Kynurenine Promotes Phosphate-Induced Endothelial Calcification via Endothelial-to-Mesenchymal Transition, Osteoblastic Differentiation and AhR Activation
Abstract
1. Introduction
2. Results
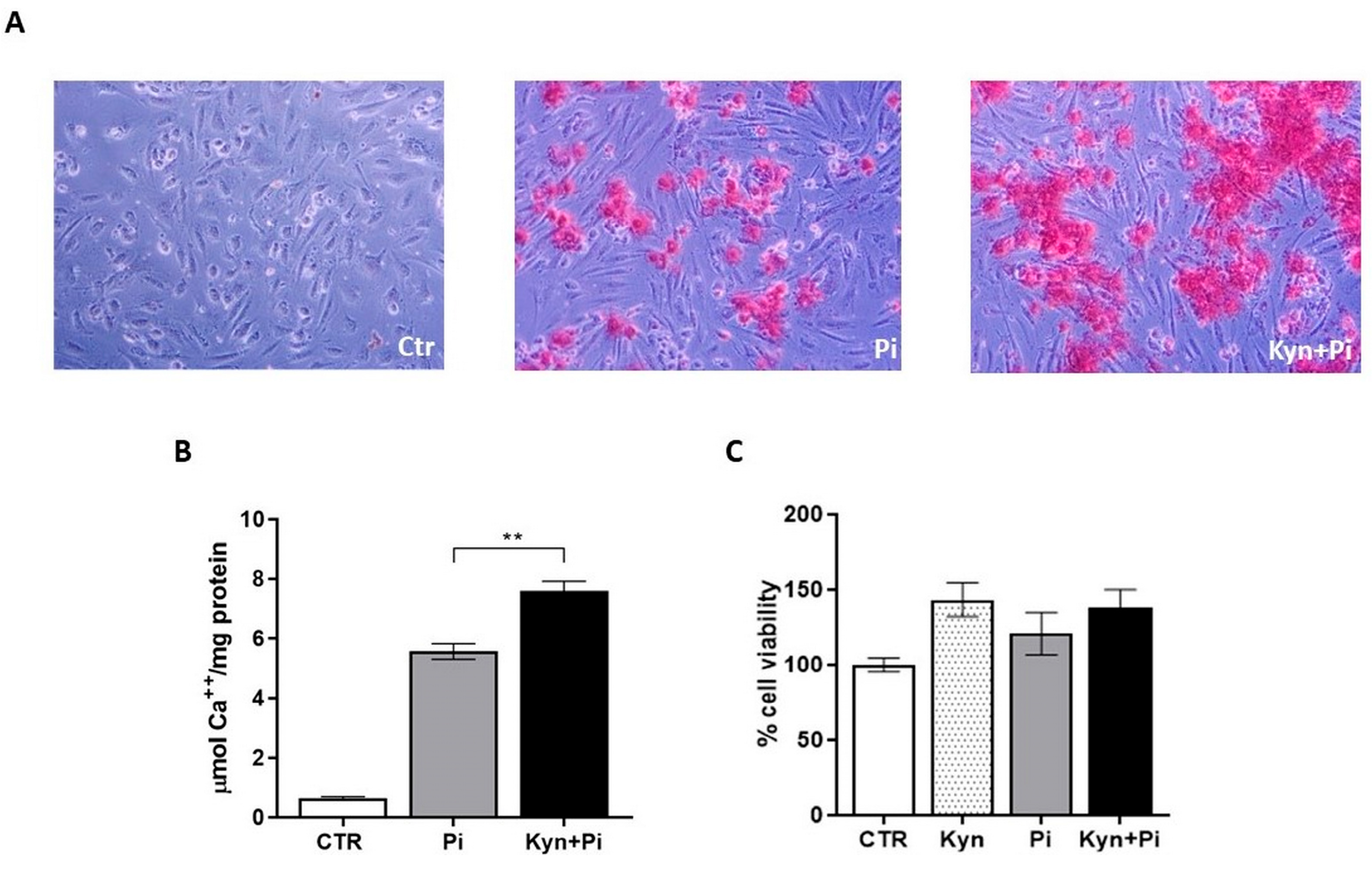
2.1. Effect of Kynurenine on Pi-Induced Endothelial Calcification
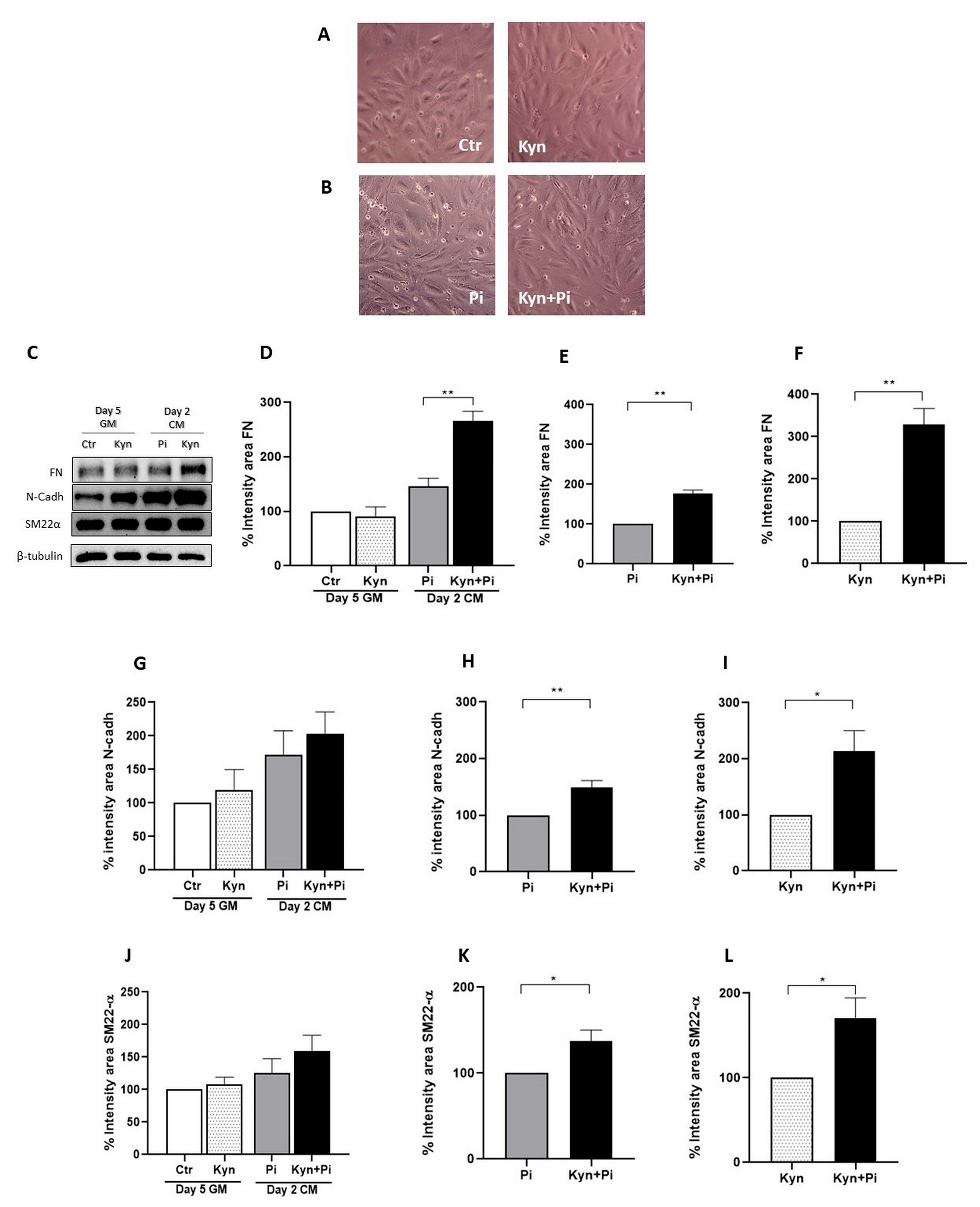
2.2. Effect of Kynurenine on Pi-Induced Endothelial-to-Mesenchymal Transition
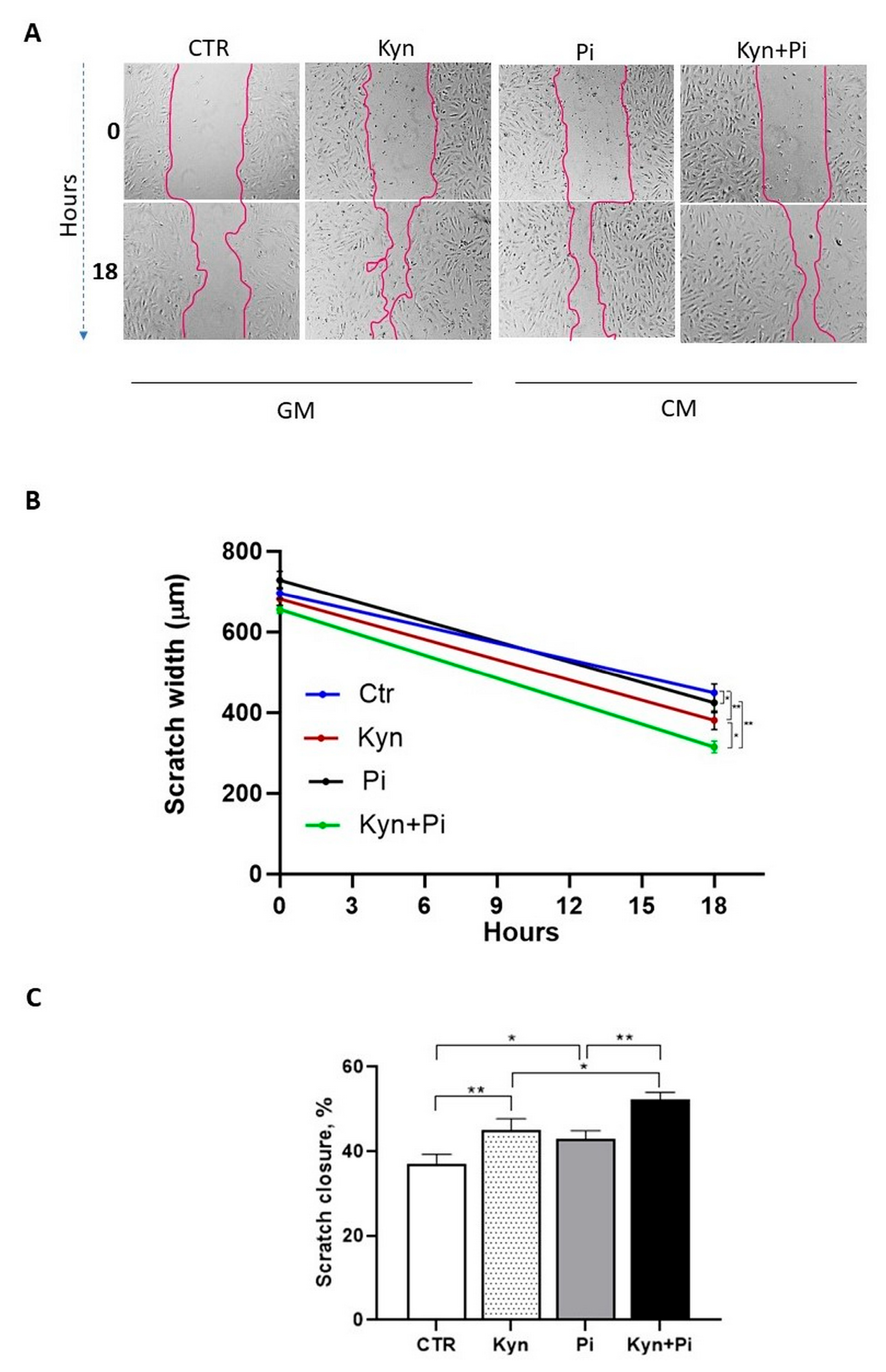
2.3. Effect of Kynurenine on Pi-Induced Migratory Capability
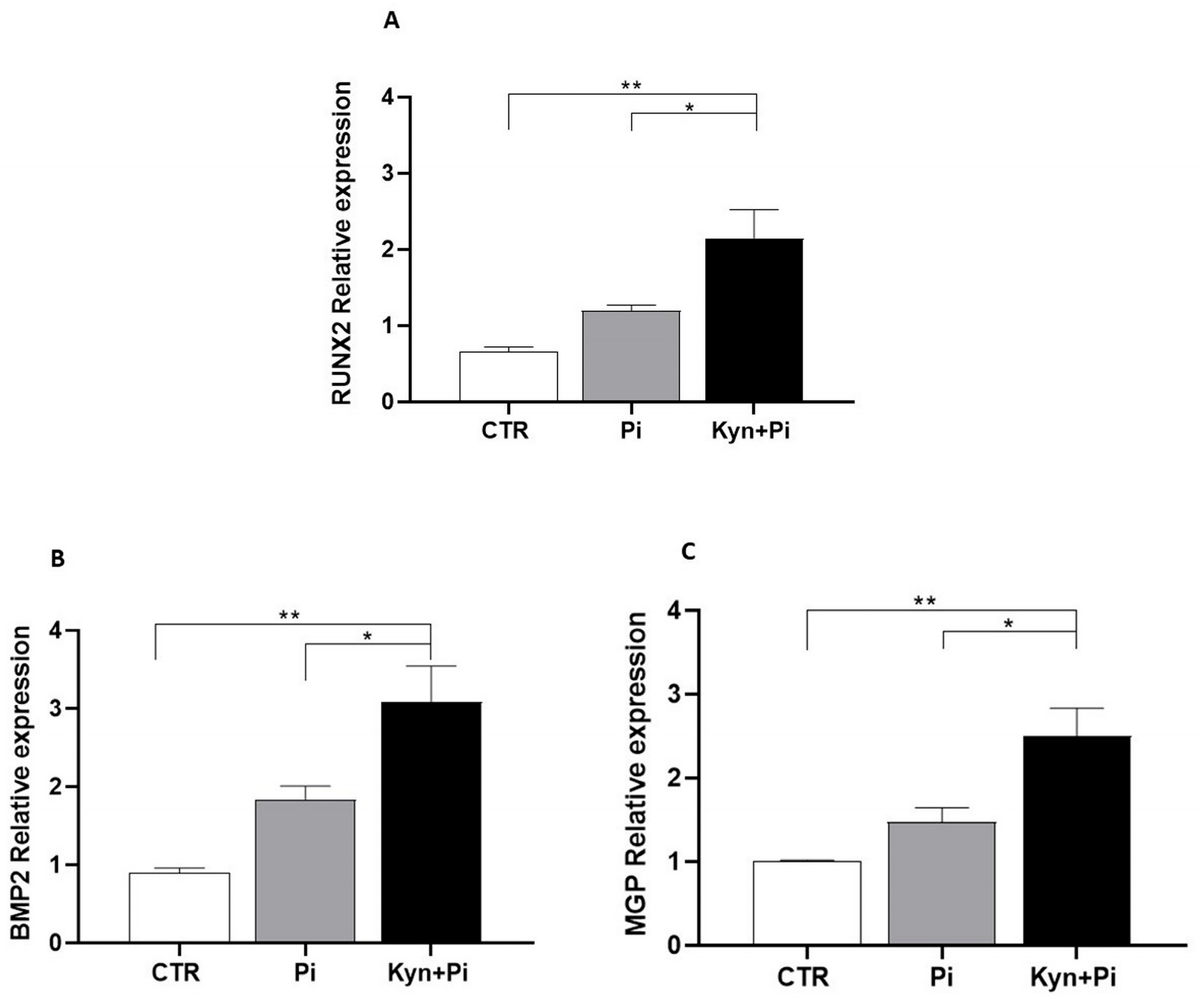
2.4. Effect of Kynurenine on Pi-Induced Osteoblastic Differentiation
2.5. Effect of Co-Stimulation with Kynurenine and Pi on AhR Pathway
3. Discussion
4. Materials and Methods
4.1. Endothelial Cell Culture and Induction of Calcification
4.2. Alizarin Red Staining and Quantification of Calcium Deposition by De-Staining
4.3. Cell Proliferation Assay
4.4. Endothelial Cells Scratch Wound Healing Assay
4.5. RNA Extraction and RT-PCR
4.6. Protein Extraction and Western Blotting
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESRD | End-stage renal disease |
| VC | Vascular calcification |
| EndMT | Endothelial-to-mesenchymal transition |
| Pi | High phosphate |
| Kyn | Kynurenine |
| FN | Fibronectin |
| N-Cadh | N-cadherin |
| SM22α | Smooth muscle 22 α |
| AhR | Aryl hydrocarbon receptor |
References
- Mor, A.; Kalaska, B.; Pawlak, D. Kynurenine Pathway in Chronic Kidney Disease: What’s Old, What’s New, and What’s Next? Int. J. Tryptophan. Res. 2020, 13, 1178646920954882. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Liu, X.; Xie, C.; Shi, J. The Role of the Kynurenine Pathway in Cardiovascular Disease. Front. Cardiovasc. Med. 2024, 11, 1406856. [Google Scholar] [CrossRef]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular Disease in Dialysis Patients. Nephrol. Dial. Transplant. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Ahmadmehrabi, S.; Tang, W.H.W. Hemodialysis-induced cardiovascular disease. Semin. Dial. 2018, 31, 258–267. [Google Scholar] [CrossRef]
- Kaisar, M.; Isbel, N.; Johnson, D.W. Cardiovascular disease in patients with chronic kidney disease. A clinical review. Minerva Urol. Nefrol. 2007, 59, 281–297. [Google Scholar] [CrossRef]
- Ciceri, P.; Artioli, L.; Molinaro, M.; Falleni, M.; Tosi, D.; Martinelli, C.; Santo, N.; Rondinone, O.; Miozzo, M.; Bianchi, P.; et al. High-Phosphate Causes Endothelial Extracellular Matrix Calcification by Inducing Endothelial Cell Mesenchymal Transition and Osteoblastic Differentiation. Am. J. Physiol.-Ren. Physiol. 2025, 328, F861–F875. [Google Scholar] [CrossRef]
- Bouabdallah, J.; Zibara, K.; Issa, H.; Lenglet, G.; Kchour, G.; Caus, T.; Six, I.; Choukroun, G.; Kamel, S.; Bennis, Y. Endothelial cells exposed to phosphate and indoxyl sulphate promote vascular calcification through interleukin-8 secretion. Nephrol. Dial. Transplant. 2019, 34, 1125–1134. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Tang, R.N.; Wang, L.T.; Liu, B.C. Role of crosstalk between endothelial cells and smooth muscle cells in vascular calcification in chronic kidney disease. Cell Prolif. 2021, 54, e12980. [Google Scholar] [CrossRef]
- Jiang, H.; Li, L.; Zhang, L.; Zang, G.; Sun, Z.; Wang, Z. Role of endothelial cells in vascular calcification. Front. Cardiovasc. Med. 2022, 9, 895005. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, P.; Tettamanti, G.; Galassi, A.; Magagnoli, L.; Fabresse, N.; Alvarez, J.-C.; Massy, Z.A.; Messa, P.; Cozzolino, M. Pro-Calcifying Analysis of Uraemic Serum from Patients Treated with Medium Cut-off Membrane in a Prospective, Cross-over Study. Clin. Kidney J. 2021, 14, 1798–1807. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Maffei Faccioli, F.; Cara, A.; Boni Brivio, G.; Rivela, F.; Ciceri, P.; Magagnoli, L.; Galassi, A.; Barbuto, S.; Speciale, S.; et al. Future Treatment of Vascular Calcification in Chronic Kidney Disease. Expert Opin. Pharmacother. 2023, 24, 2041–2057. [Google Scholar] [CrossRef]
- Reiss, A.B.; Miyawaki, N.; Moon, J.; Kasselman, L.J.; Voloshyna, I.; D’Avino, R., Jr.; De Leon, J. CKD, arterial calcification, atherosclerosis and bone health: Inter-relationships and controversies. Atherosclerosis 2018, 278, 49–59. [Google Scholar] [CrossRef]
- Shen, Y. Pathogenesis and Mechanism of Uremic Vascular Calcification. Cureus 2024, 16, e64771. [Google Scholar] [CrossRef]
- Curaj, A.; Vanholder, R.; Loscalzo, J.; Quach, K.; Wu, Z.; Jankowski, V.; Jankowski, J. Cardiovascular Consequences of Uremic Metabolites: An Overview of the Involved Signaling Pathways. Circ. Res. 2024, 134, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Baaten, C.C.F.M.J.; Vondenhoff, S.; Noels, H. Endothelial Cell Dysfunction and Increased Cardiovascular Risk in Patients with Chronic Kidney Disease. Circ. Res. 2023, 132, 970–992. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Jumabay, M.; Ly, A.; Radparvar, M.; Cubberly, M.R.; Boström, K.I. A Role for the Endothelium in Vascular Calcification. Circ. Res. 2013, 113, 495–504. [Google Scholar] [CrossRef]
- Zakrocka, I.; Załuska, W. Kynurenine Pathway in Kidney Diseases. Pharmacol. Rep. 2022, 74, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Castro-Portuguez, R.; Sutphin, G.L. Kynurenine Pathway, NAD+ Synthesis, and Mitochondrial Function: Targeting Tryptophan Metabolism to Promote Longevity and Healthspan. Exp. Gerontol. 2020, 132, 110841. [Google Scholar] [CrossRef]
- Solvang, S.-E.H.; Hodge, A.; Watne, L.O.; Cabral-Marques, O.; Nordrehaug, J.E.; Giles, G.G.; Dugué, P.-A.; Nygård, O.; Ueland, P.M.; McCann, A.; et al. Kynurenine Pathway Metabolites in the Blood and Cerebrospinal Fluid Are Associated with Human Aging. Oxidative Med. Cell. Longev. 2022, 2022, 5019752. [Google Scholar] [CrossRef]
- El Chamieh, C.; Larabi, I.A.; Alencar De Pinho, N.; Lambert, O.; Combe, C.; Fouque, D.; Frimat, L.; Jacquelinet, C.; Laville, M.; Laville, S.; et al. Study of the Association between Serum Levels of Kynurenine and Cardiovascular Outcomes and Overall Mortality in Chronic Kidney Disease. Clin. Kidney J. 2024, 17, sfad248. [Google Scholar] [CrossRef]
- Pawlak, K.; Myśliwiec, M.; Pawlak, D. Kynurenine Pathway—A New Link between Endothelial Dysfunction and Carotid Atherosclerosis in Chronic Kidney Disease Patients. Adv. Med. Sci. 2010, 55, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Arinze, N.V.; Yin, W.; Lotfollahzadeh, S.; Napoleon, M.A.; Richards, S.; Walker, J.A.; Belghasem, M.; Ravid, J.D.; Hassan Kamel, M.; Whelan, S.A.; et al. Tryptophan Metabolites Suppress the Wnt Pathway and Promote Adverse Limb Events in Chronic Kidney Disease. J. Clin. Investig. 2022, 132, e142260. [Google Scholar] [CrossRef] [PubMed]
- Kolachalama, V.B.; Shashar, M.; Alousi, F.; Shivanna, S.; Rijal, K.; Belghasem, M.E.; Walker, J.; Matsuura, S.; Chang, G.H.; Gibson, C.M.; et al. Uremic Solute-Aryl Hydrocarbon Receptor-Tissue Factor Axis Associates with Thrombosis after Vascular Injury in Humans. J. Am. Soc. Nephrol. 2018, 29, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hatabayashi, K.; Arita, M.; Yajima, N.; Takenaka, C.; Suzuki, T.; Takahashi, M.; Oshima, Y.; Hara, K.; Kagawa, K.; et al. Kynurenine Signaling through the Aryl Hydrocarbon Receptor Maintains the Undifferentiated State of Human Embryonic Stem Cells. Sci. Signal. 2019, 12, eaaw3306. [Google Scholar] [CrossRef]
- Candellier, A.; Issa, N.; Grissi, M.; Brouette, T.; Avondo, C.; Gomila, C.; Blot, G.; Gubler, B.; Touati, G.; Bennis, Y.; et al. Indoxyl-Sulfate Activation of the AhR- NF-κB Pathway Promotes Interleukin-6 Secretion and the Subsequent Osteogenic Differentiation of Human Valvular Interstitial Cells from the Aortic Valve. J. Mol. Cell. Cardiol. 2023, 179, 18–29. [Google Scholar] [CrossRef]
- Delgado-Marin, M.; Sánchez-Esteban, S.; Cook-Calvete, A.; Jorquera-Ortega, S.; Zaragoza, C.; Saura, M. Indoxyl Sulfate-Induced Valve Endothelial Cell Endothelial-to-Mesenchymal Transition and Calcification in an Integrin-Linked Kinase-Dependent Manner. Cells 2024, 13, 481. [Google Scholar] [CrossRef]
- Nguyen, C.; Edgley, A.J.; Kelly, D.J.; Kompa, A.R. Aryl Hydrocarbon Receptor Inhibition Restores Indoxyl Sulfate-Mediated Endothelial Dysfunction in Rat Aortic Rings. Toxins 2022, 14, 100. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molinaro, M.; Cozzolino, M.; Ciceri, P. Kynurenine Promotes Phosphate-Induced Endothelial Calcification via Endothelial-to-Mesenchymal Transition, Osteoblastic Differentiation and AhR Activation. Toxins 2025, 17, 421. https://doi.org/10.3390/toxins17080421
Molinaro M, Cozzolino M, Ciceri P. Kynurenine Promotes Phosphate-Induced Endothelial Calcification via Endothelial-to-Mesenchymal Transition, Osteoblastic Differentiation and AhR Activation. Toxins. 2025; 17(8):421. https://doi.org/10.3390/toxins17080421
Chicago/Turabian StyleMolinaro, Martina, Mario Cozzolino, and Paola Ciceri. 2025. "Kynurenine Promotes Phosphate-Induced Endothelial Calcification via Endothelial-to-Mesenchymal Transition, Osteoblastic Differentiation and AhR Activation" Toxins 17, no. 8: 421. https://doi.org/10.3390/toxins17080421
APA StyleMolinaro, M., Cozzolino, M., & Ciceri, P. (2025). Kynurenine Promotes Phosphate-Induced Endothelial Calcification via Endothelial-to-Mesenchymal Transition, Osteoblastic Differentiation and AhR Activation. Toxins, 17(8), 421. https://doi.org/10.3390/toxins17080421





