Effect of Expanded Hemodialysis with the Theranova Dialyzer on the Platelet-to-Lymphocyte Ratio and Inflammatory Markers
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Comparison of Changes in Inflammation-Related Markers
2.3. Factors Associated with the Reduction in PLR
3. Discussion
4. Conclusions
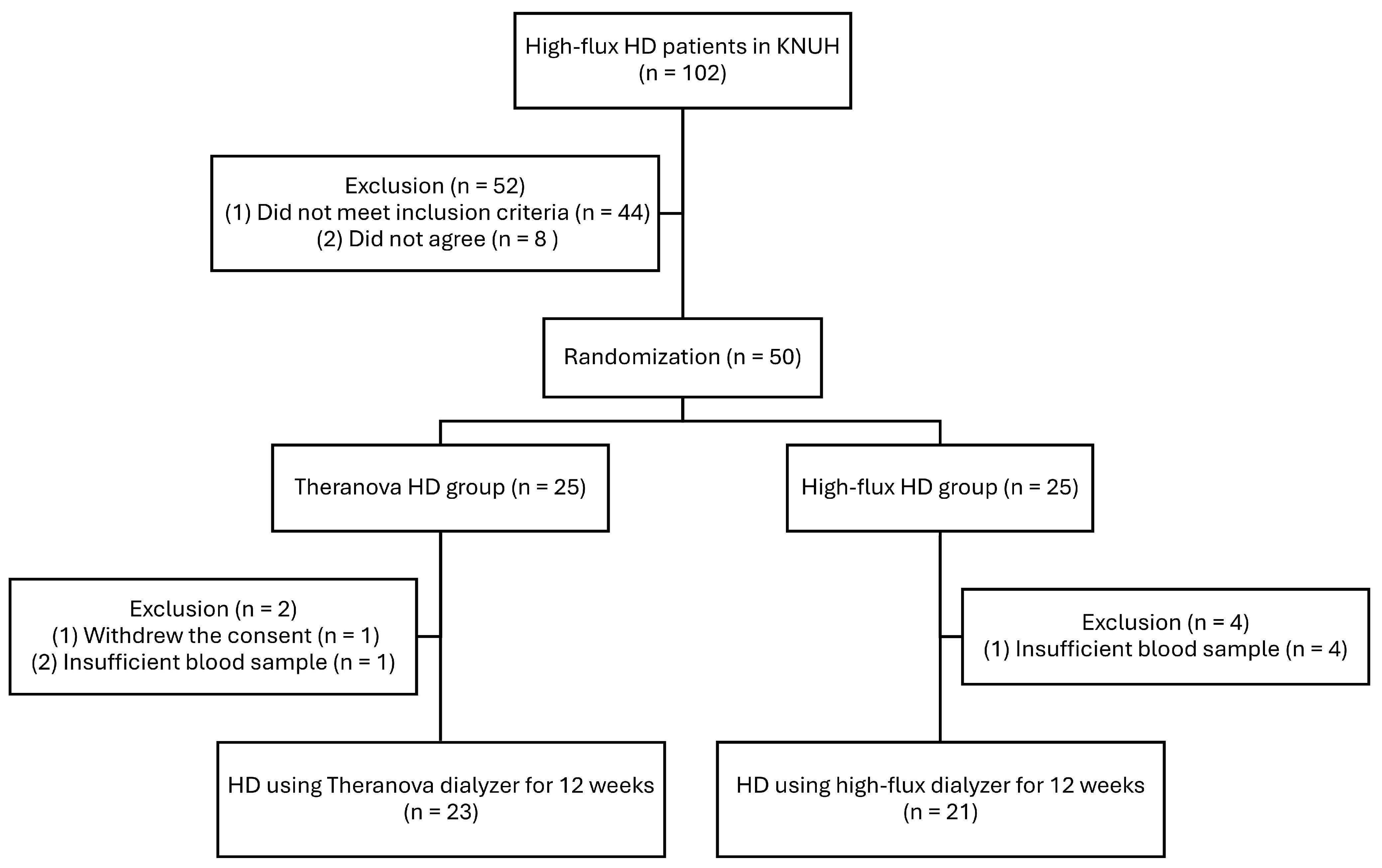
5. Materials and Methods
5.1. Patients and Study Design
5.2. Data Collection and Analyses
5.3. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| DBP | Diastolic blood pressure |
| ELISA | Enzyme-linked immunosorbent assay |
| ESKD | End-stage kidney disease |
| FGF-23 | Fibroblast growth factor 23 |
| HD | Hemodialysis |
| HDx | Expanded hemodialysis |
| IL-6 | Interleukin 6 |
| KNUH | Kyungpook National University Hospital |
| MCO | Medium cut-off |
| NA | Not applicable |
| PLR | Platelet-to-lymphocyte ratio |
| SBP | Systolic blood pressure |
| spKt/V | Single pool Kt/V |
| TNF-α | Tumor necrosis factor-α |
References
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33, iii35–iii40. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Herrlinger, S.; Pruy, A.; Metzger, T.; Wanner, C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999, 55, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Ahbap, E.; Sakaci, T.; Kara, E.; Sahutoglu, T.; Koc, Y.; Basturk, T.; Sevinc, M.; Akgol, C.; Kayalar, A.O.; Ucar, Z.A.; et al. Neutrophil-to-lymphocyte ratio and platelet-tolymphocyte ratio in evaluation of inflammation in end-stage renal disease. Clin. Nephrol. 2016, 85, 199–208. [Google Scholar] [CrossRef]
- Mureșan, A.V.; Russu, E.; Arbănași, E.M.; Kaller, R.; Hosu, I.; Arbănași, E.M.; Voidăzan, S.T. The Predictive Value of NLR, MLR, and PLR in the Outcome of End-Stage Kidney Disease Patients. Biomedicines 2022, 10, 1272. [Google Scholar] [CrossRef]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Ortiz, A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef]
- Wolley, M.; Jardine, M.; Hutchison, C.A. Exploring the Clinical Relevance of Providing Increased Removal of Large Middle Molecules. Clin. J. Am. Soc. Nephrol. 2018, 13, 805–814. [Google Scholar] [CrossRef]
- Sun, J.; Axelsson, J.; Machowska, A.; Heimbürger, O.; Bárány, P.; Lindholm, B.; Lindström, K.; Stenvinkel, P.; Qureshi, A.R. Biomarkers of Cardiovascular Disease and Mortality Risk in Patients with Advanced CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Munoz Mendoza, J.; Isakova, T.; Cai, X.; Bayes, L.Y.; Faul, C.; Scialla, J.J.; Lash, J.P.; Chen, J.; He, J.; Navaneethan, S.; et al. Inflammation and elevated levels of fibroblast growth factor 23 are independent risk factors for death in chronic kidney disease. Kidney Int. 2017, 91, 711–719. [Google Scholar] [CrossRef]
- Zweigart, C.; Boschetti-de-Fierro, A.; Hulko, M.; Nilsson, L.G.; Beck, W.; Storr, M.; Krause, B. Medium cut-off membranes—Closer to the natural kidney removal function. Int. J. Artif. Organs 2017, 40, 328–334. [Google Scholar] [CrossRef]
- Taymez, D.G.; Ucar, E.; Turkmen, K.; Ucar, R.; Afsar, B.; Gaipov, A.; Turk, S. The Predictive Value of Platelet/Lymphocyte Ratio in Hemodialysis Patients With Erythropoietin Resistance. Ther. Apher. Dial. 2016, 20, 118–121. [Google Scholar] [CrossRef]
- Zickler, D.; Schindler, R.; Willy, K.; Martus, P.; Pawlak, M.; Storr, M.; Hulko, M.; Boehler, T.; Glomb, M.A.; Liehr, K.; et al. Medium Cut-Off (MCO) Membranes Reduce Inflammation in Chronic Dialysis Patients-A Randomized Controlled Clinical Trial. PLoS ONE 2017, 12, e0169024. [Google Scholar] [CrossRef]
- Yeter, H.H.; Korucu, B.; Akcay, O.F.; Derici, K.; Derici, U.; Arinsoy, T. Effects of medium cut-off dialysis membranes on inflammation and oxidative stress in patients on maintenance hemodialysis. Int. Urol. Nephrol. 2020, 52, 1779–1789. [Google Scholar] [CrossRef]
- Lim, J.H.; Jeon, Y.; Yook, J.M.; Choi, S.Y.; Jung, H.Y.; Choi, J.Y.; Park, S.H.; Kim, C.D.; Kim, Y.L.; Cho, J.H. Medium cut-off dialyzer improves erythropoiesis stimulating agent resistance in a hepcidin-independent manner in maintenance hemodialysis patients: Results from a randomized controlled trial. Sci. Rep. 2020, 10, 16062. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Seo, Y.J.; Jeon, Y.; Jeon, Y.H.; Jung, H.Y.; Choi, J.Y.; Park, S.H.; Kim, C.D.; Kang, S.H.; Ryu, J.H.; et al. Expanded Hemodialysis with Theranova Dialyzer and Residual Kidney Function in Patients Starting Long-Term Hemodialysis: A Randomized Controlled Trial. J. Am. Soc. Nephrol. 2025, 36, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, A.; de la Flor, J.C.; Coll, E.; Iglesias, E.; Reque, J.; Valga, F. Expanded hemodialysis: What’s up, Doc? Clin. Kidney J. 2023, 16, 1071–1080. [Google Scholar] [CrossRef]
- Maduell, F.; Broseta, J.J.; Rodríguez-Espinosa, D.; Del Risco, J.; Rodas, L.M.; Arias-Guillén, M.; Vera, M.; Fontseré, N.; Salgado, M.D.C.; Rico, N. Comparison of four medium cut-off dialyzers. Clin. Kidney J. 2022, 15, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, A.; Vega, A.; Linares, T.; Abad, S.; Macías, N.; Aragoncillo, I.; Torres, E.; Hernández, A.; Barbieri, D.; Luño, J. Evaluation of the efficacy of a medium cut-off dialyser and comparison with other high-flux dialysers in conventional haemodialysis and online haemodiafiltration. Clin. Kidney J. 2018, 11, 742–746. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.H.; Kim, T.Y.; Park, H.Y.; Jung, K.S.; Lee, M.H.; Jhee, J.H.; Lee, J.E.; Choi, H.Y.; Park, H.C. Removal of large middle molecules via haemodialysis with medium cut-off membranes at lower blood flow rates: An observational prospective study. BMC Nephrol. 2019, 21, 2. [Google Scholar] [CrossRef]
- Sanabria, R.M.; Hutchison, C.A.; Vesga, J.I.; Ariza, J.G.; Sanchez, R.; Suarez, A.M. Expanded Hemodialysis and Its Effects on Hospitalizations and Medication Usage: A Cohort Study. Nephron 2021, 145, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Park, Y.; Yook, J.M.; Choi, S.Y.; Jung, H.Y.; Choi, J.Y.; Park, S.H.; Kim, C.D.; Kim, Y.L.; Cho, J.H. Randomized controlled trial of medium cut-off versus high-flux dialyzers on quality of life outcomes in maintenance hemodialysis patients. Sci. Rep. 2020, 10, 7780. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, J.; Martin-Malo, A.; Pedrini, L.; Basci, A.; Canaud, B.; Fouque, D.; Haage, P.; Konner, K.; Kooman, J.; Pizzarelli, F.; et al. EBPG guideline on dialysis strategies. Nephrol. Dial. Transplant. 2007, 22 (Suppl. S2), ii5–ii21. [Google Scholar] [CrossRef] [PubMed]

| Theranova (n = 23) | High-Flux (n = 21) | p | |
|---|---|---|---|
| Age, years | 62.9 ± 13.5 | 62.6 ± 15.6 | 0.95 |
| Sex, male, n (%) | 16 (69.6) | 13 (61.9) | 0.59 |
| Body mass index, kg/m2 | 22.0 ± 2.6 | 22.1 ± 4.0 | 0.94 |
| Dialysis vintage, months | 85.8 ± 49.6 | 78.8 ± 47.9 | 0.64 |
| Primary renal disease, n (%) | |||
| Diabetes mellitus | 10 (43.5) | 11 (52.4) | 0.56 |
| Hypertension | 3 (13.0) | 3 (14.3) | >0.99 |
| Glomerulonephritis | 9 (39.1) | 4 (19.0) | 0.15 |
| Others | 1 (4.3) | 3 (14.3) | 0.34 |
| Comorbid conditions, n (%) | |||
| Diabetes | 11 (47.8) | 13 (61.9) | 0.35 |
| Hypertension | 19 (82.6) | 17 (81.0) | >0.99 |
| Cardiovascular disease | 9 (39.1) | 4 (19.0) | 0.15 |
| Pre-dialysis SBP (mmHg) | 145.2 ± 18.1 | 145.9 ± 20.3 | 0.91 |
| Pre-dialysis DBP (mmHg) | 68.8 ± 17.4 | 67.4 ± 15.2 | 0.77 |
| Blood flow rate (mL/min) | 246.1 ± 21.1 | 237.6 ± 19.7 | 0.18 |
| Dialysate flow rate (mL/min) | 500 | 500 | NA |
| Dialysis time (min) | 241.2 ± 6.2 | 237.0 ± 13.1 | 0.18 |
| Target body weight (kg) | 61.2 ± 7.7 | 57.3 ± 10.1 | 0.15 |
| spKt/V | 1.62 ± 0.21 | 1.67 ± 0.21 | 0.40 |
| Laboratory findings | |||
| Hemoglobin (g/dL) | 10.6 ± 0.9 | 10.6 ± 1.2 | 0.87 |
| Sodium (mEq/L) | 137.0 ± 2.8 | 136.9 ± 2.7 | 0.91 |
| Potassium (mEq/L) | 4.9 ± 0.7 | 4.5 ± 0.7 | 0.09 |
| Albumin (g/dL) | 4.2 ± 0.3 | 4.1 ± 0.4 | 0.40 |
| Calcium (mg/dL) | 9.3 ± 0.7 | 9.0 ± 0.4 | 0.10 |
| Phosphate (mg/dL) | 3.9 ± 0.9 | 4.2 ± 0.9 | 0.35 |
| Intact parathyroid hormone (pg/mL) | 178.6 ± 125.9 | 187.2 ± 124.2 | 0.82 |
| Baseline | 12-Week | |||||
|---|---|---|---|---|---|---|
| Theranova | High-Flux | p | Theranova | High-Flux | p | |
| White blood cell count (×109/L) | 6.5 ± 1.7 | 6.6 ± 1.9 | 0.82 | 6.4 ± 1.5 | 6.9 ± 2.1 | 0.37 |
| Absolute neutrophil count (×109/L) | 4.4 ± 1.5 | 4.3 ± 1.5 | 0.82 | 4.1 ± 1.3 | 4.7 ± 1.8 | 0.19 |
| Lymphocyte count (×109/L) | 1.3 ± 4.3 | 1.4 ± 3.5 | 0.83 | 1.5 ± 5.1 | 1.3 ± 3.6 | 0.30 |
| Platelet count (×109/L) | 192.3 ± 99.4 | 192.4 ± 51.5 | 1.00 | 179.6 ± 81.0 | 204.3 ± 42.3 | 0.22 |
| Neutrophil-to-lymphocyte ratio | 3.78 ± 2.74 | 3.26 ± 1.16 | 0.43 | 3.06 ± 1.17 | 3.64 ± 1.43 | 0.15 |
| Platelet-to-lymphocyte ratio | 155.4 ± 78.8 | 144.9 ± 31.0 | 0.56 | 125.5 ± 43.5 | 157.4 ± 32.4 | 0.01 |
| IL-6 (pg/mL) | 4.7 (2.3, 8.1) | 5.4 (3.6, 8.4) | 0.26 | 5.4 (2.7, 8.0) | 5.2 (3.7, 10.8) | 0.44 |
| TNF-α (pg/mL) | 17.8 ± 5.1 | 18.0 ± 4.9 | 0.89 | 16.1 ± 3.5 | 19.0 ± 5.2 | 0.04 |
| FGF-23 (pg/mL) | 553.9 (188.2, 2087.3) | 369.8 (255.7, 763.5) | 0.37 | 632.4 (162.9, 1635.8) | 396.2 (150.2, 1008.2) | 0.66 |
| B | SE | β | p | |
|---|---|---|---|---|
| Theranova dialyzer | −20.41 | 9.30 | −0.32 | 0.04 |
| Age | −0.07 | 0.30 | −0.03 | 0.82 |
| Female | 13.68 | 8.73 | 0.20 | 0.13 |
| Diabetes mellitus | 1.74 | 8.95 | 0.03 | 0.85 |
| Cardiovascular disease | 27.83 | 9.56 | 0.40 | 0.01 |
| Neutrophil percentage ratio of 12-week to baseline | 72.68 | 44.25 | 0.24 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.H.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; Lim, J.-H.; Cho, J.-H. Effect of Expanded Hemodialysis with the Theranova Dialyzer on the Platelet-to-Lymphocyte Ratio and Inflammatory Markers. Toxins 2025, 17, 521. https://doi.org/10.3390/toxins17110521
Jeon YH, Jung H-Y, Choi J-Y, Park S-H, Kim C-D, Kim Y-L, Lim J-H, Cho J-H. Effect of Expanded Hemodialysis with the Theranova Dialyzer on the Platelet-to-Lymphocyte Ratio and Inflammatory Markers. Toxins. 2025; 17(11):521. https://doi.org/10.3390/toxins17110521
Chicago/Turabian StyleJeon, You Hyun, Hee-Yeon Jung, Ji-Young Choi, Sun-Hee Park, Chan-Duck Kim, Yong-Lim Kim, Jeong-Hoon Lim, and Jang-Hee Cho. 2025. "Effect of Expanded Hemodialysis with the Theranova Dialyzer on the Platelet-to-Lymphocyte Ratio and Inflammatory Markers" Toxins 17, no. 11: 521. https://doi.org/10.3390/toxins17110521
APA StyleJeon, Y. H., Jung, H.-Y., Choi, J.-Y., Park, S.-H., Kim, C.-D., Kim, Y.-L., Lim, J.-H., & Cho, J.-H. (2025). Effect of Expanded Hemodialysis with the Theranova Dialyzer on the Platelet-to-Lymphocyte Ratio and Inflammatory Markers. Toxins, 17(11), 521. https://doi.org/10.3390/toxins17110521








