Disease: How to Reconcile it with Obesity Management. Kidney Int. Rep. 2017, 2, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P. Obesity and Metabolic Health in CKD. Clin. J. Am. Soc. Nephrol. 2025, 20, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Palmer, B.F.; Clegg, D.J. Novel Hypothesis for the “Obesity Paradox” in ESKD Subjects on Dialysis. Clin. J. Am. Soc. Nephrol. 2025, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Guzman, A.; Hernandez-Coronado, C.G.; Rosales-Torres, A.M.; Hernandez-Medrano, J.H. Leptin regulates neuropeptides associated with food intake and GnRH secretion. Ann. Endocrinol. 2019, 80, 38–46. [Google Scholar] [CrossRef]
- Christou, G.A.; Kiortsis, D.N. The role of adiponectin in renal physiology and development of albuminuria. J. Endocrinol. 2014, 221, R49–R61. [Google Scholar] [CrossRef]
- Zoccali, C.; D’Arrigo, G.; Leonardis, D.; Pizzini, P.; Postorino, M.; Tripepi, G.; Mallamaci, F. Neuropeptide Y predicts cardiovascular events in chronic kidney disease patients: A cohort study. J. Hypertens. 2019, 37, 1359–1365. [Google Scholar] [CrossRef]
- Lee, Y.J.; Cho, S.; Kim, S.R. The association between serum adiponectin levels and nutritional status of hemodialysis patients. Ren. Fail. 2011, 33, 506–511. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; Humphreys, M.H.; Kopple, J.D. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003, 63, 793–808. [Google Scholar] [CrossRef]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Shimizu, H.; Shimomura, Y.; Nakanishi, Y.; Futawatari, T.; Ohtani, K.; Sato, N.; Mori, M. Estrogen increases in vivo leptin production in rats and human subjects. J. Endocrinol. 1997, 154, 285–292. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakaya, S.; Kumai, T.; Watanabe, M.; Tateishi, T.; Shimizu, H.; Kobayashi, S. Effects of estrogen on serum leptin levels and leptin mRNA expression in adipose tissue in rats. Horm. Res. 2001, 56, 98–104. [Google Scholar] [CrossRef]
- Geer, E.B.; Shen, W. Gender differences in insulin resistance, body composition, and energy balance. Gend. Med. 2009, 6 (Suppl. S1), 60–75. [Google Scholar] [CrossRef]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef] [PubMed]
- Tsao, T.S.; Tomas, E.; Murrey, H.E.; Hug, C.; Lee, D.H.; Ruderman, N.B.; Heuser, J.E.; Lodish, H.F. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J. Biol. Chem. 2003, 278, 50810–50817. [Google Scholar] [CrossRef]
- Richards, A.A.; Stephens, T.; Charlton, H.K.; Jones, A.; Macdonald, G.A.; Prins, J.B.; Whitehead, J.P. Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: Evidence for regulation of multimerization by alterations in posttranslational modifications. Mol. Endocrinol. 2006, 20, 1673–1687. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Charlesworth, J.A.; Kelly, J.J.; Peake, P.W. The effect of renal transplantation on adiponectin and its isoforms and receptors. Metabolism 2007, 56, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Shlipak, M.G.; Mathew, R.O.; Ndumele, C.E. The Cardiovascular-Kidney-Metabolic Health Framework: Implications for Nephrology. Clin. J. Am. Soc. Nephrol. 2025, 20, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
| LEPTIN-TO-ADIPONECTIN RATIO CATEGORY | |||||
|---|---|---|---|---|---|
| Overall | Tertile 1 | Tertile 2 | Tertile 3 | p-Value * | |
| No. of patients (%) | 448 (100) | 149 (33) | 150 (33) | 149 (33) | N/A |
| Leptin/adiponectin range (pg/mL) | <0.1–102.0 | <0.1–<0.5 | ≥0.5–<3.2 | ≥3.20–102.0 | N/A |
| Age (years) Mean ± SD | 55.1 ± 14.3 | 52.8 ± 14.4 | 56.5 ± 14.9 | 56.1 ± 13.4 | 0.08 |
| Female (%) | 44 | 35 | 41 | 57 | <0.001 |
| Black race (%) | 31 | 36 | 27 | 30 | 0.18 |
| Hispanic ethnicity (%) | 50 | 49 | 55 | 46 | 0.29 |
| Diabetes (%) | 55 | 48 | 55 | 60 | 0.11 |
| AVF/AVG (%) | 81 | 77 | 76 | 88 | 0.03 |
| Vintage, months Median (IQR) | 48.3 (27.6, 84.3) | 53.0 (32.3, 89.9) | 48.9 (23.9, 86.8) | 42.3 (27.5, 78.2) | 0.21 |
| Laboratory Tests Median (IQR) | |||||
| Albumin (g/dL) | 3.9 (3.7, 4.1) | 4.0 (3.8, 4.2) | 4.0 (3.7, 4.2) | 3.9 (3.7, 4.0) | 0.14 |
| Creatinine (mg/dL) | 10.0 (8.2, 12.3) | 10.6 (8.4, 13.3) | 9.3 (7.8, 11.2) | 10.0 (8.3, 11.9) | 0.02 |
| Corrected calcium (mg/dL) | 9.1 (8.7, 9.5) | 9.0 (8.5, 9.5) | 9.1 (8.7, 9.4) | 9.1 (8.8, 9.5) | 0.38 |
| Phosphorus (mg/dL) | 5.2 (4.3, 6.3) | 5.3 (4.4, 6.8) | 5.1 (4.1, 6.4) | 5.0 (4.3, 6.0) | 0.06 |
| Intact PTH (pg/mL) | 385 (263, 579) | 435 (279, 663) | 362 (256, 510) | 392 (256, 580) | 0.12 |
| Hemoglobin (g/dL) | 10.7 (10.1, 11.4) | 10.7 (10.1, 11.2) | 10.7 (10.1, 11.5) | 10.8 (10.2, 11.4) | 0.67 |
| Ferritin (ng/mL) | 638 (415, 865) | 699 (404, 873) | 599 (413, 843) | 622 (429, 845) | 0.92 |
| nPCR (g/kg/day) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.2) | 0.68 |
| Adiponectin (mcg/mL) | 15.1 (9.1, 24.2) | 24.5 (18.0, 32.7) | 14.9 (10.4, 22.6) | 8.3 (5.1, 12.8) | <0.001 |
| Leptin (mcg/mL) | 16.5 (5.7, 54.6) | 3.4 (0.8, 6.4) | 16.7 (11.0, 23.1) | 112.0 (49.6, 168.4) | <0.001 |
| IL-6 | 2.3 (1.3, 4.2) | 2.8 (1.4, 6.3) | 2.1 (1.0, 3.8) | 2.2 (1.4, 4.0) | 0.004 |
| Unadjusted | Case-Mix Adjusted * | Case-Mix + Laboratory ** | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (∆10 years) | 1.05 (0.91, 1.20) | 0.51 | 0.96 (0.82, 1.13) | 0.63 | 0.96 (0.80, 1.14) | 0.62 |
| Vintage (∆1 year) | 1.01 (0.96, 1.06) | 0.65 | 1.00 (0.95, 1.06) | 0.91 | 1.01 (0.95, 1.07) | 0.75 |
| Female | 3.66 (2.42, 5.53) | <0.001 | 3.79 (2.48, 5.80) | <0.001 | 3.58 (2.23, 5.77) | <0.001 |
| Black race | 0.96 (0.63, 1.47) | 0.85 | 0.74 (0.41, 1.31) | 0.30 | 0.65 (0.35, 1.22) | 0.18 |
| Hispanic | 0.81 (0.55, 1.20) | 0.30 | 0.66 (0.39, 1.13) | 0.13 | 0.61 (0.35, 1.07) | 0.08 |
| Diabetes | 1.27 (0.85, 1.89) | 0.24 | 1.55 (0.96, 2.50) | 0.07 | 1.69 (1.04, 2.76) | 0.04 |
| AVF/AVG (vs. catheter) | 2.01 (1.07, 3.78) | 0.03 | 2.23 (1.14, 4.37) | 0.02 | 2.20 (1.10, 4.39) | 0.03 |
| Serum albumin (∆0.5 g/dL) | 0.72 (0.54, 0.96) | 0.03 | 0.74 (0.53, 1.02) | 0.07 | 0.59 (0.41, 0.84) | 0.004 |
| Creatinine (mg/dL) | 0.96 (0.90, 1.03) | 0.29 | 1.05 (0.95, 1.15) | 0.35 | 1.04 (0.94, 1.15) | 0.45 |
| Calcium (mg/dL) | 1.10 (0.79, 1.52) | 0.57 | 0.97 (0.68, 1.39) | 0.86 | 1.01 (0.70, 1.46) | 0.98 |
| Phosphorus (mg/dL) | 0.90 (0.78, 1.03) | 0.11 | 0.92 (0.80, 1.07) | 0.28 | 0.91 (0.77, 1.07) | 0.26 |
| PTH (pg/mL) | 1.00 (1.00, 1.00) | 0.61 | 1.00 (1.00, 1.00) | 0.69 | 1.00 (1.00, 1.00) | 0.58 |
| Hemoglobin (g/dL) | 1.04 (0.86, 1.27) | 0.67 | 1.16 (0.94, 1.44) | 0.17 | 1.15 (0.92, 1.44) | 0.22 |
| Ferritin (ng/mL) | 1.00 (1.00, 1.00) | 0.73 | 1.00 (1.00, 1.00) | 0.23 | 1.00 (1.00, 1.00) | 0.45 |
| nPCR (g/kg/day) | 1.32 (0.64, 2.74) | 0.45 | 1.24 (0.55, 2.79) | 0.60 | 1.15 (0.48, 2.71) | 0.76 |
| Adiponectin (∆10) | 0.46 (0.36, 0.59) | <0.001 | 0.30 (0.22, 0.41) | <0.001 | 0.30 (0.22, 0.41) | <0.001 |
| IL-6 (∆5) | 0.57 (0.40, 0.82) | 0.002 | 0.57 (0.39, 0.85) | 0.006 | 0.50 (0.33, 0.77) | 0.002 |
| Unadjusted | Case-Mix Adjusted * | Case-Mix + Laboratory ** | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (∆10 years) | 1.06 (0.92, 1.22) | 0.40 | 1.06 (0.91, 1.24) | 0.46 | 1.02 (0.86, 1.21) | 0.80 |
| Vintage (∆1 year) | 1.10 (1.05, 1.16) | <0.001 | 1.10 (1.04, 1.16) | <0.001 | 1.10 (1.04, 1.16) | <0.001 |
| Female | 1.93 (1.30, 2.87) | 0.001 | 1.89 (1.26, 2.84) | 0.002 | 1.94 (1.24, 3.05) | 0.004 |
| Black race | 1.46 (0.96, 2.21) | 0.08 | 1.58 (0.90, 2.78) | 0.11 | 1.83 (1.01, 3.34) | 0.05 |
| Hispanic | 0.95 (0.64, 1.41) | 0.81 | 1.36 (0.79, 2.32) | 0.27 | 1.40 (0.81, 2.43) | 0.23 |
| Diabetes | 0.92 (0.62, 1.36) | 0.66 | 1.03 (0.66, 1.63) | 0.89 | 0.94 (0.59, 1.49) | 0.78 |
| AVF/AVG | 0.98 (0.54, 1.77) | 0.95 | 0.82 (0.44, 1.54) | 0.54 | 0.85 (0.45, 1.62) | 0.62 |
| Serum albumin (∆0.5 g/dL) | 0.78 (0.58, 1.03) | 0.08 | 0.81 (0.60, 1.10) | 0.18 | 0.92 (0.66, 1.28) | 0.63 |
| Creatinine (mg/dL) | 0.94 (0.87, 1.01) | 0.07 | 0.96 (0.88, 1.04) | 0.29 | 0.96 (0.88, 1.05) | 0.35 |
| Calcium (mg/dL) | 1.07 (0.78, 1.47) | 0.69 | 0.98 (0.70, 1.36) | 0.88 | 0.97 (0.69, 1.35) | 0.84 |
| Phosphorus (mg/dL) | 1.01 (0.89, 1.15) | 0.91 | 1.05 (0.91, 1.20) | 0.51 | 1.05 (0.91, 1.22) | 0.50 |
| PTH (pg/mL) | 1.00 (1.00, 1.00) | 0.73 | 1.00 (1.00, 1.00) | 0.88 | 1.00 (1.00, 1.00) | 0.89 |
| Hemoglobin (g/dL) | 0.86 (0.71, 1.04) | 0.13 | 0.88 (0.72, 1.08) | 0.24 | 0.92 (0.75, 1.14) | 0.46 |
| Ferritin (ng/mL) | 1.00 (1.00, 1.00) | 0.13 | 1.00 (1.00, 1.00) | 0.31 | 1.00 (1.00, 1.00) | 0.40 |
| nPCR (g/kg/day) | 1.33 (0.65, 2.73) | 0.44 | 1.61 (0.75, 3.43) | 0.22 | 1.96 (0.89, 4.34) | 0.10 |
| Leptin (∆25) | 0.74 (0.66, 0.84) | <0.001 | 0.66 (0.57, 0.76) | <0.001 | 0.66 (0.57, 0.76) | <0.001 |
| IL-6 (∆5) | 1.40 (1.07, 1.83) | 0.01 | 1.57 (1.18, 2.09) | 0.002 | 1.55 (1.15, 2.08) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, T.-A.V.; You, A.S.; Kalantar, S.S.; Yoon, J.; Narasaki, Y.; Sy, J.; Hanna, R.; Daza, A.; Guerrero, Y.; Dang, A.; et al. Leptin and Adiponectin as Uremic Adipokines: Associations with Survival in a Prospective Hemodialysis Cohort. Toxins 2025, 17, 525. https://doi.org/10.3390/toxins17110525
Bui T-AV, You AS, Kalantar SS, Yoon J, Narasaki Y, Sy J, Hanna R, Daza A, Guerrero Y, Dang A, et al. Leptin and Adiponectin as Uremic Adipokines: Associations with Survival in a Prospective Hemodialysis Cohort. Toxins. 2025; 17(11):525. https://doi.org/10.3390/toxins17110525
Chicago/Turabian StyleBui, Thuy-Anh V., Amy S. You, Sara S. Kalantar, Jihoon Yoon, Yoko Narasaki, John Sy, Ramy Hanna, Andrea Daza, Yalitzi Guerrero, Anyssa Dang, and et al. 2025. "Leptin and Adiponectin as Uremic Adipokines: Associations with Survival in a Prospective Hemodialysis Cohort" Toxins 17, no. 11: 525. https://doi.org/10.3390/toxins17110525
APA StyleBui, T.-A. V., You, A. S., Kalantar, S. S., Yoon, J., Narasaki, Y., Sy, J., Hanna, R., Daza, A., Guerrero, Y., Dang, A., Arora, R., Nguyen, D. V., Kalantar-Zadeh, K., & Rhee, C. M. (2025). Leptin and Adiponectin as Uremic Adipokines: Associations with Survival in a Prospective Hemodialysis Cohort. Toxins, 17(11), 525. https://doi.org/10.3390/toxins17110525






