One Bloom Is Not Like the Other—Distinct Environmental Drivers Result in Domoic Acid Events in Monterey Bay, California
Abstract
1. Introduction
2. Results
2.1. Toxin Events
2.2. Physical Environment
2.3. Biological Environment
2.4. Chemical Environment
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Online Data Acquisition
5.2. SPATT Sampling
5.3. Untargeted LCMS/MS
5.4. Dereplication and Molecular Networking
5.5. Statistics and Visualization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| ASP | Amnesiac shellfish poisoning |
| BEUTI | Biologically effective upwelling transport index |
| CCS | California current system |
| Chl | Chlorophyll |
| cDA | Cellular domoic acid |
| DA | Domoic acid |
| ENSO | El Niño Southern Oscillation |
| ESI | Electrospray ionization |
| FBMN | Feature based molecular networking |
| GF/F | Glass fiber filter |
| GNPS | Global natural products social molecular networking |
| HABs | Harmful algal blooms |
| LCMS/MS | Liquid chromotagraphy tandem mass spectrometry |
| MeOH | Methanol |
| MQ | Milli-Q water |
| NDBC | National data buoy center |
| NPA | Natural product atlas |
| PCA | Principal component analysis |
| pDA | Particulate domoic acid |
| RAI | Relative abundant index |
| SCW | Santa Cruz Municipal Wharf |
| SPATT | Solid-phase adsorption toxin tracking |
References
- Tsikoti, C.; Genitsaris, S. Review of Harmful Algal Blooms in the Coastal Mediterranean Sea, with a Focus on Greek Waters. Diversity 2021, 13, 396. [Google Scholar] [CrossRef]
- Anderson, C.R.; Berdalet, E.; Kudela, R.M.; Cusack, C.K.; Silke, J.; O’Rourke, E.; Dugan, D.; McCammon, M.; Newton, J.A.; Moore, S.K.; et al. Scaling Up From Regional Case Studies to a Global Harmful Algal Bloom Observing System. Front. Mar. Sci. 2019, 6, 250. [Google Scholar] [CrossRef]
- Petroff, R.; Hendrix, A.; Shum, S.; Grant, K.S.; Lefebvre, K.A.; Burbacher, T.M. Public Health Risks Associated with Chronic, Low-Level Domoic Acid Exposure: A Review of the Evidence. Pharmacol. Ther. 2021, 227, 107865. [Google Scholar] [CrossRef]
- Bates, S.S.; Hubbard, K.A.; Lundholm, N.; Montresor, M.; Leaw, C.P. Pseudo-Nitzschia, Nitzschia, and Domoic Acid: New Research since 2011. Harmful Algae 2018, 79, 3–43. [Google Scholar] [CrossRef]
- Bargu, S.; Powell, C.L.; Wang, Z.; Doucette, G.J.; Silver, M.W. Note on the Occurrence of Pseudo-nitzschia Australis and Domoic Acid in Squid from Monterey Bay, CA (USA). Harmful Algae 2008, 7, 45–51. [Google Scholar] [CrossRef]
- Bernstein, S.; Ruiz-Cooley, R.I.; Kudela, R.; Anderson, C.R.; Dunkin, R.; Field, J.C. Stable Isotope Analysis Reveals Differences in Domoic Acid Accumulation and Feeding Strategies of Key Vectors in a California Hotspot for Outbreaks. Harmful Algae 2021, 110, 102117. [Google Scholar] [CrossRef]
- Kvrgić, K.; Lešić, T.; Džafić, N.; Pleadin, J. Occurrence and Seasonal Monitoring of Domoic Acid in Three Shellfish Species from the Northern Adriatic Sea. Toxins 2022, 14, 33. [Google Scholar] [CrossRef]
- Scholin, C.A.; Gulland, F.; Doucette, G.J.; Benson, S.; Busman, M.; Chavez, F.P.; Cordaro, J.; DeLong, R.; De Vogelaere, A.; Harvey, J.; et al. Mortality of Sea Lions along the Central California Coast Linked to a Toxic Diatom Bloom. Nature 2000, 403, 80–84. [Google Scholar] [CrossRef]
- Work, T.M.; Barr, B.; Beale, A.M.; Fritz, L.; Michael, A.; Wright, J.L.C.; Url, S. Epidemiology of Domoic Acid Poisoning in Brown Pelicans (Pelecanus Occidentalis ) and Brandt’ s Cormorants (Phalacrocorax Penicillatus ) in California. J. Zoo Wildl. Med. 1993, 24, 54–62. [Google Scholar]
- Moriarty, M.E.; Tinker, M.T.; Miller, M.A.; Tomoleoni, J.A.; Staedler, M.M.; Fujii, J.A.; Batac, F.I.; Dodd, E.M.; Kudela, R.M.; Zubkousky-White, V.; et al. Exposure to Domoic Acid Is an Ecological Driver of Cardiac Disease in Southern Sea Otters. Harmful Algae 2021, 101, 101973. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Quakenbush, L.; Frame, E.; Huntington, K.B.; Sheffield, G.; Stimmelmayr, R.; Bryan, A.; Kendrick, P.; Ziel, H.; Goldstein, T.; et al. Prevalence of Algal Toxins in Alaskan Marine Mammals Foraging in a Changing Arctic and Subarctic Environment. Harmful Algae 2016, 55, 13–24. [Google Scholar] [CrossRef]
- Mazzillo, F.F.M.; Pomeroy, C.; Kuo, J.; Ramondi, P.T.; Prado, R.; Silver, M.W. Angler Exposure to Domoic Acid via Consumption of Contaminated Fishes. Aquat. Biol. 2010, 9, 1–12. [Google Scholar] [CrossRef]
- Dyson, K.; Huppert, D.D. Regional Economic Impacts of Razor Clam Beach Closures Due to Harmful Algal Blooms (HABs) on the Pacific Coast of Washington. Harmful Algae 2010, 9, 264–271. [Google Scholar] [CrossRef]
- McCabe, R.M.; Hickey, B.M.; Kudela, R.M.; Lefebvre, K.A.; Adams, N.G.; Bill, B.D.; Gulland, F.M.D.; Thomson, R.E.; Cochlan, W.P.; Trainer, V.L. An Unprecedented Coastwide Toxic Algal Bloom Linked to Anomalous Ocean Conditions. Geophys. Res. Lett. 2016, 43, 10366–10376. [Google Scholar] [CrossRef]
- Kenitz, K.M.; Anderson, C.R.; Carter, M.L.; Eggleston, E.; Seech, K.; Shipe, R.; Smith, J.; Orenstein, E.C.; Franks, P.J.S.; Jaffe, J.S.; et al. Environmental and Ecological Drivers of Harmful Algal Blooms Revealed by Automated Underwater Microscopy. Limnol. Oceanogr. 2023, 68, 598–615. [Google Scholar] [CrossRef]
- Chavez, F.P.; Messié, M. A Comparison of Eastern Boundary Upwelling Ecosystems. Prog. Oceanogr. 2009, 83, 80–96. [Google Scholar] [CrossRef]
- Skogsberg, T. Hydrography of Monterey Bay, California. Thermal Conditions, 1929–1933. Trans. Am. Philos. Soc. 1936, 29, 125–152. [Google Scholar] [CrossRef]
- Kudela, R.; Chavez, F. The Impact of Coastal Runoff on Ocean Color during an El Niño Year in Central California. Deep Sea Res. Part II Top. Stud. Oceanogr. 2004, 51, 1173–1185. [Google Scholar] [CrossRef]
- Sandoval-Belmar, M.; Smith, J.; Moreno, A.R.; Anderson, C.; Kudela, R.M.; Sutula, M.; Kessouri, F.; Caron, D.A.; Chavez, F.P.; Bianchi, D. A Cross-Regional Examination of Patterns and Environmental Drivers of Pseudo-nitzschia Harmful Algal Blooms along the California Coast. Harmful Algae 2023, 126, 102435. [Google Scholar] [CrossRef]
- Schnetzer, A.; Jones, B.H.; Schaffner, R.A.; Cetinic, I.; Fitzpatrick, E.; Miller, P.E.; Seubert, E.L.; Caron, D.A. Coastal Upwelling Linked to Toxic Pseudo-nitzschia Australis Blooms in Los Angeles Coastal Waters, 2005–2007. J. Plankton Res. 2013, 35, 1080–1092. [Google Scholar] [CrossRef]
- Brunson, J.K.; Thukral, M.; Ryan, J.P.; Anderson, C.R.; Kolody, B.C.; James, C.C.; Chavez, F.P.; Leaw, C.P.; Rabines, A.J.; Venepally, P.; et al. Molecular Forecasting of Domoic Acid during a Pervasive Toxic Diatom Bloom. Proc. Natl. Acad. Sci. USA 2024, 121, e2319177121. [Google Scholar] [CrossRef]
- Hasle, G.R. Are Most of the Domoic Acid-Producing Species of the Diatom Genus Pseudo-nitzschia Cosmopolites? Harmful Algae 2002, 1, 137–146. [Google Scholar] [CrossRef]
- Harðardóttir, S.; Pančić, M.; Tammilehto, A.; Krock, B.; Møller, E.; Nielsen, T.; Lundholm, N. Dangerous Relations in the Arctic Marine Food Web: Interactions between Toxin Producing Pseudo-nitzschia Diatoms and Calanus Copepodites. Mar. Drugs 2015, 13, 3809–3835. [Google Scholar] [CrossRef]
- Lundholm, N.; Krock, B.; John, U.; Skov, J.; Cheng, J.; Pančić, M.; Wohlrab, S.; Rigby, K.; Nielsen, T.G.; Selander, E.; et al. Induction of Domoic Acid Production in Diatoms—Types of Grazers and Diatoms Are Important. Harmful Algae 2018, 79, 64–73. [Google Scholar] [CrossRef]
- Sison-Mangus, M.P.; Jiang, S.; Kudela, R.M.; Mehic, S. Phytoplankton-Associated Bacterial Community Composition and Succession during Toxic Diatom Bloom and Non-Bloom Events. Front. Microbiol. 2016, 7, 1433. [Google Scholar] [CrossRef]
- Graham, W.M.; Largier, J.L. Upwelling Shadows as Nearshore Retention Sites: The Example of Northern Monterey Bay. Cont. Shelf Res. 1997, 17, 509–532. [Google Scholar] [CrossRef]
- Schulien, J.A.; Peacock, M.B.; Hayashi, K.; Raimondi, P.; Kudela, R.M. Phytoplankton and Microbial Abundance and Bloom Dynamics in the Upwelling Shadow of Monterey Bay, California, from 2006 to 2013. Mar. Ecol. Prog. Ser. 2017, 572, 43–56. [Google Scholar] [CrossRef]
- Trapp, A.; Hayashi, K.; Fiechter, J.; Kudela, R.M. What Happens in the Shadows—Influence of Seasonal and Non-Seasonal Dynamics on Domoic Acid Monitoring in the Monterey Bay Upwelling Shadow. Harmful Algae 2023, 129, 102522. [Google Scholar] [CrossRef]
- MacKenzie, L.A. In Situ Passive Solid-Phase Adsorption of Micro-Algal Biotoxins as a Monitoring Tool. Curr. Opin. Biotechnol. 2010, 21, 326–331. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Chinain, M. Solid-phase Adsorption Toxin Tracking (Spatt) Technology for the Monitoring of Aquatic Toxins: A Review. Toxins 2018, 10, 167. [Google Scholar] [CrossRef]
- Thukral, M.; Allen, A.E.; Petras, D. Progress and Challenges in Exploring Aquatic Microbial Communities Using Non-Targeted Metabolomics. ISME J. 2023, 17, 2147–2159. [Google Scholar] [CrossRef]
- Bogdanov, A.; Salib, M.N.; Chase, A.B.; Hammerlindl, H.; Muskat, M.N.; Luedtke, S.; Da Silva, E.B.; O’Donoghue, A.J.; Wu, L.F.; Altschuler, S.J.; et al. Small Molecule in Situ Resin Capture Provides a Compound First Approach to Natural Product Discovery. Nat. Commun. 2024, 15, 5230. [Google Scholar] [CrossRef]
- Ryan, J.P.; Kudela, R.M.; Birch, J.M.; Blum, M.; Bowers, H.A.; Chavez, F.P.; Doucette, G.J.; Hayashi, K.; Marin, R.; Mikulski, C.M.; et al. Causality of an Extreme Harmful Algal Bloom in Monterey Bay, California, during the 2014–2016 Northeast Pacific Warm Anomaly. Geophys. Res. Lett. 2017, 44, 5571–5579. [Google Scholar] [CrossRef]
- Paduan, J.D.; Cook, M.S.; Tapia, V.M. Patterns of Upwelling and Relaxation around Monterey Bay Based on Long-Term Observations of Surface Currents from High Frequency Radar. Deep Sea Res. Part II Top. Stud. Oceanogr. 2018, 151, 129–136. [Google Scholar] [CrossRef]
- Trainer, V.L.; Hickey, B.M.; Horner, R.A. Biological and Physical Dynamics of Domoic Acid Production off the Washington Coast. Limnol. Oceanogr. 2002, 47, 1438–1446. [Google Scholar] [CrossRef]
- Tweddle, J.; Strutton, P.; Foley, D.; O’Higgins, L.; Wood, A.; Scott, B.; Everroad, R.; Peterson, W.; Cannon, D.; Hunter, M.; et al. Relationships among Upwelling, Phytoplankton Blooms, and Phycotoxins in Coastal Oregon Shellfish. Mar. Ecol. Prog. Ser. 2010, 405, 131–145. [Google Scholar] [CrossRef]
- Bowers, H.A.; Ryan, J.P.; Hayashi, K.; Woods, A.L.; Marin, R.; Smith, G.J.; Hubbard, K.A.; Doucette, G.J.; Mikulski, C.M.; Gellene, A.G.; et al. Diversity and Toxicity of Pseudo-nitzschia Species in Monterey Bay: Perspectives from Targeted and Adaptive Sampling. Harmful Algae 2018, 78, 129–141. [Google Scholar] [CrossRef]
- Lecher, A.L.; Mackey, K.; Kudela, R.; Ryan, J.; Fisher, A.; Murray, J.; Paytan, A. Nutrient Loading through Submarine Groundwater Discharge and Phytoplankton Growth in Monterey Bay, CA. Environ. Sci. Technol. 2015, 49, 6665–6673. [Google Scholar] [CrossRef]
- Lane, J.Q.; Raimondi, P.T.; Kudela, R.M. Development of a Logistic Regression Model for the Prediction of Toxigenic Pseudo-nitzschia Blooms in Monterey Bay, California. Mar. Ecol. Prog. Ser. 2009, 383, 37–51. [Google Scholar] [CrossRef]
- Lelong, A.; Hégaret, H.; Soudant, P.; Bates, S.S. Pseudo-nitzschia (Bacillariophyceae) Species, Domoic Acid and Amnesic Shellfish Poisoning: Revisiting Previous Paradigms. Phycologia 2012, 51, 168–216. [Google Scholar] [CrossRef]
- Pednekar, S.M.; Bates, S.S.; Kerkar, V.; Matondkar, S.G.P. Environmental Factors Affecting the Distribution of Pseudo-nitzschia in Two Monsoonal Estuaries of Western India and Effects of Salinity on Growth and Domoic Acid Production by P. pungens. Estuaries Coasts 2018, 41, 1448–1462. [Google Scholar] [CrossRef]
- Ryan, J.P.; Fischer, A.M.; Kudela, R.M.; Gower, J.F.R.; King, S.A.; Marin, R.; Chavez, F.P. Influences of Upwelling and Downwelling Winds on Red Tide Bloom Dynamics in Monterey Bay, California. Cont. Shelf Res. 2009, 29, 785–795. [Google Scholar] [CrossRef]
- Ryan, J.P.; Gower, J.F.R.; King, S.A.; Bissett, W.P.; Fischer, A.M.; Kudela, R.M.; Kolber, Z.; Mazzillo, F.; Rienecker, E.V.; Chavez, F.P. A Coastal Ocean Extreme Bloom Incubator. Geophys. Res. Lett. 2008, 35, L12602. [Google Scholar] [CrossRef]
- Quijano-Scheggia, S.I.; Olivos-Ortiz, A.; Garcia-Mendoza, E.; Sánchez-Bravo, Y.; Sosa-Avalos, R.; Salas Marias, N.; Lim, H.C. Phylogenetic Relationships of Pseudo-nitzschia Subpacifica (Bacillariophyceae) from the Mexican Pacific, and Its Production of Domoic Acid in Culture. PLoS ONE 2020, 15, e0231902. [Google Scholar] [CrossRef]
- Heaney, S.I.; Eppley, R.W. Light, Temperature and Nitrogen as Interacting Factors Affecting Diel Vertical Migrations of Dinoflagellates in Culture. J. Plankton Res. 1981, 3, 331–344. [Google Scholar] [CrossRef]
- Haroardóttir, S.; Wohlrab, S.; Hjort, D.M.; Krock, B.; Nielsen, T.G.; John, U.; Lundholm, N. Transcriptomic Responses to Grazing Reveal the Metabolic Pathway Leading to the Biosynthesis of Domoic Acid and Highlight Different Defense Strategies in Diatoms. BMC Mol. Biol. 2019, 20, 7. [Google Scholar] [CrossRef]
- Selander, E.; Berglund, E.C.; Engström, P.; Berggren, F.; Eklund, J.; Harðardóttir, S.; Lundholm, N.; Grebner, W.; Andersson, M.X. Copepods Drive Large-Scale Trait-Mediated Effects in Marine Plankton. Sci. Adv. 2019, 5, 3–9. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between Diatoms and Bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef]
- Koester, I.; Quinlan, Z.A.; Nothias, L.; White, M.E.; Rabines, A.; Petras, D.; Brunson, J.K.; Dührkop, K.; Ludwig, M.; Böcker, S.; et al. Illuminating the Dark Metabolome of Pseudo-nitzschia–Microbiome Associations. Environ. Microbiol. 2022, 24, 5408–5424. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takata, Y.; Kodama, M. Direct Contact between Pseudo-nitzschia Multiseries and Bacteria Is Necessary for the Diatom to Produce a High Level of Domoic Acid. Fish. Sci. 2009, 75, 771–776. [Google Scholar] [CrossRef]
- Sison-Mangus, M.P.; Jiang, S.; Tran, K.N.; Kudela, R.M. Host-Specific Adaptation Governs the Interaction of the Marine Diatom, Pseudo-nitzschia and Their Microbiota. ISME J. 2014, 8, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Brunson, J.K.; McKinnie, S.M.K.; Chekan, J.R.; McCrow, J.P.; Miles, Z.D.; Bertrand, E.M.; Bielinski, V.A.; Luhavaya, H.; Oborník, M.; Smith, G.J.; et al. Biosynthesis of the Neurotoxin Domoic Acid in a Bloom-Forming Diatom. Science 2018, 361, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Steen, A.D.; Kusch, S.; Abdulla, H.A.; Cakić, N.; Coffinet, S.; Dittmar, T.; Fulton, J.M.; Galy, V.; Hinrichs, K.-U.; Ingalls, A.E.; et al. Analytical and Computational Advances, Opportunities, and Challenges in Marine Organic Biogeochemistry in an Era of “Omics”. Front. Mar. Sci. 2020, 7, 718. [Google Scholar] [CrossRef]
- Zendong, Z.; Bertrand, S.; Herrenknecht, C.; Abadie, E.; Jauzein, C.; Lemée, R.; Gouriou, J.; Amzil, Z.; Hess, P. Passive Sampling and High Resolution Mass Spectrometry for Chemical Profiling of French Coastal Areas with a Focus on Marine Biotoxins. Environ. Sci. Technol. 2016, 50, 8522–8529. [Google Scholar] [CrossRef]
- Tatters, A.O.; Smith, J.; Kudela, R.M.; Hayashi, K.; Howard, M.D.; Donovan, A.R.; Loftin, K.A.; Caron, D.A. The Tide Turns: Episodic and Localized Cross-Contamination of a California Coastline with Cyanotoxins. Harmful Algae 2021, 103, 102003. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Jacox, M.G.; Edwards, C.A.; Hazen, E.L.; Bograd, S.J. Coastal Upwelling Revisited: Ekman, Bakun, and Improved Upwelling Indices for the U.S. West Coast. J. Geophys. Res. Oceans 2018, 123, 7332–7350. [Google Scholar] [CrossRef]
- Miller, P.E.; Scholin, C.A. Identification and enumeration of cultured and wild Pseudo-nitzschia (bacillariophyceae) using species-specific LSU rRNA-targeted fluorescent probes and filter-based whole cell hybridization. J. Phycol. 1998, 34, 371–382. [Google Scholar] [CrossRef]
- Howard, M.D.A.; Hayashi, K.; Smith, J.; Kudela, R.; Caron, D. Standard Operating Procedure for Solid Phase Adsorption Toxin Testing (SPATT) Assemblage and Extraction of HAB Toxins; University of California and University of Southern California: Santa Cruz, CA, USA, 2018. [Google Scholar]
- Bateman, R.H.; Carruthers, R.; Hoyes, J.B.; Jones, C.; Langridge, J.I.; Millar, A.; Vissers, J.P.C. A Novel Precursor Ion Discovery Method on a Hybrid Quadrupole Orthogonal Acceleration Time-of-Flight (Q-TOF) Mass Spectrometer for Studying Protein Phosphorylation. J. Am. Soc. Mass Spectrom. 2002, 13, 792–803. [Google Scholar] [CrossRef]
- van Santen, J.A.; Poynton, E.F.; Iskakova, D.; McMann, E.; Alsup, T.A.; Clark, T.N.; Fergusson, C.H.; Fewer, D.P.; Hughes, A.H.; McCadden, C.A.; et al. The Natural Products Atlas 2.0: A Database of Microbially-Derived Natural Products. Nucleic Acids Res. 2022, 50, D1317–D1323. [Google Scholar] [CrossRef]
- Wolf, S.; Schmidt, S.; Müller-Hannemann, M.; Neumann, S. In Silico Fragmentation for Computer Assisted Identification of Metabolite Mass Spectra. BMC Bioinform. 2010, 11, 148. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- R Core Team. R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Kassambra, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses, R Package Version 1.0.7. [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Soft. 2008, 25, 1–18. [Google Scholar] [CrossRef]



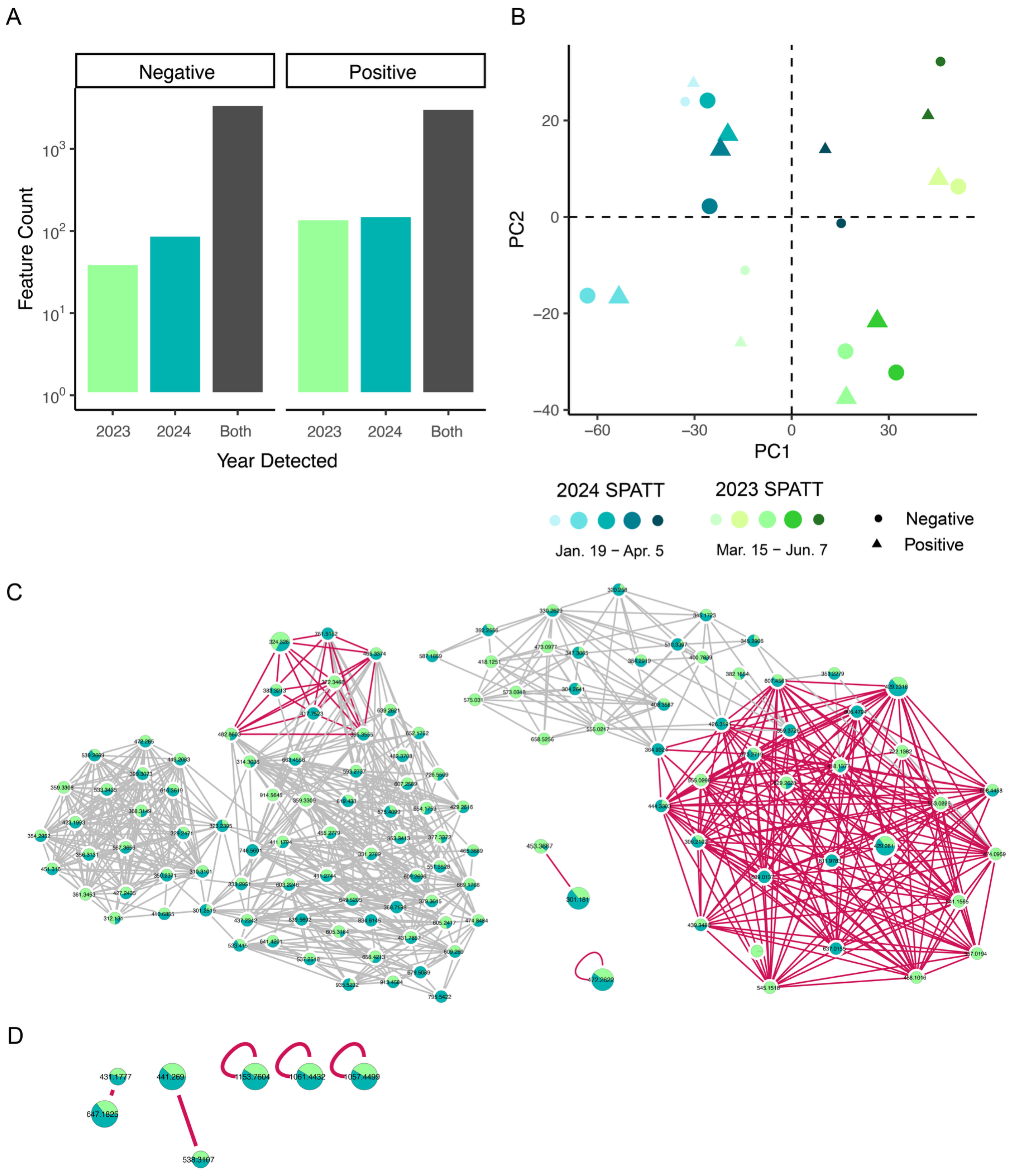

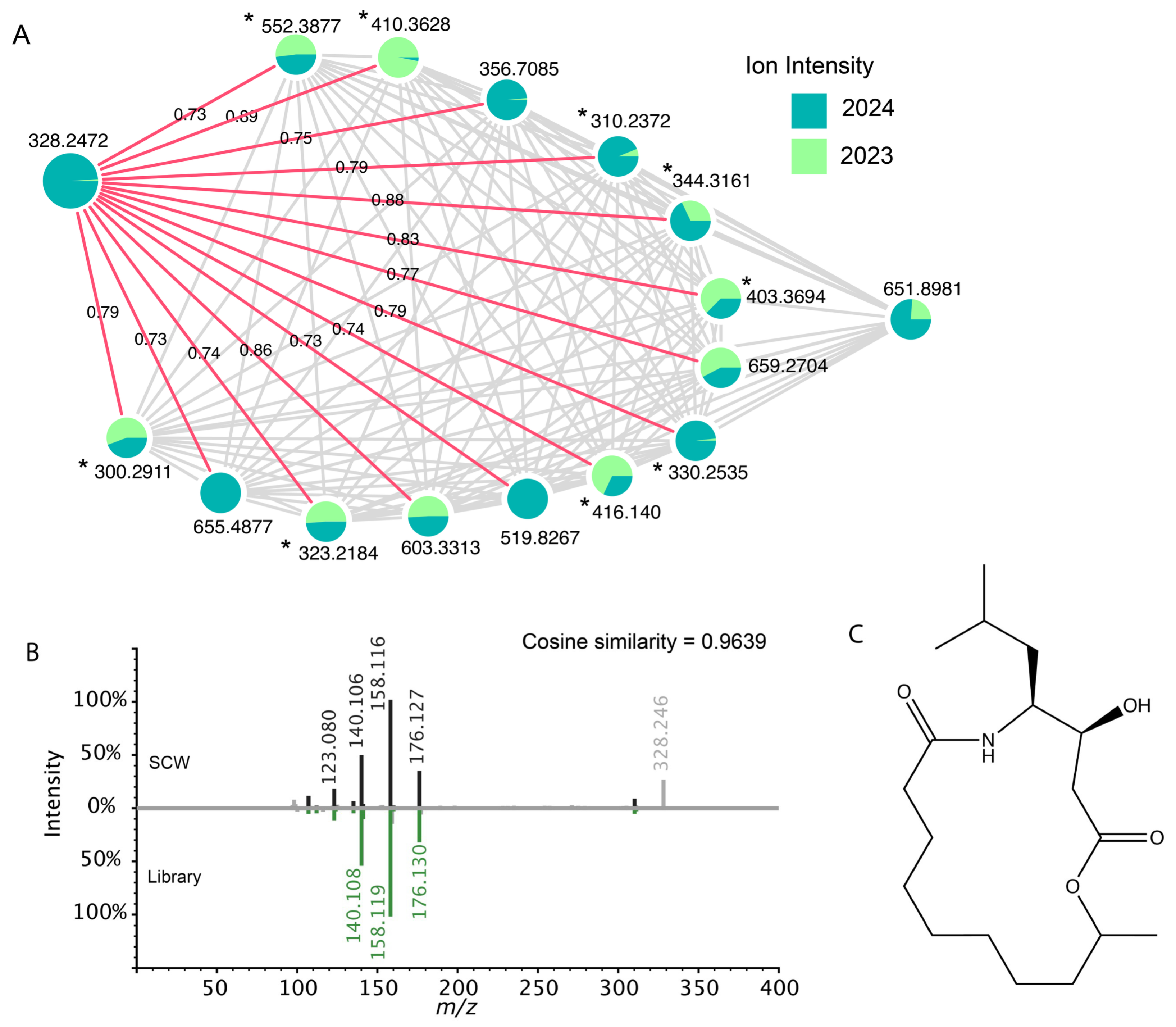

| Toxin Event | Event Mean (µg L−1) | Event Duration (Days) |
|---|---|---|
| April–May 2023 | 0.16 | 35 |
| February–March 2024 | 0.12 | 35 |
| March–June 2014 | 2.41 (Max) | 85 |
| May–September 2016 | 0.73 | 133 (Max) |
| Mean | 0.29 | 27 |
| ESI 2023 | Only 2023 | Only 2024 | Both Years | m/z Range | Intensity |
|---|---|---|---|---|---|
| Positive | 148 | 161 | 3269 | 300−1195 | 1.3−183,267.6 |
| Negative | 42 | 93 | 3646 | 300−1199 | 0.2−1,888,801.9 |
| Total | 6915 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trapp, A.; Baker, A.; Hayashi, K.; Kudela, R.M. One Bloom Is Not Like the Other—Distinct Environmental Drivers Result in Domoic Acid Events in Monterey Bay, California. Toxins 2025, 17, 511. https://doi.org/10.3390/toxins17100511
Trapp A, Baker A, Hayashi K, Kudela RM. One Bloom Is Not Like the Other—Distinct Environmental Drivers Result in Domoic Acid Events in Monterey Bay, California. Toxins. 2025; 17(10):511. https://doi.org/10.3390/toxins17100511
Chicago/Turabian StyleTrapp, Aubrey, Andrew Baker, Kendra Hayashi, and Raphael M. Kudela. 2025. "One Bloom Is Not Like the Other—Distinct Environmental Drivers Result in Domoic Acid Events in Monterey Bay, California" Toxins 17, no. 10: 511. https://doi.org/10.3390/toxins17100511
APA StyleTrapp, A., Baker, A., Hayashi, K., & Kudela, R. M. (2025). One Bloom Is Not Like the Other—Distinct Environmental Drivers Result in Domoic Acid Events in Monterey Bay, California. Toxins, 17(10), 511. https://doi.org/10.3390/toxins17100511






