The Effective Way of Botulinum Toxin Injection to Reduce Bite Force: Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Changes in Bite Force According to Injection Sites
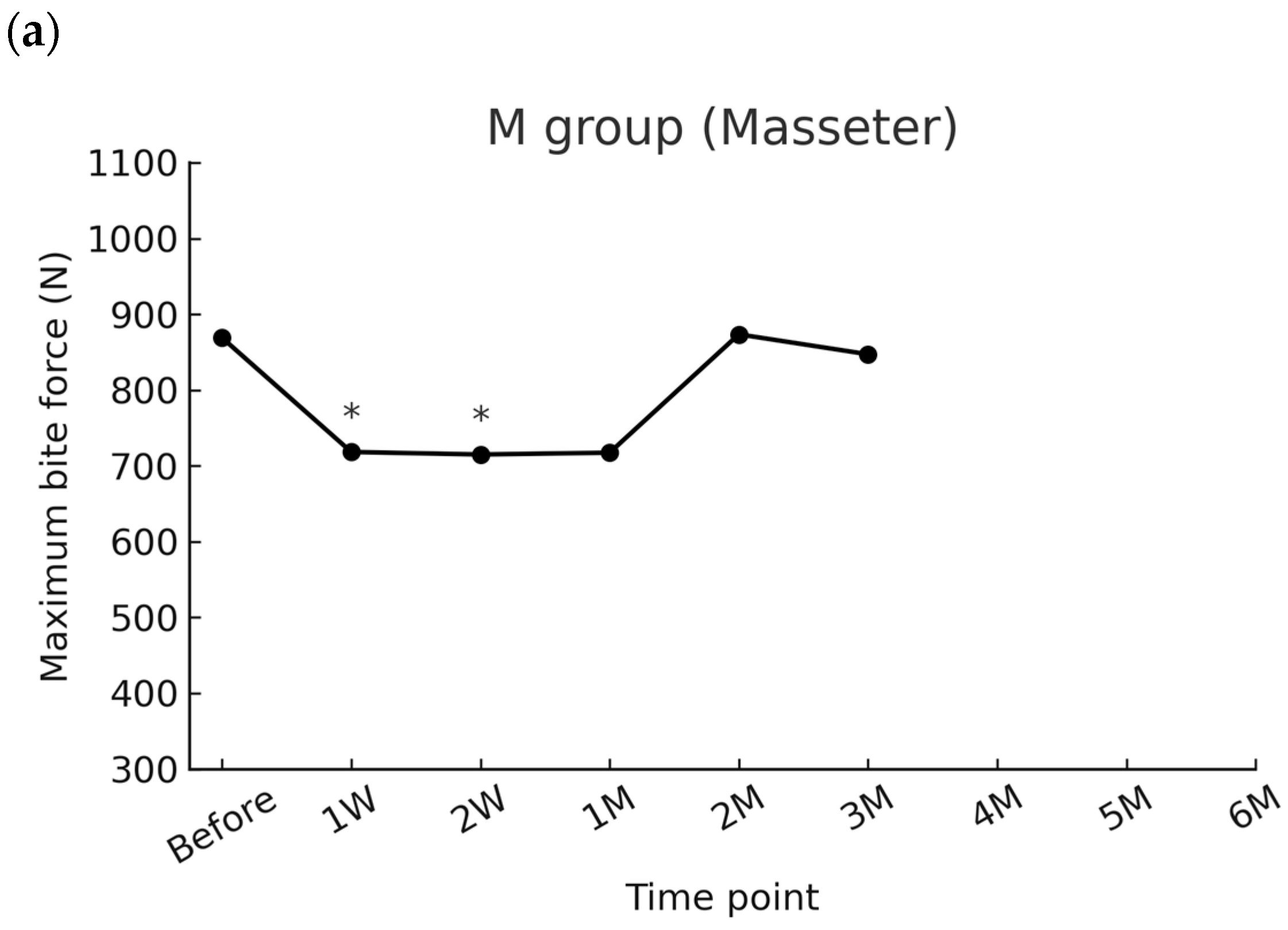
2.1.1. Masseter Group (M)
2.1.2. Masseter and Temporalis Group (MT)
2.1.3. Masseter, Temporalis, and Medial Pterygoid Group (MTP)
2.2. Comparison Among Groups
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Participants
5.2. Intervention
5.3. Bite Force Measurement
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT-A | Botulinum Toxin Type A |
| M | Masseter |
| MT | Masseter and temporalis |
| MTP | Masseter, temporalis, and medial pterygoid |
| U | Unit |
Appendix A
| Group | Time Point | Mean Difference (N) | 95% CI | Cohen’s d |
|---|---|---|---|---|
| M group | 1 week | −150.6 | −271.0 to −30.2 | 0.65 |
| 2 weeks | −153.9 | −285.2 to −22.6 | 0.61 | |
| 1 months | −151.5 | −340.2 to +37.2 | 0.42 | |
| 2 months | 4.6 | −157.8 to +166.9 | 0.02 | |
| 3 months | −21.7 | −211.4 to +168.0 | 0.07 | |
| MT group | 1 week | −225.5 | −311.0 to −140.0 | 1.89 |
| 2 weeks | −312.9 | −468.4 to −157.4 | 1.44 | |
| 1 months | −150.6 | −316.9 to +15.7 | 0.65 | |
| 2 months | −32 | −225.1 to +161.0 | 0.14 | |
| 3 months | −20.9 | −210.5 to +168.7 | 0.09 | |
| 4 months | 96.8 | −112.3 to +305.9 | 0.3 | |
| MTP group | 1 week | −430.2 | −636.7 to −223.7 | 1.49 |
| 2 weeks | −513.4 | −720.7 to −306.1 | 1.77 | |
| 1 months | −490.4 | −703.3 to −277.5 | 1.65 | |
| 2 months | −415.5 | −622.3 to −208.7 | 1.44 | |
| 3 months | −361 | −577.1 to −144.9 | 1.2 | |
| 4 months | −286.2 | −492.0 to −80.4 | 0.95 | |
| 5 months | −156.4 | −386.7 to +73.9 | 0.45 | |
| 6 months | −67.5 | −288.4 to +153.4 | 0.21 |
References
- Delcanho, R.; Manfredini, D.; Guarda-Nardini, L.; Val, M. Botulinum Toxin for Treating Temporomandibular Disorders: What Is the Evidence? J. Oral Facial Pain Headache 2022, 36, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Pihut, M.; Szewczyk, M.; Ferendiuk, E.; Kasprzyk, K.; Więckiewicz, M. The Efficiency of Botulinum Toxin Type A for the Treatment of Masseter Muscle Pain in Patients with Temporomandibular Joint Dysfunction and Tension-Type Headache. J. Headache Pain 2016, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, G.; DeSantis, L.; Goodacre, C. Bruxism: Best Evidence Consensus Statement. J. Prosthodont. 2021, 30 (Suppl. 1), 91–101. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Botulinum Neurotoxin Therapy for Lingual Dystonia Using an Individualized Injection Method Based on Clinical Features. Toxins 2019, 11, 51. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum Neurotoxin Injection for the Treatment of Recurrent Temporomandibular Joint Dislocation with and without Neurogenic Muscular Hyperactivity. Toxins 2018, 10, 174. [Google Scholar] [CrossRef]
- Zheng, Z.; Hao, Y.; Yin, J.; Lei, X.; Cheng, B.; Huang, W. Autogenous Fat Transplantation and Botulinum Toxin Injection into the Masseter Muscle to Create an Ideal Oval Face. Aesthet. Surg. J. 2021, 41, NP579–NP588. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Lora, V.M.; Araújo Oliveira Ferreira, D.M.; Stuginski-Barbosa, J.; Bonjardim, L.R.; Cury, A.A.D.B.; Conti, P.C.R. Botulinum Toxin Type A Applications for Masticatory Myofascial Pain and Trigeminal Neuralgia: What Is the Evidence Regarding Adverse Effects? Clin. Oral Investig. 2019, 23, 3411–3421. [Google Scholar] [CrossRef]
- Filipo, R.; Spahiu, I.; Covelli, E.; Nicastri, M.; Bertoli, G.A.; Bussu, F. Botulinum Toxin in the Treatment of Facial Synkinesis and Hyperkinesis. Laryngoscope 2012, 122, 266–270. [Google Scholar] [CrossRef]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum Neurotoxins: Genetic, Structural and Mechanistic Insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef]
- Juzans, P.; Comella, J.X.; Molgó, J.; Faille, L.; Angaut-Petit, D. Nerve Terminal Sprouting in Botulinum Type-A Treated Mouse Levator Auris Longus Muscle. Neuromuscul. Disord. 1996, 6, 177–185. [Google Scholar] [CrossRef]
- de Maio, M.; Bento, R.F. Botulinum Toxin in Facial Palsy: An Effective Treatment for Contralateral Hyperkinesis. Plast. Reconstr. Surg. 2007, 120, 917–927. [Google Scholar] [CrossRef]
- Kim, K.S.; Byun, Y.S.; Kim, Y.J.; Kim, S.T. Muscle Weakness after Repeated Injection of Botulinum Toxin Type A Evaluated According to Bite Force Measurement of Human Masseter Muscle. Dermatol. Surg. 2009, 35, 1902–1906. [Google Scholar] [CrossRef]
- Seo, K.K. Botulinum Toxin for Asians; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Kim, N.H.; Park, R.H.; Park, J.B. Botulinum Toxin Type A for the Treatment of Hypertrophy of the Masseter Muscle. Plast. Reconstr. Surg. 2010, 125, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.P.; Best, D.; Kidd, L.; Roberts, B.; Stark, S.; Weeks, P.; Whitaker, J. The Use of Botulinum Toxin Type-B in the Treatment of Patients Who Have Become Unresponsive to Botulinum Toxin Type-A—Initial Experiences. Eur. J. Neurol. 2005, 12, 947–955. [Google Scholar] [CrossRef]
- Kessler, K.R.; Skutta, M.; Benecke, R. Long-Term Treatment of Cervical Dystonia with Botulinum Toxin A: Efficacy, Safety, and Antibody Frequency. J. Neurol. 1999, 246, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Greene, P.; Fahn, S.; Diamond, B. Development of Resistance to Botulinum Toxin Type A in Patients with Torticollis. Mov. Disord. 1994, 9, 213–217. [Google Scholar] [CrossRef]
- Moussa, M.S.; Bachour, D.; Komarova, S.V. Adverse Effect of Botulinum Toxin-A Injections on Mandibular Bone: A Systematic Review and Meta-Analysis. J. Oral Rehabil. 2024, 51, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Blanksma, N.G.; Van Eijden, T.M.; Weijs, W.A. Electromyographic heterogeneity in the human masseter muscle. J. Dent. Res. 1992, 71, 47–52. [Google Scholar] [CrossRef]
- Matsunaga, K.; Usui, A.; Yamaguchi, K.; Akita, K. An Anatomical Study of the Muscles That Attach to the Articular Disc of the Temporomandibular Joint. Clin. Anat. 2009, 22, 932–940. [Google Scholar] [CrossRef]
- Shimokawa, T.; Akita, K.; Soma, K.; Sato, T. Innervation Analysis of the Small Muscle Bundles Attached to the Temporalis Muscle: Truly New Muscles or Merely Derivatives of the Temporalis Muscle? Surg. Radiol. Anat. 1998, 20, 329–334. [Google Scholar] [CrossRef]
- Shankland, W.E.; Negulesco, J.A.; O’Brian, B. The “Pre-Anterior Belly” of the Temporalis Muscle: A Preliminary Study of a Newly Described Muscle. Cranio 1996, 14, 106–112. [Google Scholar] [CrossRef]
- Akita, K.; Sakaguchi-Kuma, T.; Fukino, K.; Ono, T. Masticatory Muscles and Branches of Mandibular Nerve: Positional Relationships between Various Muscle Bundles and Their Innervating Branches. Anat. Rec. 2019, 302, 609–619. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Akita, K. Spatial Relationships between Masticatory Muscles and Their Innervating Nerves in Man with Special Reference to the Medial Pterygoid Muscle and Its Accessory Muscle Bundle. Surg. Radiol. Anat. 2004, 26, 122–127. [Google Scholar] [CrossRef]
- Jain, P.; Rathee, M. Anatomy, Head and Neck, Medial (Internal) Pterygoid Nerve. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Peck, C.C. Biomechanics of Occlusion—Implications for Oral Rehabilitation. J. Oral Rehabil. 2016, 43, 205–214. [Google Scholar] [CrossRef]
- Weijs, W.A.; Hillen, B. Cross-Sectional Areas and Estimated Intrinsic Strength of the Human Jaw Muscles. Acta Morphol. Neerl. Scand. 1985, 23, 267–274. [Google Scholar] [PubMed]
- Lee, H.J.; Jung, S.J.; Kim, S.T.; Kim, H.J. Ultrasonographic Considerations for Safe and Efficient Botulinum Neurotoxin Injection in Masseteric Hypertrophy. Toxins 2021, 13, 28. [Google Scholar] [CrossRef]
- Chang, C.S.; Lin, S.; Wallace, C.G.; Hsiao, Y.C.; Lin, C.M.; Kang, G.C.W.; Chen, J.P. Masseter Muscle Volume Changes Evaluated by 3-Dimensional Computed Tomography after Repeated Botulinum Toxin A Injections in Patients with Square Facial Morphology. Ann. Plast. Surg. 2019, 82 (Suppl. 1), S29–S32. [Google Scholar] [CrossRef]
- Koc, D.; Dogan, A.; Bek, B. Bite Force and Influential Factors on Bite Force Measurements: A Literature Review. Eur. J. Dent. 2010, 4, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Braun, S.; Bantleon, H.P.; Hnat, W.P.; Freudenthaler, J.W.; Marcotte, M.R.; Johnson, B.E. A Study of Bite Force, Part 1: Relationship to Various Physical Characteristics. Angle Orthod. 1995, 65, 367–372. [Google Scholar] [PubMed]
- Kim, K.S.; Choi, J.H.; Kim, S.T.; Kim, C.Y.; Ahn, H.J. Bite Force, Occlusal Contact Area and Occlusal Pressure of Patients with Temporomandibular Joint Internal Derangement. J. Oral Med. Pain 2006, 31, 265–274. [Google Scholar]
- Malcangi, G.; Patano, A.; Pezzolla, C.; Riccaldo, L.; Mancini, A.; Di Pede, C.; Inchingolo, A.D.; Inchingolo, F.; Bordea, I.R.; Dipalma, G.; et al. Bruxism and Botulinum Injection: Challenges and Insights. J. Clin. Med. 2023, 12, 4586. [Google Scholar] [CrossRef]
- Yacoub, S.; Ons, G.; Khemiss, M. Efficacy of Botulinum Toxin Type A in Bruxism Management: A Systematic Review. Dent. Med. Probl. 2025, 62, 145–160. [Google Scholar] [CrossRef]
- Taylor, A.B.; Holmes, M.A.; Laird, M.F.; Terhune, C.E. Jaw-Muscle Structure and Function in Primates: Insights into Muscle Performance and Feeding-System Behaviors. Evol. Anthropol. 2025, 34, e22053. [Google Scholar] [CrossRef] [PubMed]
- Angst, L.; Koolstra, J.H.; Wiedemeier, D.; van Sluijs, R.M.; Pulfer, A.M.; Gallo, L.M.; Colombo, V. Masticatory Muscles Activation and TMJ Space During Asymmetrically Loaded Jaw Closing. Ann. Biomed. Eng. 2024, 52, 877–887. [Google Scholar] [CrossRef]
- de Souza Nobre, B.B.; de Oliveira Resende Machado, L.; Poluha, R.L.; Câmara-Souza, M.B.; Carbone, A.C.; de Almeida, A.M.; Grigoriadis, A.; Kumar, A.; De la Torre Canales, G. Temporalis Muscle Changes Following Botulinum Toxin A Injections in Masseter Hypertrophy Patients: A Randomized Triple-Blinded Trial. Aesthet. Plast. Surg. 2024, 48, 3979–3987. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum Toxin Therapy for Oromandibular Dystonia and Other Movement Disorders in the Stomatognathic System. Toxins 2022, 14, 282. [Google Scholar] [CrossRef]
- Dressler, D.; Adib Saberi, F. Botulinum Toxin: Mechanisms of Action. Eur. Neurol. 2005, 53, 3–9. [Google Scholar] [CrossRef]
- Rosales, R.L.; Dressler, D. On Muscle Spindles, Dystonia and Botulinum Toxin. Eur. J. Neurol. 2010, 17 (Suppl. 1), 71–80. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Shu, X.; Leung, K.C.M.; Lo, E.C.M. Association between masticatory performance and oral conditions in adults: A systematic review and meta-analysis. J. Dent. 2023, 129, 104395. [Google Scholar] [CrossRef] [PubMed]
- Hatch, J.P.; Shinkai, R.S.; Sakai, S.; Rugh, J.D.; Paunovich, E.D. Determinants of Masticatory Performance in Dentate Adults. Arch. Oral Biol. 2001, 46, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Shin, J.H.; Kim, S.T.; Kim, C.Y. Effects of two different units of botulinum toxin type A evaluated by computed tomography and electromyographic measurement of human masseter muscle. Plast Reconstr. Surg. 2007, 119, 711–717. [Google Scholar] [CrossRef]
- Naumann, M.; Jankovic, J. Safety of Botulinum Toxin Type A: A Systematic Review and Meta-Analysis. Curr. Med. Res. Opin. 2004, 20, 981–990. [Google Scholar] [CrossRef]
- Albrecht, P.; Jansen, A.; Lee, J.-I.; Moll, M.; Ringelstein, M.; Rosenthal, D.; Bigalke, H.; Aktas, O.; Hartung, H.-P.; Hefter, H. High Prevalence of Neutralizing Antibodies after Long-Term Botulinum Neurotoxin Therapy. Neurology. 2019, 92, e48–e54. [Google Scholar] [CrossRef]
- Lange, O.; Bigalke, H.; Dengler, R.; Wegner, F.; deGroot, M.; Wohlfarth, K. Neutralizing Antibodies and Secondary Therapy Failure After Treatment With Botulinum Toxin Type A: Much Ado About Nothing? Clin. Neuropharmacol. 2009, 32, 213–218. [Google Scholar] [CrossRef]
- Tamura, K.; Shiga, H. Gender Differences in Masticatory Movement Path and Rhythm in Dentate Adults. J. Prosthodont. Res. 2014, 58, 237–242. [Google Scholar] [CrossRef]
- Shiga, H.; Kobayashi, Y.; Katsuyama, H.; Yokoyama, M.; Arakawa, I. Gender Difference in Masticatory Performance in Dentate Adults. J. Prosthodont. Res. 2012, 56, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Cho, E.S.; Kim, S.T.; Ahn, H.J. Change of Distribution and Timing of Bite Force after Botulinum Toxin Type A Injection Evaluated by a Computerized Occlusion Analysis System. Yonsei Med. J. 2014, 55, 1123–1129. [Google Scholar] [CrossRef]
- Cruse, B.; Dharmadasa, T.; White, E.; Hollis, C.; Evans, A.; Sharmin, S.; Kalincik, T.; Kiers, L. Efficacy of Botulinum Toxin Type A in the Targeted Treatment of Sleep Bruxism: A Double-Blind, Randomised, Placebo-Controlled, Cross-Over Study. BMJ Neurol. Open 2022, 4, e000328. [Google Scholar] [CrossRef] [PubMed]
- Shim, Y.J.; Lee, M.K.; Kato, T.; Park, H.U.; Heo, K.; Kim, S.T. Effects of Botulinum Toxin on Jaw Motor Events during Sleep in Sleep Bruxism Patients: A Polysomnographic Evaluation. J. Clin. Sleep. Med. 2014, 10, 291–298. [Google Scholar] [CrossRef]
- Dadgardoust, P.D.; Rosales, R.L.; Asuncion, R.M.; Dressler, D. Botulinum Neurotoxin A Therapy Efficacy and Safety for Oromandibular Dystonia: A Meta-Analysis. J. Neural. Transm. 2019, 126, 141–148. [Google Scholar] [CrossRef] [PubMed]




| Variables | M (n = 15) | MT (n = 10) | MTP (n = 10) | p-Value |
|---|---|---|---|---|
| Age (years) | 28.9 ± 9.7 | 30.2 ± 8.9 | 32.2 ± 6.9 | 0.661 |
| Baseline bite force (N) | 868.9 ± 323.3 | 737.7 ± 309.9 | 1000.4 ± 313.9 | 0.196 |
| Time Point | Mean (N) | SD (N) | Raw p-Value * | Adjusted p-Value ** |
|---|---|---|---|---|
| Before | 868.9 | 323.3 | Reference | Reference |
| 1 week | 718.3 | 245.7 | 0.004 | 0.024 |
| 2 weeks | 715.0 | 320.9 | 0.003 | 0.023 |
| 1 month | 717.4 | 290.0 | 0.017 | 0.103 |
| 2 months | 873.5 | 269.6 | 0.093 | 0.620 |
| 3 months | 847.2 | 285.5 | 0.500 | 1.000 |
| Time Point | Mean (N) | SD (N) | Raw p-Value * | Adjusted p-Value ** |
|---|---|---|---|---|
| Before | 737.7 | 309.9 | Reference | Reference |
| 1 week | 512.2 | 233.4 | 0.0002 | 0.0013 |
| 2 weeks | 424.8 | 207.3 | 0.0014 | 0.0083 |
| 1 month | 587.1 | 281.7 | 0.0708 | 0.4248 |
| 2 months | 643.3 | 365.9 | 0.7063 | 1.0000 |
| 3 months | 716.8 | 325.8 | 0.8490 | 1.0000 |
| 4 months | 834.5 | 284.9 | 0.1523 | 1.0000 |
| Time Point | Mean (N) | SD (N) | Raw p-Value * | Adjusted p-Value ** |
|---|---|---|---|---|
| Before | 1000.4 | 313.9 | Reference | Reference |
| 1 week | 570.2 | 261.1 | <0.0001 | <0.0001 |
| 2 weeks | 487.0 | 263.6 | 0.0001 | 0.0008 |
| 1 month | 510.0 | 280.5 | 0.0002 | 0.0016 |
| 2 months | 584.9 | 262.0 | 0.0002 | 0.0016 |
| 3 months | 639.4 | 289.8 | 0.0006 | 0.0032 |
| 4 months | 714.2 | 259.6 | 0.0010 | 0.0080 |
| 5 months | 844.0 | 347.1 | 0.0180 | 0.1896 |
| 6 months | 932.9 | 253.5 | 0.5283 | 1.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.-H.; Jung, J.-K.; Byun, J.-S.; Kim, J.R. The Effective Way of Botulinum Toxin Injection to Reduce Bite Force: Preliminary Study. Toxins 2025, 17, 519. https://doi.org/10.3390/toxins17100519
Kang K-H, Jung J-K, Byun J-S, Kim JR. The Effective Way of Botulinum Toxin Injection to Reduce Bite Force: Preliminary Study. Toxins. 2025; 17(10):519. https://doi.org/10.3390/toxins17100519
Chicago/Turabian StyleKang, Kun-Hwa, Jae-Kwang Jung, Jin-Seok Byun, and Ji Rak Kim. 2025. "The Effective Way of Botulinum Toxin Injection to Reduce Bite Force: Preliminary Study" Toxins 17, no. 10: 519. https://doi.org/10.3390/toxins17100519
APA StyleKang, K.-H., Jung, J.-K., Byun, J.-S., & Kim, J. R. (2025). The Effective Way of Botulinum Toxin Injection to Reduce Bite Force: Preliminary Study. Toxins, 17(10), 519. https://doi.org/10.3390/toxins17100519





