Self-Sufficient Aflatoxin Decontamination System: MOF-Based Composite Membrane with Peroxidase-Mimic and Controlled H2O2 Generation
Abstract
1. Introduction
2. Results and Discussion
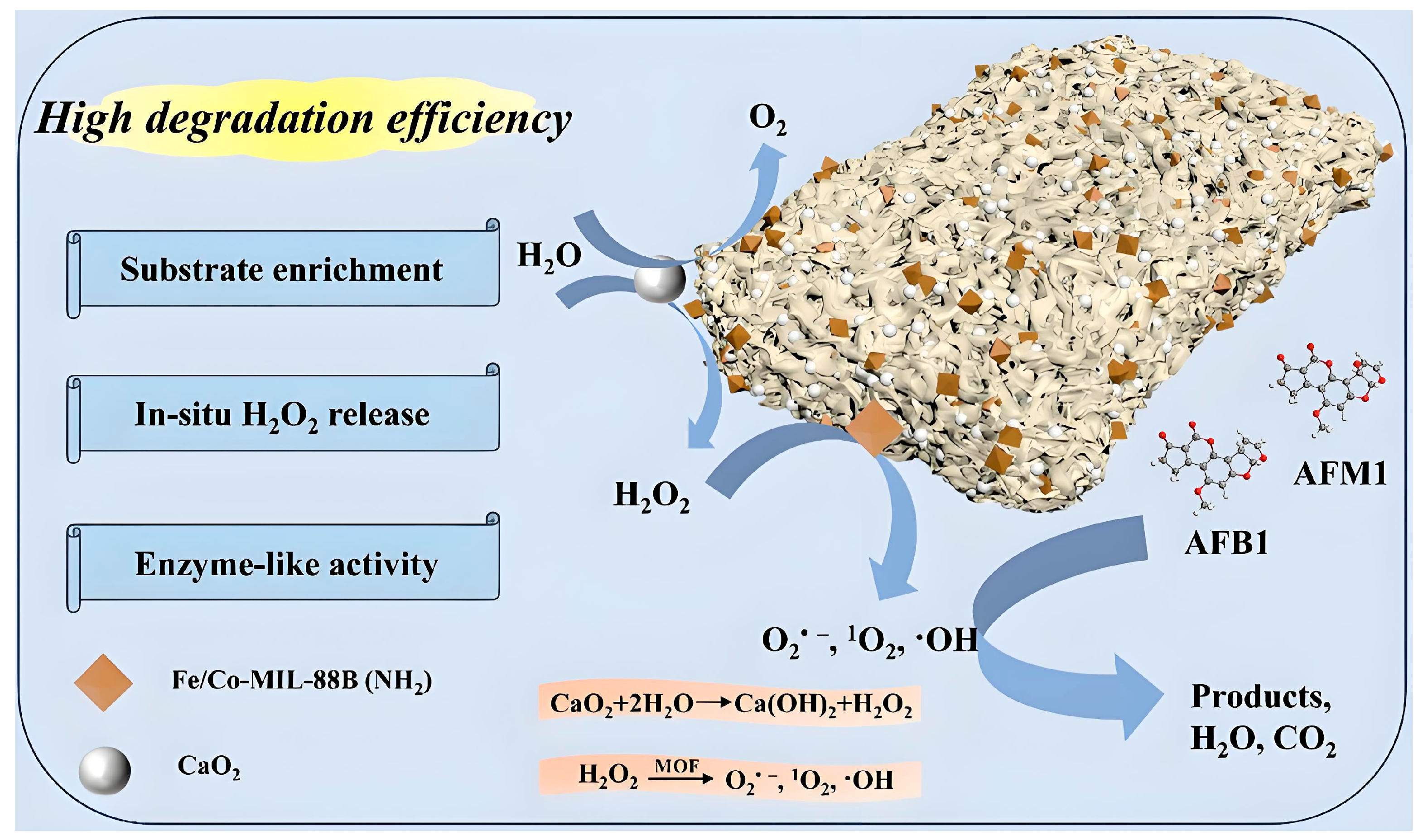
2.1. Principles of Aflatoxin Degradation
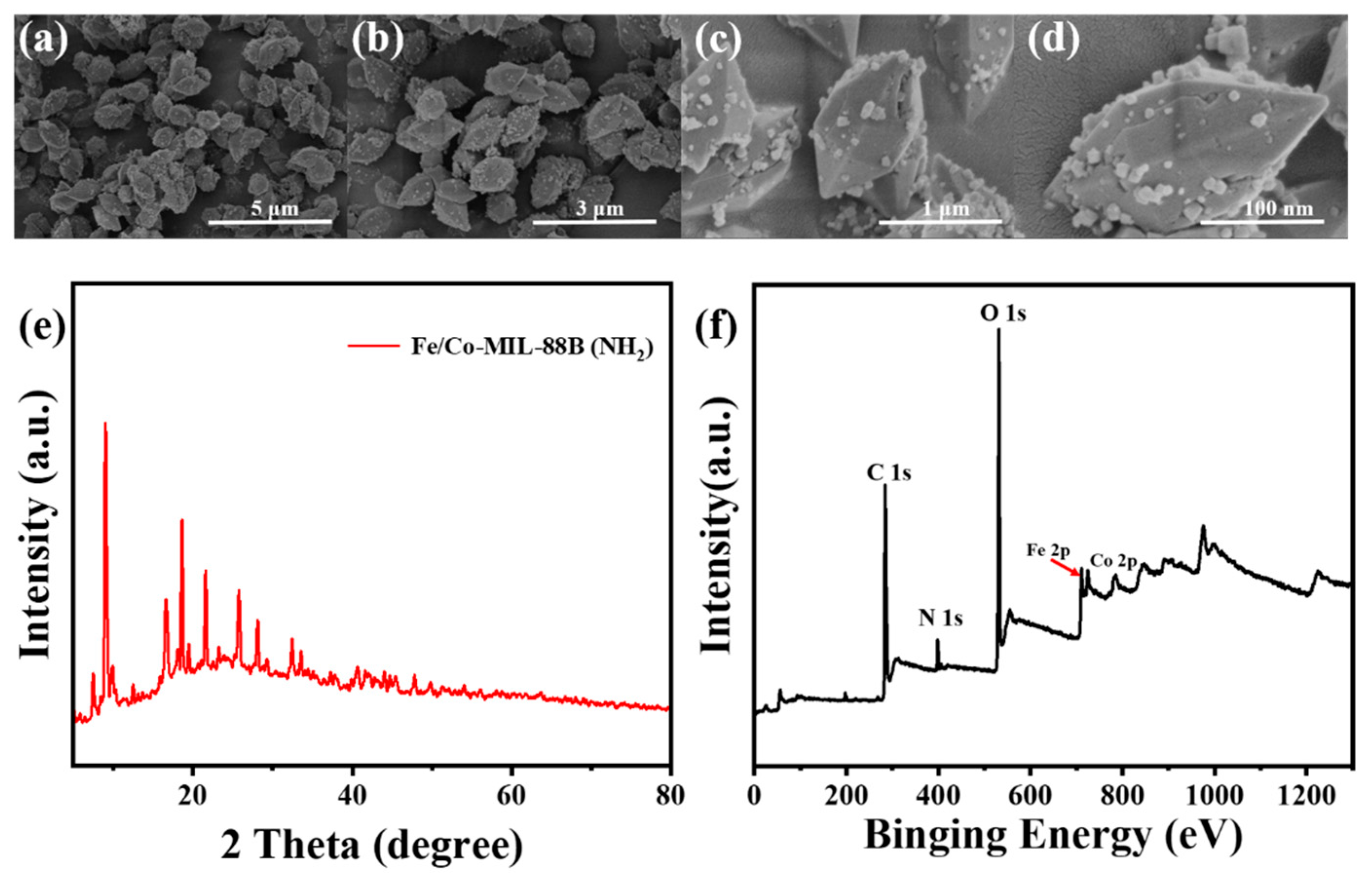
2.2. Characterization of MOF
2.3. Characterization of Membranes
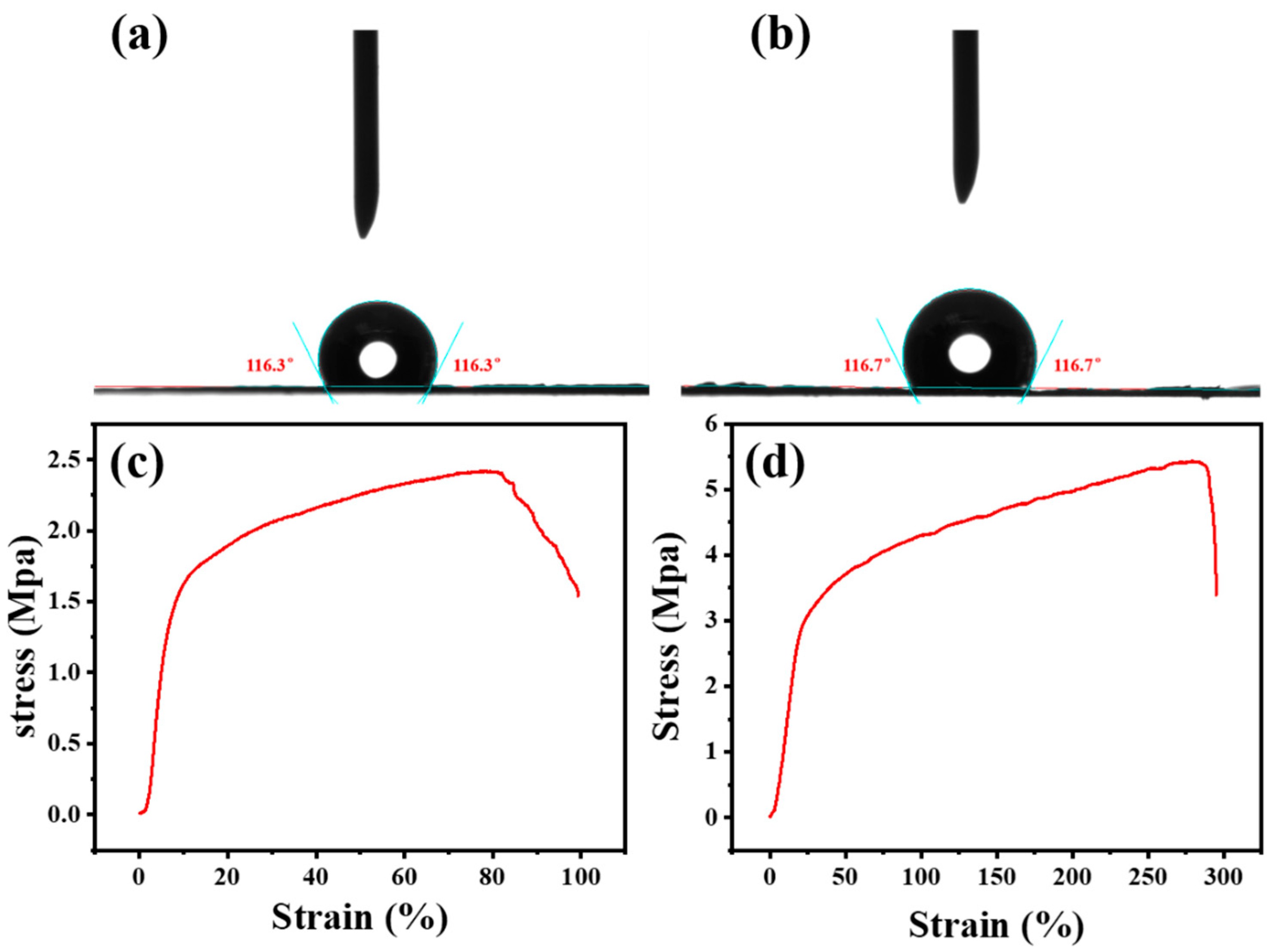
2.4. Mechanical Properties of the Membrane
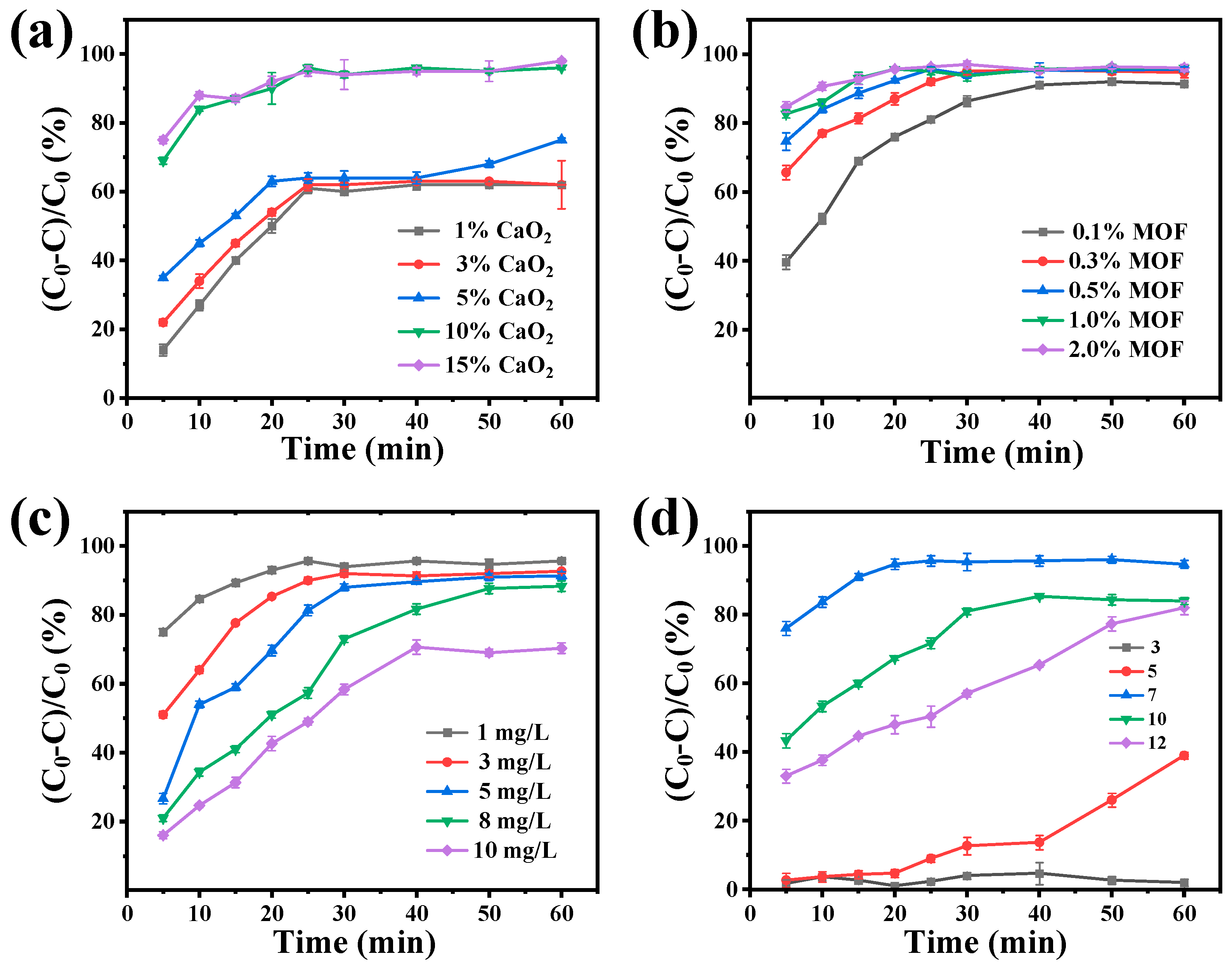
2.5. Degradation Condition Experiment
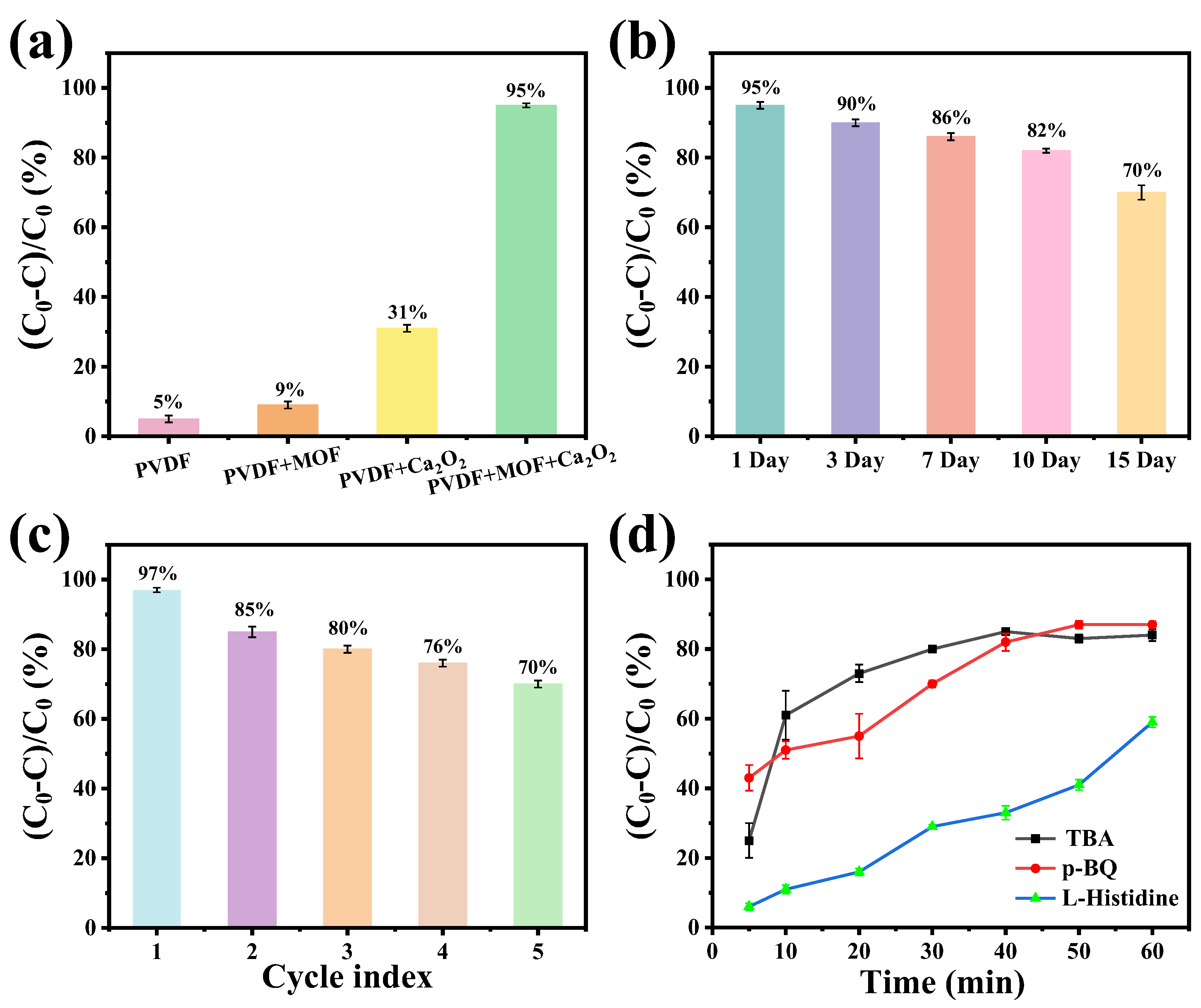
2.6. Comparison of Degradation Performance of Different Membrane Compositions
2.7. Stability and Cyclability of Membranes
2.8. Comparison of Metal Leaching Performance
2.9. Degradation Mechanism
2.10. Degradation Products
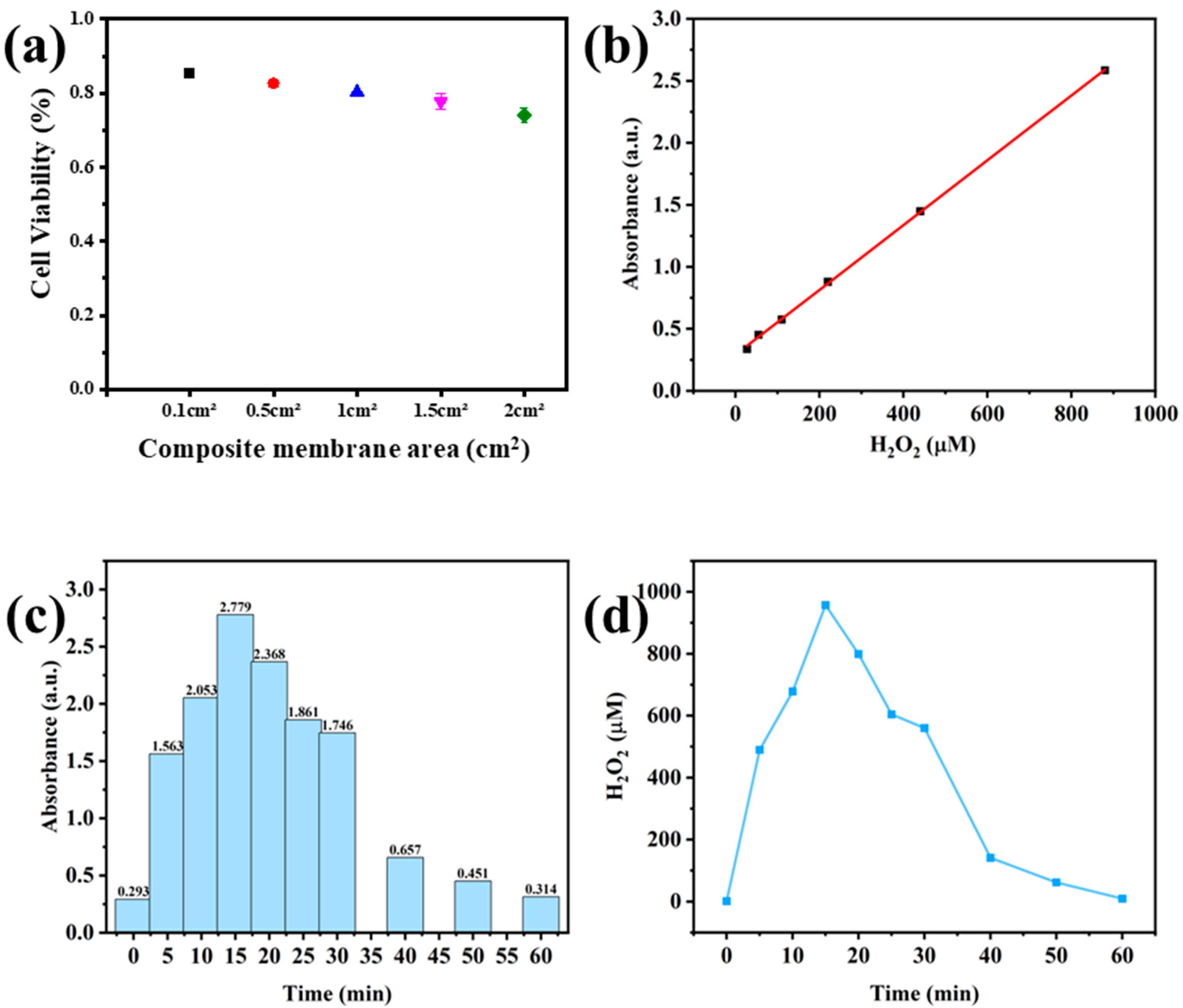
2.11. Safety Test of Composite Membrane
2.12. Actual Test of the Composite Membrane
3. Conclusions
4. Experimental Section
4.1. Materials
4.2. Preparation of Fe/Co-MIL-88B(NH2)
4.3. Membrane Preparation
4.4. Characterization
4.5. Degradability Test
4.6. Metal Leaching Test
4.7. Analytical Method
4.8. Safety Experiment
4.9. Actual Test
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ji, J.; Xie, W. Detoxification of Aflatoxin B1 by magnetic graphene composite adsorbents from contaminated oils. J. Hazard. Mater. 2020, 381, 120915. [Google Scholar] [CrossRef]
- Ren, Z.; Luo, J.; Wan, Y. Enzyme-Like Metal-Organic Frameworks in Polymeric Membranes for Efficient Removal of Aflatoxin B1. ACS Appl. Mater. Inter. 2019, 11, 30542–30550. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Man, Y.; Jiang, X.; Zhao, R.; Xiang, G.; He, L. Construction of a controllable and dispersed Fe3O4-based catalyst using ZIFs as a spatial support for highly catalytic degradation of aflatoxin B1. Appl. Catal. B Environ. Energy 2022, 318, 121818. [Google Scholar] [CrossRef]
- Cao, H.; Liang, D.; Tang, K.; Sun, Y.; Xu, Y.; Miao, M.; Zhao, Y. SERS and MRS signals engineered dual-mode aptasensor for simultaneous distinguishment of aflatoxin subtypes. J. Hazard. Mater. 2024, 462, 132810. [Google Scholar] [CrossRef]
- Turna, N.; Wu, F. Aflatoxin M1 in milk: A global occurrence, intake, & exposure assessment. Trends Food Sci. Tech. 2021, 110, 183–192. [Google Scholar]
- Guo, L.; Zhang, J.; Bao, Y.; Zhang, Y.; Zhang, D.; Ma, X.; Zhang, J. Label-free and highly sensitive detection of aflatoxin B1 by Ag IANPs via surface-enhanced Raman spectroscopy. Food Chem. 2024, 458, 140231. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Sartori, A.; Caetano-Silva, M.; Alencar, S.; Calori, M.; Augusto, P. Ozone processing of peanut “milk”: Degradation of aflatoxins, impact on quality attributes and the potential effect on peanut allergens. J. Clean. Prod. 2023, 405, 136950. [Google Scholar] [CrossRef]
- Nguyen, T.; Palmer, J.; Loo, T.; Shilton, A.; Petcu, M.; Newson, H.; Flint, S. Investigation of UV light treatment (254 nm) on the reduction of aflatoxin M1 in skim milk and degradation products after treatment. Food Chem. 2022, 390, 133165. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Herrera-Balandrano, D.; Shi, X.; Chen, X.; Liu, F.; Laborda, P. Occurrence of aflatoxins in water and decontamination strategies: A review. Water Res. 2023, 232, 119703. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, Y.; Feng, Y.; Peng, Q.; Li, Y.; He, C.; Fang, Z.; Xiao, Y.; Fang, W. A cost-saving, safe, and highly efficient natural mediator for laccase application on aflatoxin detoxification. Food Chem. 2024, 455, 139862. [Google Scholar] [CrossRef]
- Nguyen, T.; Flint, S.; Palmer, J. Control of aflatoxin M1 in milk by novel methods: A review. Food Chem. 2020, 311, 125984. [Google Scholar] [CrossRef]
- Song, C.; Yang, J.; Wang, Y.; Ding, G.; Guo, L.; Qin, J. Mechanisms and transformed products of aflatoxin B1 degradation under multiple treatments: A review. Crit. Rev. Food Sci. 2024, 64, 2263–2275. [Google Scholar] [CrossRef] [PubMed]
- Sert, D.; Mercan, E. Effects of ozone treatment to milk and whey concentrates on degradation of antibiotics and aflatoxin and physicochemical and microbiological characteristics. LWT Food Sci. Technol. 2021, 144, 111226. [Google Scholar] [CrossRef]
- Ding, S.; Barr, J.; Lyu, Z.; Zhang, F.; Wang, M.; Tieu, P.; Li, X.; Engelhard, M.; Feng, Z.; Beckman, S.; et al. Effect of Phosphorus Modulation in Iron Single-Atom Catalysts for Peroxidase Mimicking. Adv. Mater. 2024, 36, e2209633. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Gao, Q.; Xiao, L.; Wang, W.; Gu, Y.; Huang, H.; Tan, Y.; Tang, D.; Guo, S. Precise Tuning of the D-Band Center of Dual-Atomic Enzymes for Catalytic Therapy. J. Am. Chem. Soc. 2024, 146, 10023–10031. [Google Scholar] [CrossRef]
- Shan, J.; Jin, X.; Zhang, C.; Huang, M.; Xing, J.; Li, Q.; Cui, Y.; Niu, Q.; Chen, X.; Wang, X. Metal natural product complex Ru-procyanidins with quadruple enzymatic activity combat infections from drug-resistant bacteria. Acta Pharm. Sin. B 2024, 14, 2298–2316. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, M.; Zhou, L.; Huang, Y.; Srivastava, S.; Kumar, A.; Liu, J. Prospects, advances and biological applications of MOF-based platform for the treatment of lung cancer. Biomater. Sci 2024, 12, 3725–3744. [Google Scholar]
- Sun, Y.; Ding, S.; Zhao, X.; Sun, D.; Yang, Y.; Chen, M.; Zhu, C.; Jiang, B.; Gu, Q.; Liu, H.; et al. Self-reinforced MOF-based nanogel alleviates osteoarthritis by Long-Acting Drug Release. Adv. Mater. 2024, 36, e2401094. [Google Scholar] [CrossRef]
- Kirchon, A.; Feng, L.; Drake, H.; Joseph, E.; Zhou, H. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Tibbetts, I.; Kostakis, G. Recent Bio-Advances in Metal-Organic Frameworks. Molecules 2020, 25, 1291. [Google Scholar] [CrossRef]
- Tansell, A.; Jones, C.; Easun, T. MOF the beaten track: Unusual structures and uncommon applications of metal-organic frameworks. Chem. Cent. J. 2017, 11, 100. [Google Scholar] [CrossRef]
- Zhang, S.; Qin, S.; Xu, G.; Xie, W.; Xu, N.; Jiang, N.; Xu, Y.; Hu, X.; Su, Z. Co-based MOF as an efficient catalyst by peroxymonosulfate activation for degradation of tetracycline: Synthesis and performance. Crystengcomm 2024, 26, 3744–3753. [Google Scholar] [CrossRef]
- Choi, G.; Mandal, M.; Jung, H.; Panda, J.; Kwon, Y.; Zhang, K.; Vivek, E.; Shon, M.; Ravi, K.; Baek, K.; et al. Post-synthetic modifications (PSM)-induced defects in hybrid metal-organic frameworks (MOFs) to unleash potential in gas separation membrane applications. J. Mater. Sci. Technol. 2024, 201, 95–118. [Google Scholar] [CrossRef]
- Li, X.; Zheng, H.; Chen, J.; Xu, M.; Bai, Y.; Liu, T. MIL-101 (Fe)@Ag Rapid Synergistic Antimicrobial and Biosafety Evaluation of Nanomaterials. Molecules 2022, 27, 3497. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; He, D.; Shentu, J.; Yang, S.; Yang, Y.; Wang, Y.; Zhang, R.; Wang, K.; Qian, J.; Long, L. Smartphone-assisted colorimetric and near-infrared ratiometric fluorescent sensor for on-spot detection of H2O2 in food samples. Chem. Eng. J. 2023, 472, 144900. [Google Scholar] [CrossRef]
- Pan, J.; Yang, X.; Zhou, J.; Cheng, W.; Cheng, K. Novel ZIF-8/ZnS hollow polyhedral heterostructures derived from ZIF-8 with enhanced photocatalytic activity for degradation of aflatoxin B1. Prog. Nat. Sci. 2023, 33, 575–580. [Google Scholar] [CrossRef]
- Yuan, X.; Zhu, W.; Tang, K.; Zhang, P.; Lou, W. Closed-loop peroxide supply strategy: Engineering photo-nanozyme cascade systems for ultrafast degradation of mycotoxins in water. J. Environ. Chem. Eng. 2025, 13, 117909. [Google Scholar] [CrossRef]
- Zhu, X.; Wei, J.; Xu, S.; Zhu, Y.; Shen, W.; Wu, L. Metal-organic framework incorporated fungal mycelium membrane for synergistic mycotoxin degradation via adsorption, oxidation, and photocatalysis. Food Chem. 2025, 480, 143861. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, X.; Xiong, J.; Cao, Y.; Ta, N.; Gao, R.; Lou, W. Engineering the primary and second coordination sphere of metal-organic framework boosts the peroxidase-like activity. Chem. Eng. J. 2025, 522, 166966. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, Y.; Wang, D.; Zhu, M.; Sun, X.; Jiang, S.; Fan, Y.; Zhang, D.; Zhang, L. Surface-functionalized PVDF membranes by facile synthetic Cu-MOF-74 for enhanced contaminant degradation and antifouling performance. Colloid. Surf. A 2022, 651, 129640. [Google Scholar] [CrossRef]
- Hong, W.; Li, C.; Tang, T.; Xu, H.; Yu, Y.; Liu, G.; Wang, F.; Lei, C.; Zhu, H. The photocatalytic activity of the SnO2/TiO2/PVDF composite membrane in rhodamine B degradation. New J. Chem. 2021, 45, 2631–2642. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Shao, L.; Feng, X.; Zeng, H.; Liu, Y.; Long, R.; Zhu, X. Self-cleaning photocatalytic PVDF membrane loaded with NH2-MIL-88B/CDs and Graphene oxide for MB separation and degradation. Opt. Mater. 2021, 119, 111368. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Zhang, M.; Wang, T.; Hou, X. MIL-88B(Fe)/cellulose microspheres as sorbent for the fully automated dispersive pipette extraction towards trace sulfonamides in milk samples prior to UPLC-MS/MS analysis. Anal. Chim. Acta 2022, 1232, 340420. [Google Scholar] [CrossRef]
- Wu, Y.; Fang, Y.; Fu, J.; He, L.; Kabtamu, D.; Matovic, L.; Li, F.; Li, J. Optimized scalable synthesis and granulation of MIL-88B(Fe) for efficient arsenate removal. J. Environ. Chem. Eng. 2022, 10, 108556. [Google Scholar] [CrossRef]
- Feng, J.; Wang, H.; Ma, Z. Ultrasensitive amperometric immunosensor for the prostate specific antigen by exploiting a Fenton reaction induced by a metal-organic framework nanocomposite of type Au/Fe-MOF with peroxidase mimicking activity. Microchim. Acta 2020, 187, 95. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, J.; Ma, X.; Xu, J.; Yun, Z.; Zuo, Q.; Wang, L. A novel Fe-based Bi-MOFs material for photocatalytic degradation of tetracycline: Performance, mechanism and toxicity assessment. J. Water Process Eng. 2021, 44, 102364. [Google Scholar] [CrossRef]
- Han, M.; Ren, M.; Li, Z.; Qu, L.; Yu, L. A two-dimensional thin Co-MOF nanosheet as a nanozyme with high oxidase-like activity for GSH detection. New J. Chem. 2022, 46, 10682–10689. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiao, Y.; Hong, A.; Gao, Z.; Shen, Y.; Fan, Q.; Feng, P.; Zhong, W. Bimetallic Metal-Organic Framework Fe/Co-MIL-88(NH2) Exhibiting High Peroxidase-like Activity and Its Application in Detection of Extracellular Vesicles. ACS Appl. Mater. Inter. 2022, 14, 41800–41808. [Google Scholar] [CrossRef] [PubMed]
- Soetaredjo, F.; Santoso, S.; Lunardi, V.; Kurniawan, A.; Shuwanto, H.; Lie, J.; Foe, K.; Irawaty, W.; Yuliana, M.; Putro, J.; et al. Highly efficient degradation of organic pollutant mixtures by a Fe(III)-based MOF-catalyzed Fenton-like process in subcritical water. J. Mol. Liq. 2022, 347, 117989. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Zhang, P.; Zhang, Z.; Xu, X. Metal-organic framework ZnL-PVDF supramolecular ultrafiltration membrane for enhanced separation performance. J. Phys. Chem. Solids 2022, 171, 110865. [Google Scholar] [CrossRef]
- Meng, J.; Xie, Y.; Gu, Y.; Yan, X.; Chen, Y.; Guo, X.; Lang, W. PVDF-CaAlg nanofiltration membranes with dual thin-film-composite (TFC) structure and high permeation flux for dye removal. Sep. Purif. Technol. 2021, 255, 117739. [Google Scholar] [CrossRef]
- Singh, N.; Madhav, H.; Yadav, S.; Jaiswar, G. Impact of vanadium-, sulfur-, and dysprosium-doped zinc oxide nanoparticles on various properties of PVDF/functionalized-PMMA blend nanocomposites: Structural, optical, and morphological studies. J. Appl. Polym. Sci. 2019, 136, 47116. [Google Scholar] [CrossRef]
- Shen, M.; Singh, R. Decomposing Aflatoxins in Peanuts Using Advanced Oxidation Processes by UV and H2O2. Food Bioprocess Tech. 2022, 15, 1647–1657. [Google Scholar] [CrossRef]
- Guo, R.; Li, Y.; Chen, Y.; Liu, Y.; Niu, B.; Gou, J.; Cheng, X. Efficient degradation of sulfamethoxazole by CoCu LDH composite membrane activating peroxymonosulfate with decreased metal ion leaching. Chem. Eng. J. 2021, 417, 127887. [Google Scholar] [CrossRef]
- Chen, D.; Bai, Q.; Ma, T.; Jing, X.; Tian, Y.; Zhao, R.; Zhu, G. Stable metal-organic framework fixing within zeolite beads for effectively static and continuous flow degradation of tetracycline by peroxymonosulfate activation. Chem. Eng. J. 2022, 435, 134916. [Google Scholar] [CrossRef]
- Faraji, A.R.; Gil, A.; Farahanipour, A.; Tehrani, E.; Khoramdareh, N.B.; Dashtabadi, E.; Saeedi, S. Synergic removal of Aflatoxin B1 in oily matrices by focusing on the peroxidase-like nanozymes-driven strategies: Mechanisms and intermediate toxicity, nutritional impact, advances and challenges. Trends Food Sci. Technol. 2025, 163, 105135. [Google Scholar] [CrossRef]
- Stanley, J.; Patras, A.; Pendyala, B.; Vergne, M.; Bansode, R. Performance of a UV-A LED system for degradation of aflatoxins B1 and M1 in pure water: Kinetics and cytotoxicity study. Sci. Rep. 2020, 10, 13473. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Palmer, J.; Pedley, J.; Petcu, M.; Newson, H.L.; Keener, K.; Flint, S. The effect of variations in cold plasma conditions on the detoxification of Aflatoxin M1 and degradation products. Int. Dairy. J. 2025, 160, 106103. [Google Scholar] [CrossRef]
- Kousar, K.; Naseer, F.; Abduh, M.; Kakar, S.; Gul, R.; Anjum, S.; Ahmad, T. Green synthesis of hyaluronic acid coated, thiolated chitosan nanoparticles for CD44 targeted delivery and sustained release of Cisplatin in cervical carcinoma. Front. Pharmacol. 2023, 13, 1073004. [Google Scholar] [CrossRef]
- Nicolás-Vázquez, I.; Méndez-Albores, A.; Moreno-Martínez, E.; Miranda, R.; Castro, M. Role of Lactone Ring in Structural, Electronic, and Reactivity Properties of Aflatoxin B1: A Theoretical Study. Arch. Environ. Con Tox 2010, 59, 393–406. [Google Scholar] [CrossRef]
- Nascimento, C.; Santos, P.; Pereira, E.; Rocha, F. Recent advances on determination of milk adulterants. Food Chem. 2017, 221, 1232–1244. [Google Scholar] [CrossRef]
- Peng, T.; Ye, S.; Liu, R.; Qu, J. Colorimetric and fluorescent dual-signals probes for naked-eye detection of hydrogen peroxide and applications in milk samples and in vivo. Spectrochim. Acta A 2023, 297, 122757. [Google Scholar] [CrossRef]
- Belousov, A.; Parkhacheva, A.; Suleimanov, E.; Fukina, D.; Markov, A.; Vorotyntsev, A.; Koroleva, A.; Zhizhin, E.; Shafiq, I. Design of visible light-responsive CsM0.25W1.75O6 (M = Ni, Co, Mn, Cu) β-pyrochlore oxides with enhanced photocatalytic activity towards a set of pollutants. Ceram. Int. 2024, 50, 45334–45352. [Google Scholar] [CrossRef]
- Pacheco-Alvarez, M.O.A.; Sevillano-Arredondo, R.M.; Serrano, O.; Peralta-Hernandez, J.M. Copper-PANI-graphite HB2 composite for eco-friendly efficient degradation of textile dyes: Advancements in wastewater treatment enhanced by solar radiation. Chemosphere 2024, 366, 143537. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Dourandish, Z.; Zhang, K.; Le, Q.; Jang, H.; Shokouhimehr, M. Recent advances in the aptamer-based electrochemical biosensors for detecting aflatoxin B1 and its pertinent metabolite aflatoxin M1. Sensors 2022, 20, 3256. [Google Scholar] [CrossRef]
- Kurup, A.H.; Patras, A.; Pendyala, B.; Vergne, M.J.; Bansode, R.R. Evaluation of ultraviolet-light (UV-A) emitting diodes technology on the reduction of spiked aflatoxin B1 and aflatoxin M1 in whole milk. Food Bioprocess Technol 2021, 15, 165–176. [Google Scholar] [CrossRef]
- Bodbodak, S.; Hesari, J.; Peighambardoust, S.; Mahkam, M. Selective decontamination of aflatoxin M1 in milk by molecularly imprinted polymer coated on the surface of stainless steel plate. Int. J. Dairy Technol 2018, 71, 868–878. [Google Scholar] [CrossRef]
- Sangare, L.; Zhao, Y.; Folly, Y.; Chang, J.; Li, J.; Selvaraj, J.; Liu, Y. Aflatoxin B1 Degradation by a Pseudomonas Strain. Toxins 2014, 6, 3028–3040. [Google Scholar] [CrossRef]
- Du, X.; Zheng, M.; Zhang, H.; Qiu, Y.; Ji, F.; Nie, Z.; Xia, Y. New application of a dye-decolorizing peroxidase immobilized on magnetic nanoparticles for efficient simultaneous degradation of two mycotoxins. Food Chem. 2025, 463, 141341. [Google Scholar] [CrossRef]
- Zhou, L.; Duan, X.; Dai, J.; Ma, Y.; Yang, Q.; Hou, X. A covalent-organic framework-based platform for simultaneous smartphone detection and degradation of aflatoxin B1. Talanta 2024, 278, 126505. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Zhu, W.; Zhu, X.; Zhang, J.; Yang, J.; Wang, H.; Mo, X.; Zhang, C.; Wu, L. Self-Sufficient Aflatoxin Decontamination System: MOF-Based Composite Membrane with Peroxidase-Mimic and Controlled H2O2 Generation. Toxins 2025, 17, 516. https://doi.org/10.3390/toxins17100516
Cheng X, Zhu W, Zhu X, Zhang J, Yang J, Wang H, Mo X, Zhang C, Wu L. Self-Sufficient Aflatoxin Decontamination System: MOF-Based Composite Membrane with Peroxidase-Mimic and Controlled H2O2 Generation. Toxins. 2025; 17(10):516. https://doi.org/10.3390/toxins17100516
Chicago/Turabian StyleCheng, Xiaofei, Wenzhong Zhu, Xueting Zhu, Jinmin Zhang, Jia Yang, Huali Wang, Xiaoqin Mo, Chi Zhang, and Lina Wu. 2025. "Self-Sufficient Aflatoxin Decontamination System: MOF-Based Composite Membrane with Peroxidase-Mimic and Controlled H2O2 Generation" Toxins 17, no. 10: 516. https://doi.org/10.3390/toxins17100516
APA StyleCheng, X., Zhu, W., Zhu, X., Zhang, J., Yang, J., Wang, H., Mo, X., Zhang, C., & Wu, L. (2025). Self-Sufficient Aflatoxin Decontamination System: MOF-Based Composite Membrane with Peroxidase-Mimic and Controlled H2O2 Generation. Toxins, 17(10), 516. https://doi.org/10.3390/toxins17100516



