Comprehensive Study of Some Cyanobacteria in Moscow Waterbodies (Russia), Including Characteristics of the Toxigenic Microcystis aeruginosa Strains
Abstract
1. Introduction
2. Results
2.1. Morphological Analysis
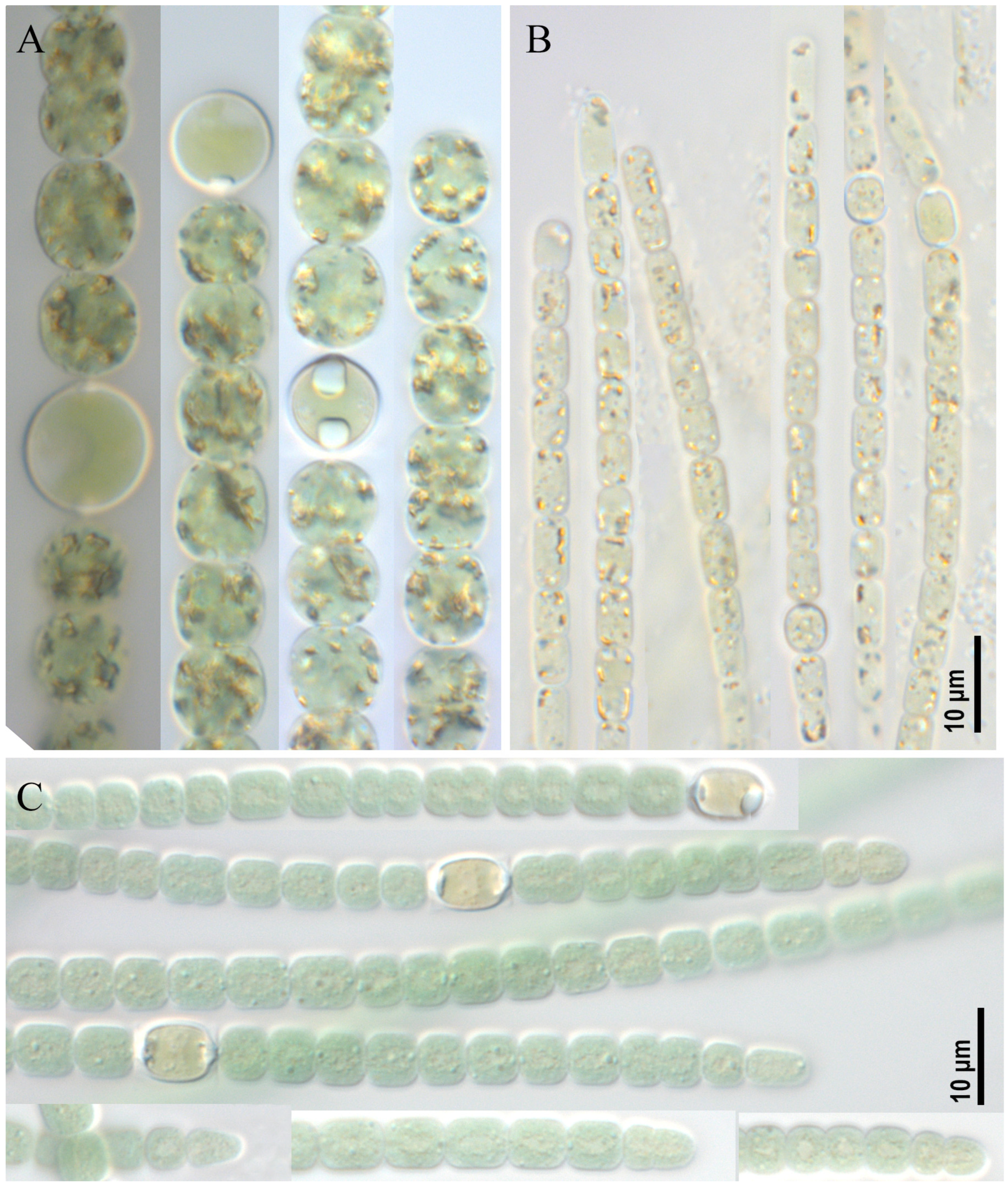
- Dolichospermum sp. strain CBMC469m (Figure 1A)
- Aphanizomenon sp. strain CBMC479m (Figure 1B)
- Anabaena sp. strain CBMC473m (Figure 1C)
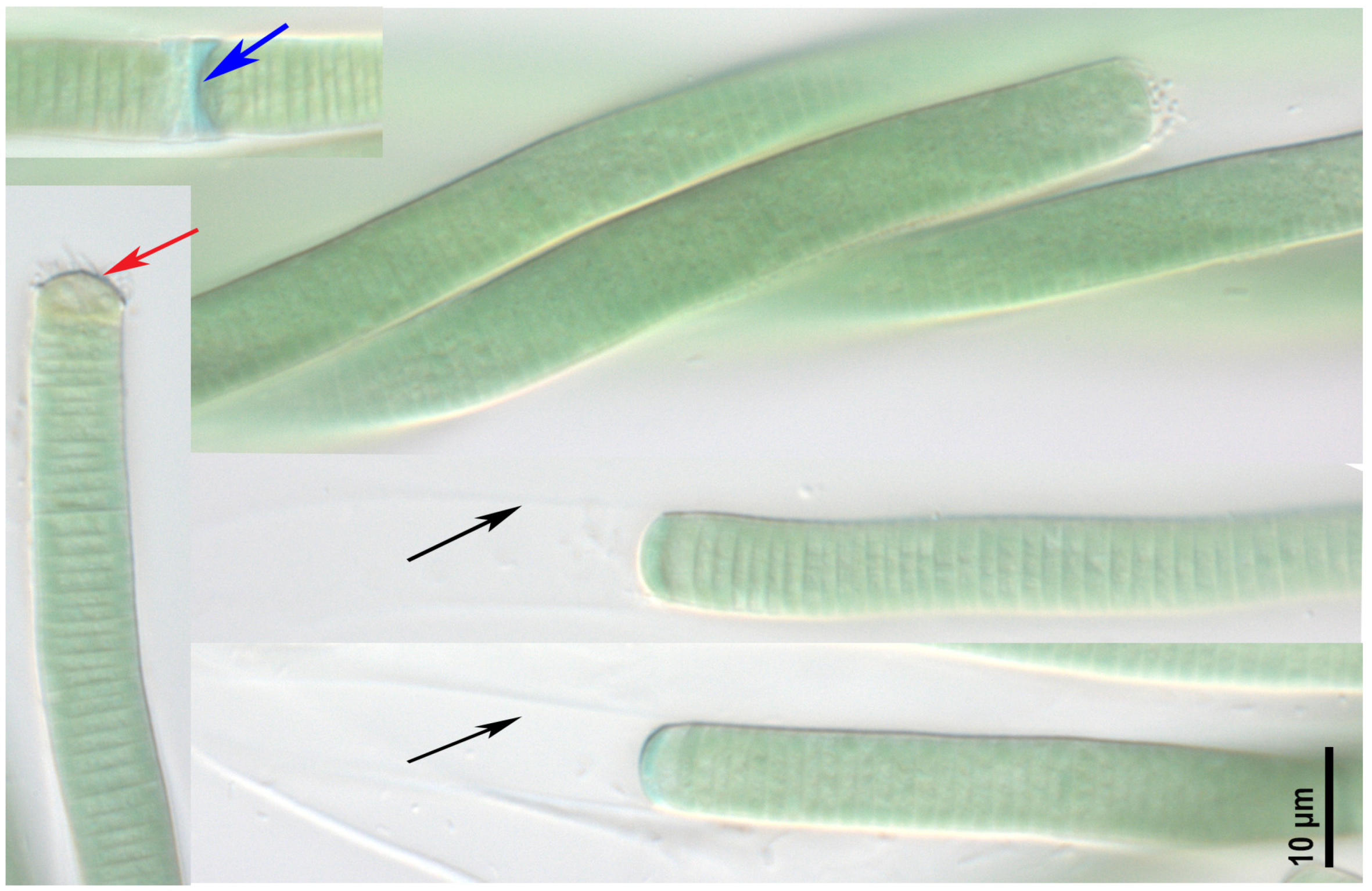
- Argonema galeatum strain CBMC475m (Figure 2)
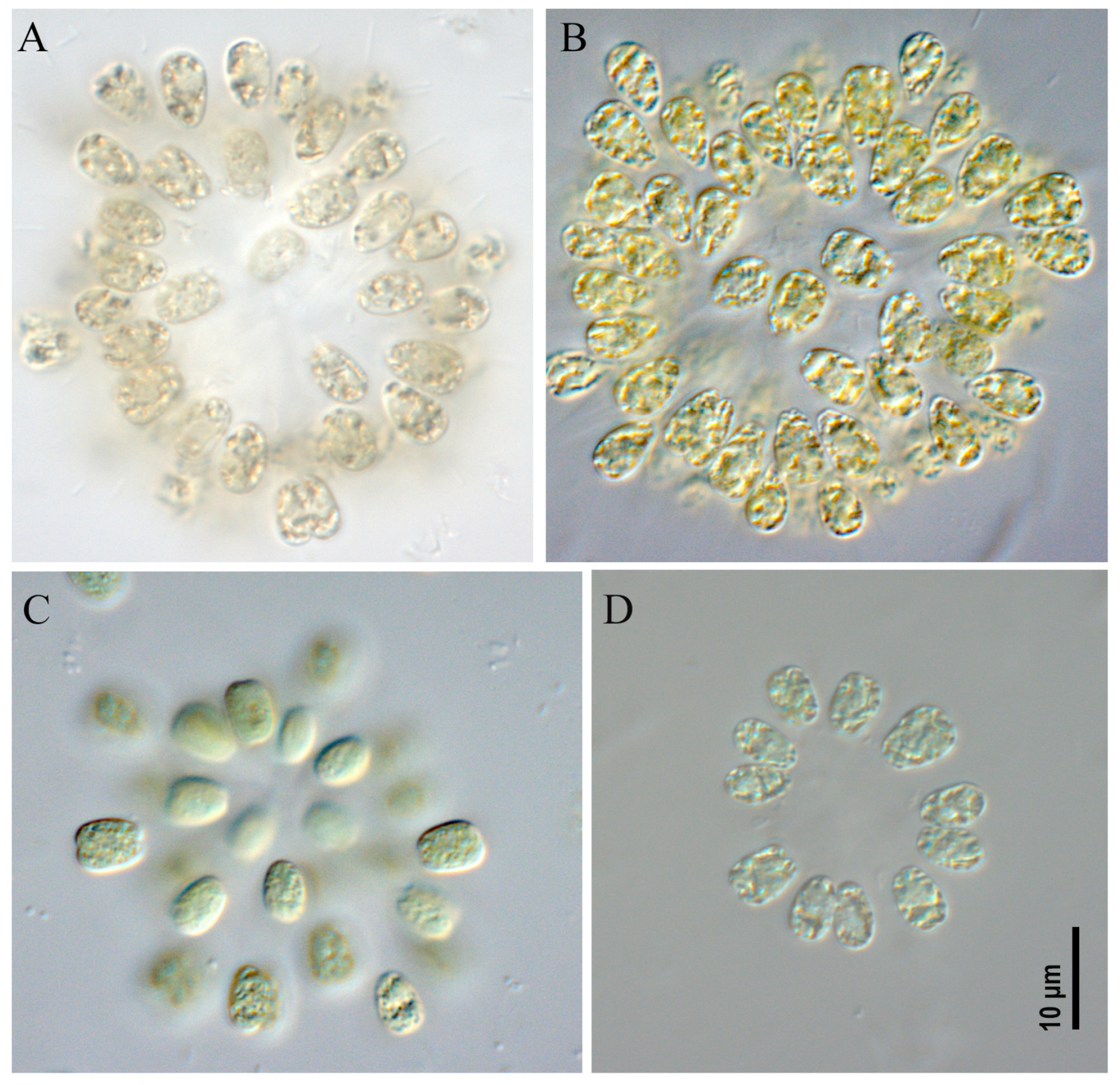
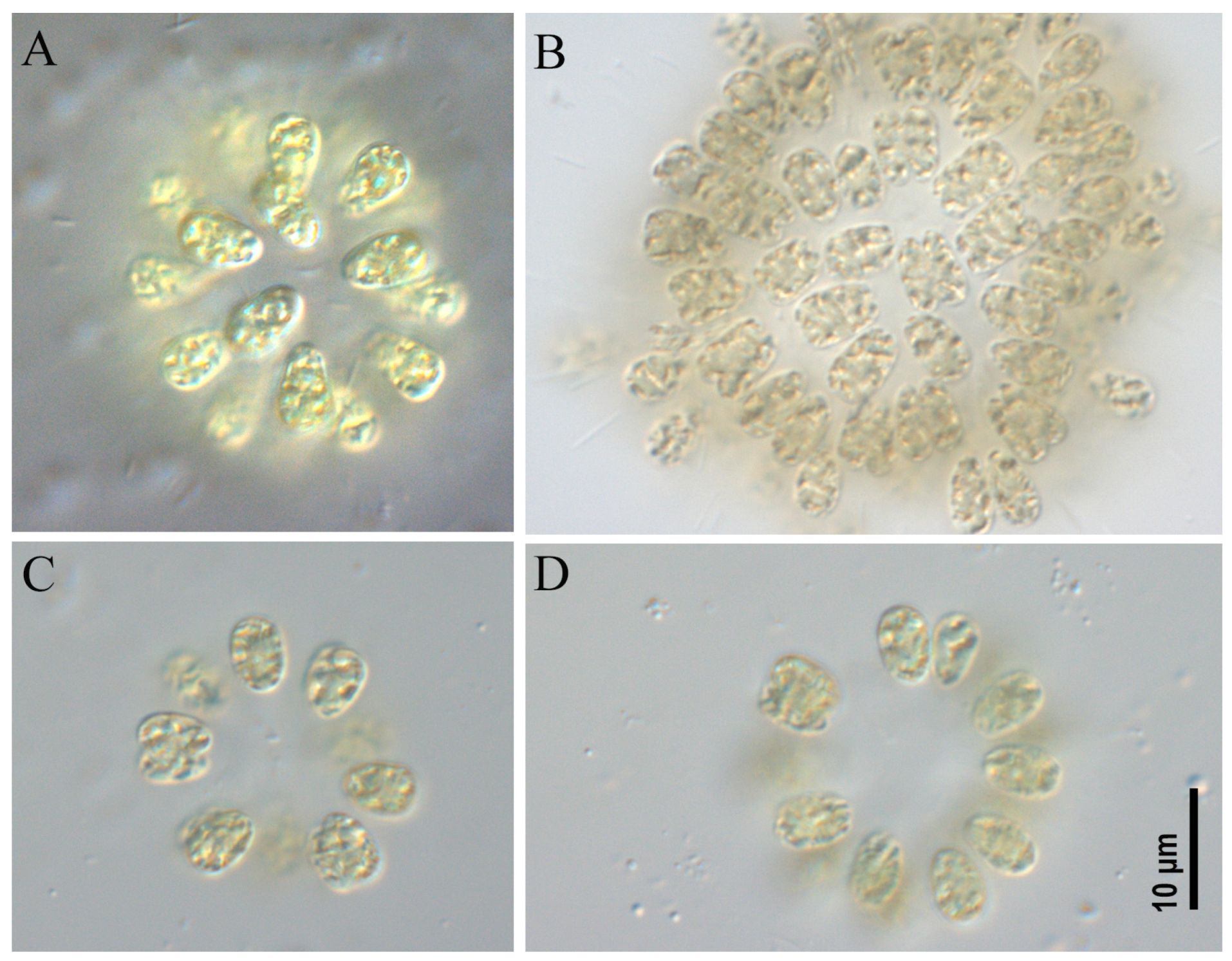
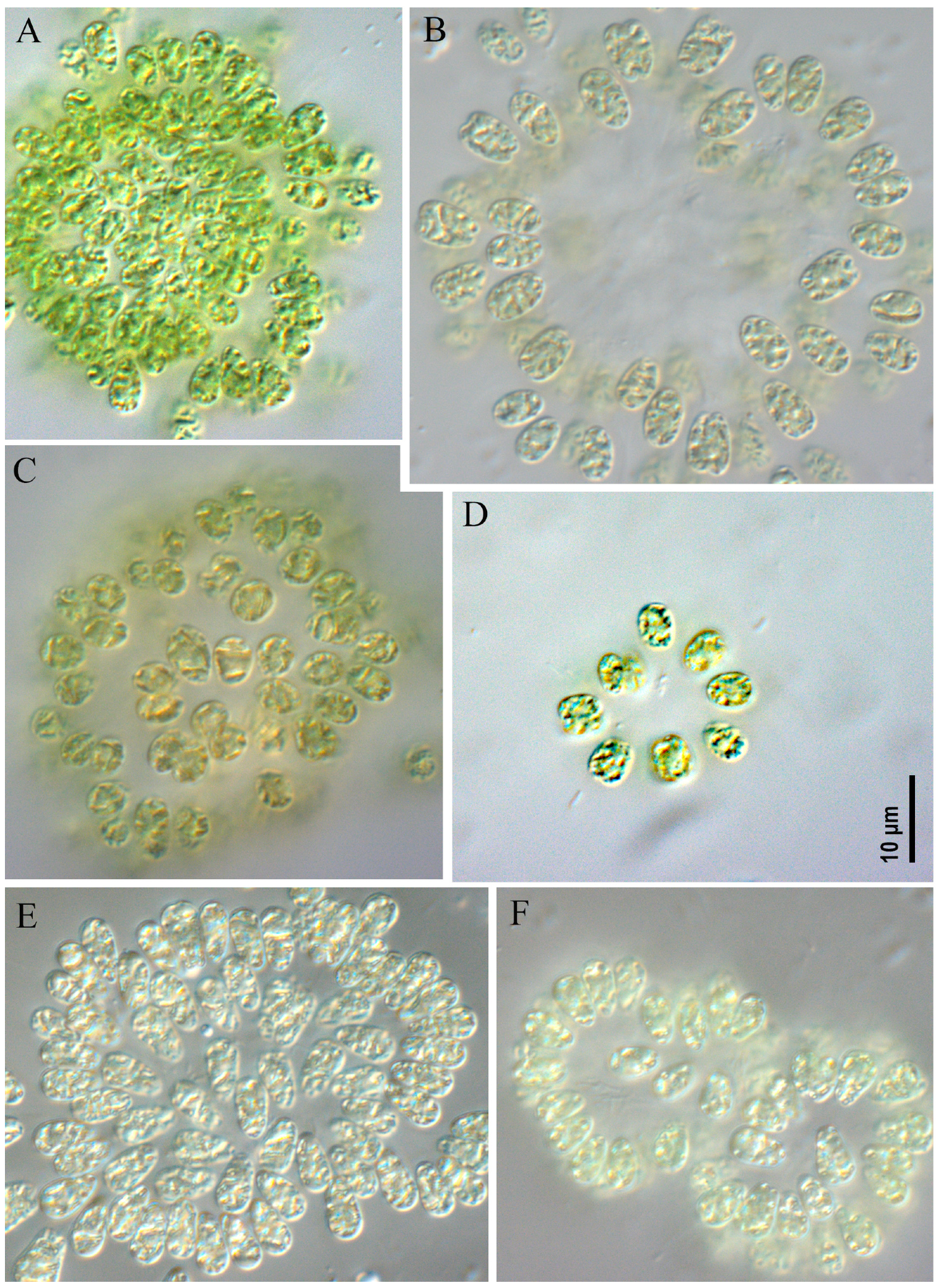
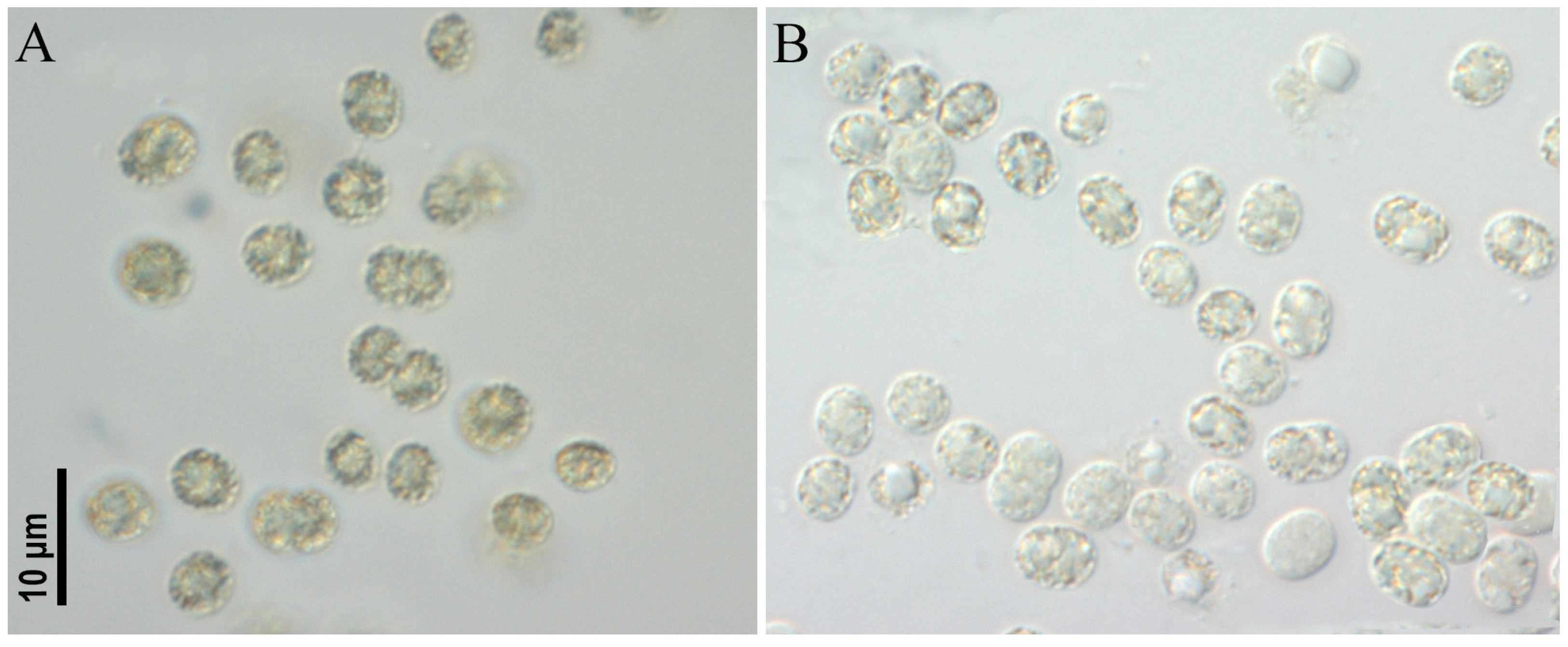
- Microcystis aeruginosa strains CBMC403m, CBMC523m (Figure 6)
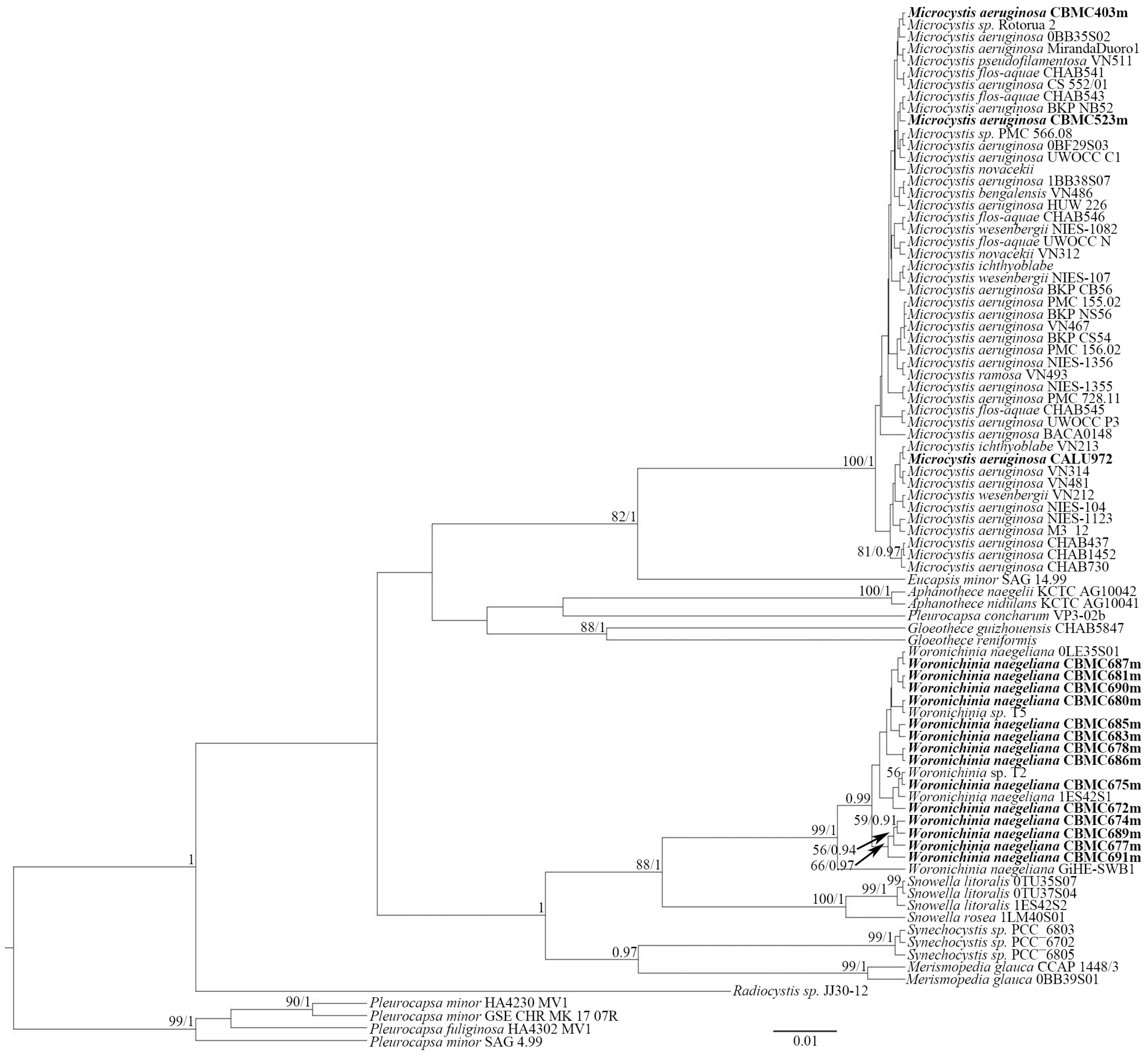
2.2. Molecular Analysis
2.3. Detection of Cyanotoxin-Producing Genes by PCR
2.4. Detection of Microcystins by HPLC-HRMS
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection and Preparation
5.2. Culturing, Microscopy and Morphological Identification
5.3. DNA Extraction and Amplification
5.4. HPLC-HRMS Toxin Analysis
5.5. Phylogenetic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANA | Anatoxin |
| BI | Bayesian Inference |
| CyanoHABs | Cyanobacterial Harmful Algal Blooms |
| CYN | Cylindrospermopsin |
| HPLC-HRMS | High Performance Liquid Chromatography–High Resolution Mass Spectrometry |
| LB | Likelihood Bootstrap |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| LOD | Limit Of Detection |
| LOQ | Limit Of Quantitation |
| MC | Microcystin |
| MC-LA | Microcystin–leucine alanine |
| MC-LF | Microcystin–leucine phenylalanine |
| MC-LR | Microcystin–leucine arginine |
| MC-LW | Microcystin–leucine tryptophan |
| MC-LY | Microcystin–leucine tyrosine |
| MC-RR | Microcystin–arginine arginine |
| MC-YR | Microcystin–tyrosine arginine |
| ML | Maximum Likelihood |
| NCBI SRA | National Center for Biotechnology Information Sequence Read Archive |
| PP | Posterior Probability |
| RAxML | Rapid Accelerationx Maximum Likelihood |
References
- Sivonen, K.; Jones, G. Cyanobacterial toxins. Toxic Cyanobacteria. In Water, a Guide to Their Health Consequences, Monitoring and Management; E. and FN Spon (on Behalf of WHO): London, UK, 1999; Volume 1, pp. 43–112. [Google Scholar]
- Mur, L.R.; Skulberg, O.M.; Utkilen, H. Cyanobacteria in the environment. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 1st ed.; Bartram, J., Chorus, I., Eds.; E. and FN Spon (on Behalf of WHO): London, UK, 1999; pp. 15–40. [Google Scholar]
- Carmichael, W. A World Overview—One-hundred-twenty-seven years of research on toxic cyanobacteria—Where do we go from here? In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: New York, NY, USA, 2008; pp. 105–125. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S., III. Ecology of Harmful Cyanobacteria. In Ecology of Harmful Algae; Granéli, E., Turner, J.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 95–109. [Google Scholar] [CrossRef]
- Melaram, R.; Newton, A.R.; Lee, A.; Herber, S.; El-Khouri, A.; Chafin, J. A review of microcystin and nodularin toxins derived from freshwater cyanobacterial harmful algal blooms and their impact on human health. ToxEHS 2024, 16, 233–241. [Google Scholar] [CrossRef]
- Kim, I.-S.; Park, H.-K. Molecular quantification of total and toxigenic Microcystis using digital-droplet-polymerase-chain-reaction-based multiplex assay. Toxins 2025, 17, 242. [Google Scholar] [CrossRef]
- Villalobos, V.I.; Morales-Torres, D.F.; Valdivia, N.; Rivera-Hechem, M.I.; Giesecke, R.; Pinones, A.; Mardones, J.I.; Garcés-Vargas, J.; Segura, J.C.; Navarro, J.M.; et al. Responses of mussel farms to harmful algal bloom governance are shaped by the scale of production: Implications for equitable blue economy. Harmful Algae 2025, 144, 102821. [Google Scholar] [CrossRef]
- Ramos, V.; Moreira, C.; Mankiewicz-Boczek, J.; Vasconcelos, V. Application of Molecular Tools in Monitoring Cyanobacteria and Their Potential Toxin Production. In Molecular Tools for the Detection and Quantification of Toxigenic Cyanobacteria; Kurmayer, R., Sivonen, K., Wilmotte, A., Salmaso, N., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 310–313. [Google Scholar] [CrossRef]
- Babanazarova, O.; Sidelev, S.; Schischeleva, S. The structure of winter phytoplankton in Lake Nero, Russia, a hypertrophic lake dominated by Planktothrix-like Cyanobacteria. Aquat. Biosyst. 2013, 9, 18. [Google Scholar] [CrossRef]
- Sidelev, S.I.; Golokolenova, T.B.; Chernova, E.N.; Russkikh, Y.V. Analysis of phytoplankton in Tsimlyansk Reservoir (Russia) for the presence of cyanobacterial hepato-and neurotoxins. Microbiology 2015, 84, 828–837. [Google Scholar] [CrossRef]
- Sidelev, S.; Koksharova, O.; Babanazarova, O.; Fastner, J.; Chernova, E.; Gusev, E. Phylogeographic, toxicological and ecological evidence for the global distribution of Raphidiopsis raciborskii and its northernmost presence in Lake Nero, Central Western Russia. Harmful Algae 2020, 98, 101889. [Google Scholar] [CrossRef]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Voyakina, E.; Babanazarova, O.; Romanov, R.; Kotovshchikov, A.; Mazur-Marzec, H. Dolichospermum and Aphanizomenon as neurotoxins producers in some Russian freshwaters. Toxicon 2017, 130, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Voyakina, E.; Zhakovskaya, Z. First observation of microcystin-and anatoxin-a-producing cyanobacteria in the easternmost part of the Gulf of Finland (the Baltic Sea). Toxicon 2019, 157, 18–24. [Google Scholar] [CrossRef]
- Chernova, E.; Sidelev, S.; Russkikh, I.; Korneva, L.; Solovyova, V.; Mineeva, N.; Stepanova, I.; Zhakovskaya, Z. Spatial distribution of cyanotoxins and ratios of microcystin to biomass indicators in the reservoirs of the Volga, Kama and Don Rivers, the European part of Russia. Limnologica 2020, 84, 125819. [Google Scholar] [CrossRef]
- Evseev, P.; Tikhonova, I.; Krasnopeev, A.; Sorokovikova, E.; Gladkikh, A.; Timoshkin, O.; Miroshnikov, K.; Belykh, O. Tychonema sp. BBK16 characterisation: Lifestyle, phylogeny and related phages. Viruses 2023, 15, 442. [Google Scholar] [CrossRef] [PubMed]
- Gabyshev, V.A.; Sidelev, S.I.; Chernova, E.N.; Gabysheva, O.I.; Voronov, I.V.; Zhakovskaya, Z.A. Limnological characterization and first data on the occurrence of toxigenic cyanobacteria and cyanotoxins in the plankton of some lakes in the permafrost zone (Yakutia, Russia). Contemp. Probl. Ecol. 2023, 16, 89–102. [Google Scholar] [CrossRef]
- Reservoirs of Moscow: Rivers, Lakes, Ponds Are Home to Red Book Animals. Available online: https://www.mos.ru/news/item/86671073/ (accessed on 15 August 2025).
- Kuz, N.V. On improvement of drinking water quality assessment (on example of cyanobacteria). Public Health Life Environ. 2014, 10, 11–13. [Google Scholar]
- Kuz, N.V.; Zholdakova, Z.I. The problem of the «flowering» of the water sources. Evaluation of the influence of water processing on the content of cyanobacterium in drinking water of economic drinking water supply of Moscow. Public Health Life Environ. 2017, 9, 35–39. [Google Scholar] [CrossRef]
- Likhacheva, N.E.; Shidlovskaya, N.A.; Kartasheva, N.V.; Khromov, V.M. Assessment of water quality in rivers based on phytoplankton indicators. In Proceedings of the All-Russian Scientific Conference “Monitoring the State and Pollution of the Environment. Main Results and Development Paths”, Moscow, Russia, 20–22 March 2017. [Google Scholar]
- Khazanova, K.P.; Rostanets, D.V.; Chamkina, A.V. Algoflora of the Moscow River as an indicator of water quality—A retrospective study at the Department of General Ecology and Hydrobiology of Moscow State University. Priroda 2024, 11, 3–17. [Google Scholar] [CrossRef]
- Gerasimova, T.N.; Pogozhev, P.I.; Sadchikov, A.P. Suppression of cyanobacteria blooming by zooplankton filter feeders. Voda Khimiya I Ecol. 2019, 7–9, 67–71. [Google Scholar]
- Li, L.; Lü, S.; Cen, J. Spatio-temporal variations of Harmful algal blooms along the coast of Guangdong, Southern China during 1980–2016. J. Oceanol. Limnol. 2019, 37, 535–551. [Google Scholar] [CrossRef]
- Feist, S.M.; Lance, R.F. Genetic detection of freshwater harmful algal blooms: A review focused on the use of environmental DNA (eDNA) in Microcystis aeruginosa and Prymnesium parvum. Harmful Algae 2021, 110, 102124. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, A.S.; Shende, V.; Chelladurai, H.M.; Dwivedi, G.; Verma, T.N.; Choudhary, T. Exploring the potential and progress of microalgae biomass production, harvesting, and pre-treatment for sustainable biofuel production: A comprehensive review. Environ. Dev. Sustain. 2025, 1–51. [Google Scholar] [CrossRef]
- Sivonen, K.; Namikoshi, M.; Evans, W.R.; Gromov, B.V.; Carmichael, W.W.; Rinehart, K.L. Isolation and structures of five microcystins from a Russian Microcystis aeruginosa strain CALU 972. Toxicon 1992, 30, 1481–1485. [Google Scholar] [CrossRef]
- Polyak, Y.; Zaytseva, T.; Medvedeva, N. Response of Toxic Cyanobacterium Microcystis aeruginosa to Environmental Pollution. Water Air Soil Pollut. 2013, 224, 1494. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium Microcystis spp. Harmful Algae 2016, 54, 4–20. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Capelli, C.; Ballot, A.; Cerasino, L.; Papini, A.; Salmaso, N. Biogeography of bloom-forming microcystin producing and non-toxigenic populations of Dolichospermum lemmermannii (Cyanobacteria). Harmful Algae 2017, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Li, C.; Peng, L.; Han, B.P. Competition between toxic and non-toxic Microcystis aeruginosa and its ecological implication. Ecotoxicology 2015, 24, 1411–1418. [Google Scholar] [CrossRef]
- Skoupý, S.; Stanojković, A.; Pavlíková, M.; Poulíčková, A.; Dvořák, P. New cyanobacterial genus Argonema is hiding in soil crusts around the world. Sci. Rep. 2022, 12, 7203. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information Sequence Read Archive. Available online: https://www.ncbi.nlm.nih.gov/sra (accessed on 15 August 2025).
- Guellati, F.Z.; Touati, H.; Seridi, L.; Djabourabi, A.; Sehili, N.; Kadri, S.E.H.; Bensouilah, M. Cyanobacteria dynamics and microcystins: An emphasis on late autumn mass development of Woronichinia and Microcystis in the Zit-Emba reservoir, North-East Algeria. Biologia 2025, 80, 1059–1068. [Google Scholar] [CrossRef]
- Mohan, R.; Thomas, L.C.; Padmakumar, K.B. Sporadic occurrence of harmful cyanobacteria Woronichinia naegeliana and its bloom dynamics from the aquatic ecosystem of South India. Biologia 2022, 77, 2967–2974. [Google Scholar] [CrossRef]
- Bober, B.; Bialczyk, J. Determination of the toxicity of the freshwater cyanobacterium Woronichinia naegeliana (Unger) Elenkin. J. Appl. Phycol. 2017, 29, 1355–1362. [Google Scholar] [CrossRef]
- Bober, B.; Lechowski, Z.; Bialczyk, J. Determination of some cyanopeptides synthesized by Woronichinia naegeliana (Chroococcales, Cyanophyceae). Phycol. Res. 2011, 59, 286–294. [Google Scholar] [CrossRef]
- Patova, E.N. Cyanoprokaryotes that cause “blooming” of water in the Harbin lakes of the Bolshezemelskaya tundra. J. Sib. Fed. Univ. Biol. 2014, 7, 282–290. [Google Scholar] [CrossRef]
- Rantala, A.; Rajaniemi-Wacklin, P.; Lyra, C.; Lepistö, L.; Rintala, J.; Mankiewicz-Boczek, J.; Sivonen, K. Detection of microcystin-producing cyanobacteria in Finnish lakes with genus-specific microcystin synthetase gene E (mcyE) PCR and associations with environmental factors. Appl. Environ. Microbiol. 2006, 72, 6101–6110. [Google Scholar] [CrossRef]
- Dreher, T.W.; Matthews, R.; Davis, E.W., II; Mueller, R.S. Woronichinia naegeliana: A common nontoxigenic component of temperate freshwater cyanobacterial blooms with 30% of its genome in transposons. Harmful Algae 2023, 125, 102433. [Google Scholar] [CrossRef]
- Temnyj Les. Available online: http://temnyjles.ru/reki/reki2-01.shtml (accessed on 15 August 2025).
- Guillard, R.R.L.; Lorenzen, C.J. Yellow-green algae with chlorophyllide c1,2. J. Phycol. 1972, 8, 10–14. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 1. Chroococcales. In Süßwasserflora von Mitteleuropa. Begründet von A. Pascher. Band 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Spektrum, Akademischer Verlag: Heidelberg/Berlin, Germany, 1999; pp. 1–548. [Google Scholar]
- Komárek, J. Süßwasserflora von Mitteleuropa. Cyanoprokaryota: Heterocystous Genera (Nostocales, Stigonematales); Springer Spektrum: Heidelberg, Germany, 2013; pp. 1–1130. [Google Scholar]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef]
- Sing, A.; Berger, A.; Schneider-Brachert, W.; Holzmann, T.; Reischl, U. Rapid detection and molecular differentiation of toxigenic Corynebacterium diphtheriae and Corynebacterium ulcerans strains by LightCycler PCR. J. Clin. Microbiol. 2011, 49, 2485–2489. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Neilan, B.A. Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria. Arch. Microbiol. 2006, 185, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Hisbergues, M.; Christiansen, G.; Rouhiainen, L.; Sivonen, K.; Börner, T. PCR-based identification of microcystin-producing genotypes of different cyanobacterial genera. Arch. Microbiol. 2003, 180, 402–410. [Google Scholar] [CrossRef]
- Liu, P.; Wei, J.; Yang, K.; Massey, I.Y.; Guo, J.; Zhang, C.; Yang, F. Isolation, molecular identification, and characterization of a unique toxic cyanobacterium Microcystis sp. found in Hunan Province, China. J. Toxicol. Environ. Health Part A 2018, 81, 1142–1149. [Google Scholar] [CrossRef]
- Genuario, D.B.; Silva-Stenico, M.E.; Welker, M.; Moraes, L.A.B.; Fiore, M.F. Characterization of a microcystin and detection of microcystin synthetase genes from a Brazilian isolate of Nostoc. Toxicon 2010, 55, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Rantala-Ylinen, A.; Känä, S.; Wang, H.; Rouhiainen, L.; Wahlsten, M.; Rizzi, E.; Berg, K.; Gugger, M.; Sivonen, K. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl. Environ. Microbiol. 2011, 77, 7271–7278. [Google Scholar] [CrossRef]
- Schembri, M.A.; Neilan, B.A.; Saint, C.P. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. Int. J. 2001, 16, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Chernova, E.; Russkikh, I.; Voyakina, E.; Zhakovskaya, Z. Occurrence of microcystins and anatoxin-a in eutrophic lakes of Saint Petersburg, Northwestern Russia. Oceanol. Hydrobiol. Stud. 2016, 45, 466–484. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 1–8. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]

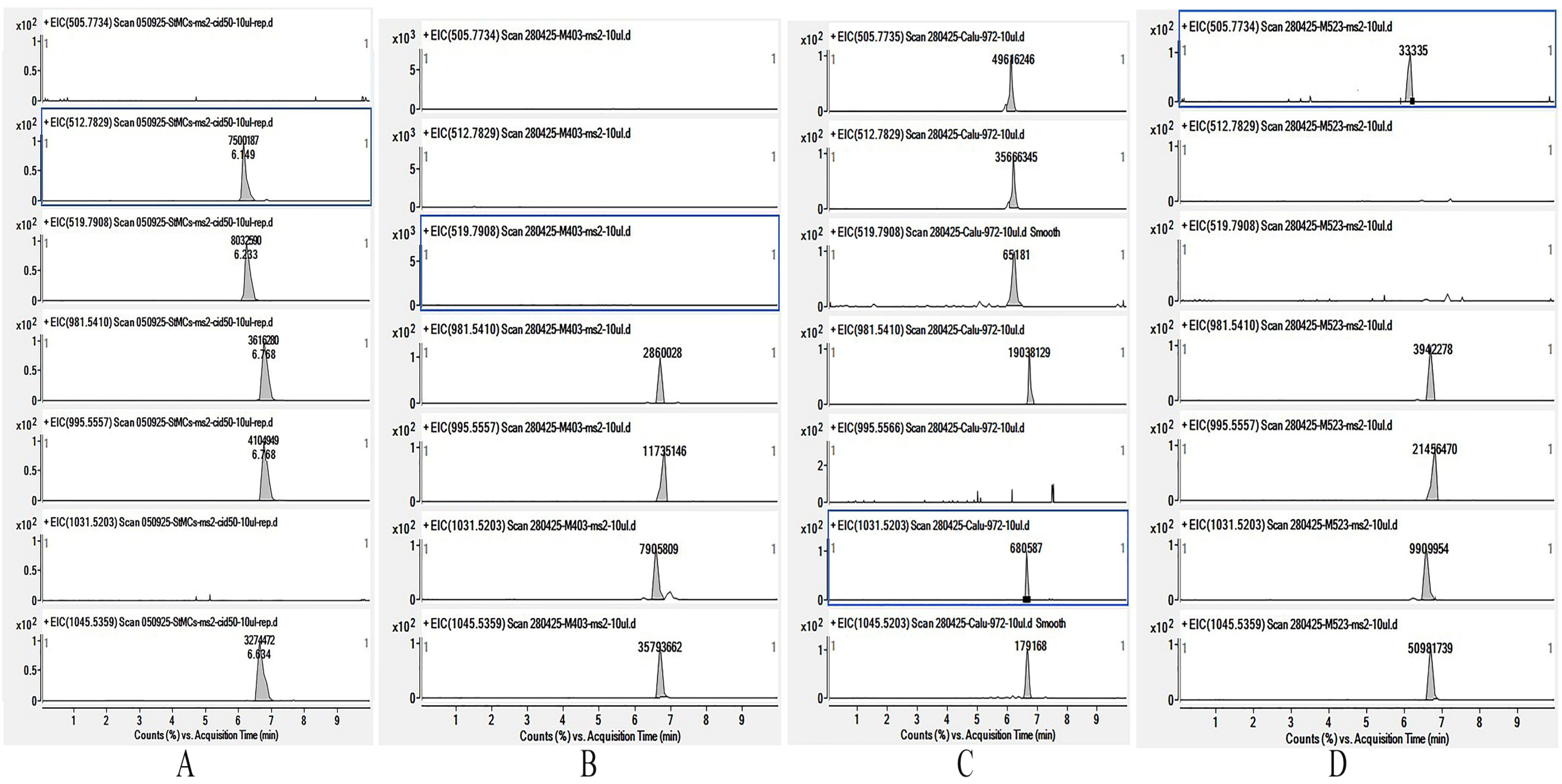
| Microcystin Congeners | Formula | m/z | CBMC403m | CALU972 | CBMC523m |
|---|---|---|---|---|---|
| [D-Asp3,Dha7]MC-RR | C47H71N13O12 | 505.7751 | <0.3 | 392.7 | <0.9 |
| [D-Asp3]MC-RR | C48H73N13O1 | 512.7829 | <0.3 | 281.4 | <0.3 |
| MC-RR | C49H75N13O12 | 519.7908 | <0.3 | 0.9 | <0.3 |
| [D-Asp3]MC-LR | C48H72N10O12 | 981.5410 | 23.5 | 147.9 | 24.2 |
| MC-LR | C49H74N10O12 | 995.55658 | 96.6 | <0.3 | 145.2 |
| [D-Asp3]MC-YR | C51H70N10O13 | 1031.5203 | 65.5 | 118.3 | 3.4 |
| MC-YR | C52H72N10O13 | 1045.5359 | 294.6 | 2.4 | 345.0 |
| Total MCs | 480.2 | 943.6 | 517.8 | ||
| MC Congener | Detected m/z | Charge of the Detected Ion | Characteristic Fragment Ions | Fragment Structure |
|---|---|---|---|---|
| MC-LR | 995.5557 | [M+H]+ | 446 553 599 | [C11H15O-Glu-Mdha-Ala]+ [Mdha-Ala-Leu-MeAsp-Arg+H]+ [Arg-Adda-Glu+H]+ |
| [D-Asp3]MC-LR | 981.5410 | [M+H]+ | 446 539 559 599 | [C11H15O-Glu-Mdha-Ala]+ [Mdha-Ala-Leu-D-Asp-Arg+H]+ [C11H15O-Glu-Mdha-Ala-Leu]+ [Arg-Adda-Glu+H]+ |
| MC-RR | 519.7908 | [M+2H]2+ | 440 453 596 599 | [Mdha-Ala-Arg-MeAsp+H]+ [Arg-Adda+H−NH3]+ [Mdha-Ala-Arg-MeAsp-Arg+H]+ [Arg-Adda-Glu+H]+ |
| [D-Asp3]MC-RR | 512.7829 | [M+2H]2+ | 426 499 582 599 | [Mdha-Ala-Arg-D-Asp-H]+ or [Dha-Ala-Arg-MeAsp+H]+ [Ala-Arg-D-Asp-Arg+H]+ [Mdha-Ala-Arg-D-Asp-Arg+H]+ [Arg-Adda-Glu+H]+ |
| [Dha,D-Asp3]MC-RR | 505.7734 | [M+2H]2+ | 412 568 599 | [Dha-Ala-Arg-D-Asp-H]+ [Dha-Ala-Arg-D-Asp-Arg+H]+ [Arg-Adda-Glu+H]+ |
| MC-YR | 1045.5359 | [M+H]+ | 446 599 603 | [C11H15O-Glu-Mdha-Ala]+ [Arg-Adda-Glu+H]+ [Mdha-Ala-Tyr-MeAsp-Arg+H]+ |
| [D-Asp3]MC-YR | 1031.5203 | [M+H]+ | 589 599 682 696 | [Mdha-Ala-Tyr-D-Asp-Arg+H]+ [Arg-Adda-Glu+H]+ [Arg-Adda-Glu-Mdha+H]+ [DAsp-Arg-Adda-Glu-H2O+H]+ |
| Sample Site ID | Name of Watercourse or Reservoir | Coordinates | Hydrological Parameters | Hydrochemical Parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Water Surface Area (km2) | Average Depth (m) | Recreational Use | Temperature (°C) | pH | Conductivity (mV) | |||
| M1 | Bolshoy Krylatsky Pond | 55.763134, 37.435047 | 0.125 | 2.5 | Yes | 23.4 | 8.09 | 134 |
| M2 | Krylatskoye Rowing Canal | 55.766868, 37.442611 | 0.18 | 3.0 | Yes | 24.8 | 8.89 | 148 |
| M4 | Moskva River, Krylatskoye district | 55.753740, 37.448831 | Length—473 km, basin area—17,600 km2, water discharge—109 m3/s | Yes | 22.1 | 7.78 | 165 | |
| M6 | Bolshoy Sadovy Pond | 55.834221, 37.539308 | 0.082 | 2.0 | Yes | 23.6 | 8.77 | 103 |
| M7 | Nizhny Fermsky Pond | 55.835205, 37.559273 | 0.067 | 1.8 | Yes | 21.5 | 8.93 | 100 |
| M9 | Unnamed pond in Odintsovsky district | 55.765066, 37.446576 | 0.015 | 1.0 | No | 25.1 | 8.50 | 154 |
| M10 | Meshchersky Pond | 55.674680, 37.410679 | 0.103 | 2.2 | Yes | 28.1 | 9.50 | 111 |
| M13 | Unnamed pond in Marfino district #1 | 55.689277, 37.365546 | 0.020 | 1.1 | No | 27.6 | 9.02 | 103 |
| M14 | Unnamed pond in Marfino district #2 | 55.668461, 37.385979 | 0.025 | 1.0 | No | 27.1 | 8.51 | 152 |
| M16 | Pervy Kamensky Pond | 55.833901, 37.608266 | 0.055 | 1.5 | Yes | 28.1 | 8.41 | 172 |
| M18 | Patriarshiy Pond | 55.763396, 37.592456 | 0.021 | 1.2 | Yes | 25.5 | 9.37 | 95 |
| M19 | Clean Pond | 55.761603, 37.644501 | 0.038 | 1.4 | Yes | 25.6 | 8.81 | 131 |
| Gene | Primer | T °C Annealing | Sequence (5′–3′) | References |
|---|---|---|---|---|
| mcyE, ndaF | HEPF | 52 | TTTGGGGTTAACTTTTTTGGGCATAGTC | [46] |
| HEPR | AATTCTTGAGGCTGTAAATCGGGTTT | |||
| mcyA | mcyACdF; | 59 | AAAAGTGTTTTATTAGCGGCTCAT | [47] |
| mcyACdR | AAAATTAAAAGCCGTATCAAA | |||
| mcyB | McyB-F; | 55 | AGACCAAAAATTAACCTATCAACAG | [48] |
| McyB-R | TACTAATCCCTATCTAAACAC | |||
| mcyE | mcyE-F2 | 56 | GAAATTTGTGTAGAAGGTGC; | [49] |
| mcyE-R4 | AATTCTAAAGCCCAAAGACG | |||
| anaC | anaC-genF | 58 | TCTGGTATTCAGTCCCCTCTAT | [50] |
| anaC-genR | CCCAATAGCCTGTCATCAA | |||
| cyrB | cyrB M13f | 58 | GGCAAATTGTGATAGCCACGAGC | [51] |
| cyrB M14r; | GATGGAACATCGCTCACTGGTG; |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kezlya, E.; Mironova, E.; Chernova, E.; Gololobova, M.; Mironov, A.; Voyakina, E.; Maltsev, Y.; Snarskaya, D.; Kulikovskiy, M. Comprehensive Study of Some Cyanobacteria in Moscow Waterbodies (Russia), Including Characteristics of the Toxigenic Microcystis aeruginosa Strains. Toxins 2025, 17, 506. https://doi.org/10.3390/toxins17100506
Kezlya E, Mironova E, Chernova E, Gololobova M, Mironov A, Voyakina E, Maltsev Y, Snarskaya D, Kulikovskiy M. Comprehensive Study of Some Cyanobacteria in Moscow Waterbodies (Russia), Including Characteristics of the Toxigenic Microcystis aeruginosa Strains. Toxins. 2025; 17(10):506. https://doi.org/10.3390/toxins17100506
Chicago/Turabian StyleKezlya, Elena, Elina Mironova, Ekaterina Chernova, Maria Gololobova, Andrei Mironov, Ekaterina Voyakina, Yevhen Maltsev, Dina Snarskaya, and Maxim Kulikovskiy. 2025. "Comprehensive Study of Some Cyanobacteria in Moscow Waterbodies (Russia), Including Characteristics of the Toxigenic Microcystis aeruginosa Strains" Toxins 17, no. 10: 506. https://doi.org/10.3390/toxins17100506
APA StyleKezlya, E., Mironova, E., Chernova, E., Gololobova, M., Mironov, A., Voyakina, E., Maltsev, Y., Snarskaya, D., & Kulikovskiy, M. (2025). Comprehensive Study of Some Cyanobacteria in Moscow Waterbodies (Russia), Including Characteristics of the Toxigenic Microcystis aeruginosa Strains. Toxins, 17(10), 506. https://doi.org/10.3390/toxins17100506







