Spider and Wasp Acylpolyamines: Venom Components and Versatile Pharmacological Leads, Probes, and Insecticidal Agents
Abstract
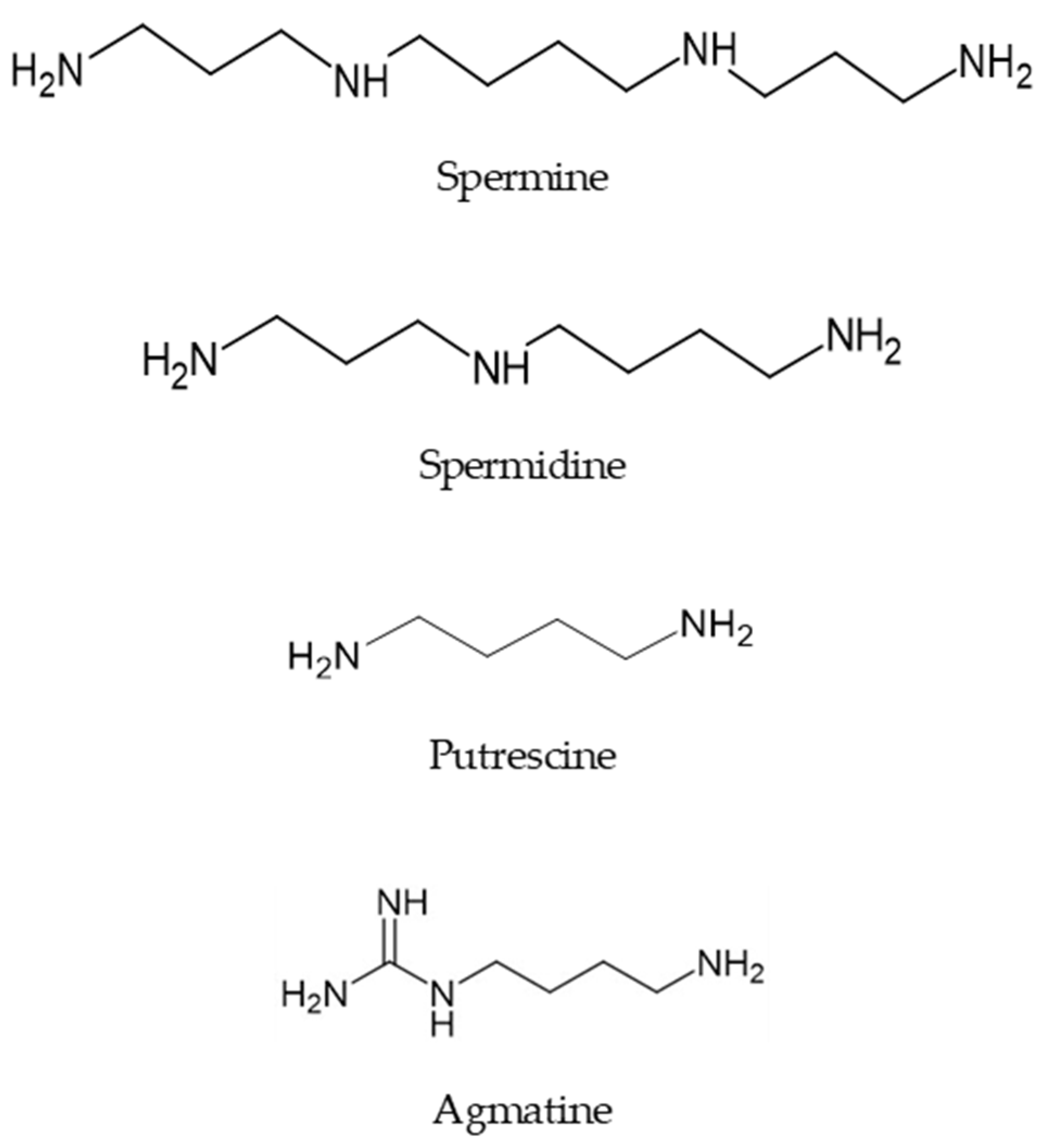
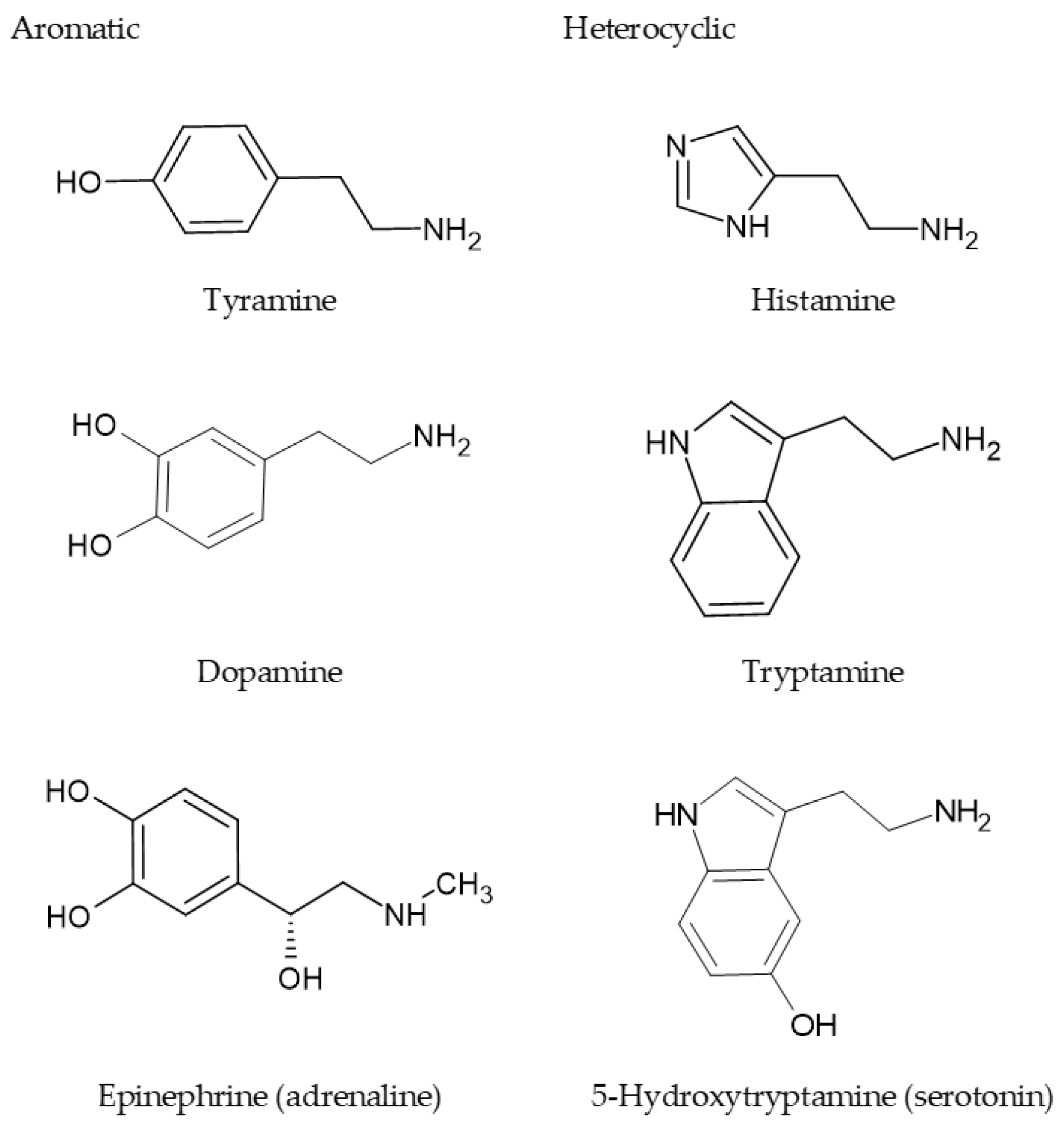
1. Introduction
2. Acylpolyamines in the Venom of Spiders and Wasp
2.1. Argiopines
2.2. Joro Spider Toxin (JSTX) and Nephila Spider Toxin (NPTX)
2.3. Philanthotoxins
3. Spider and Wasp Acylpolyamines as Versatile Pharmacological Leads, Probes, and Insecticidal Agents
3.1. Pharmacological Leads
3.2. Probes
3.3. Insecticidal Agents
3.4. Carriers
4. Conclusions
5. Material and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agostinelli, E.; Marques, M.P.; Calheiros, R.; Gil, F.P.; Tempera, G.; Viceconte, N.; Battaglia, V.; Grancara, S.; Toninello, A. Polyamines: Fundamental characters in chemistry and biology. Amino Acids 2010, 38, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Tarafdar, S.; Agarwal, S.; Tarafdar, A.; Sharma, S. Polyamines: Functions, Metabolism, and Role in Human Disease Management. Med. Sci. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Xuan, M.; Gu, X.; Li, J.; Huang, D.; Xue, C.; He, Y. Polyamines: Their significance for maintaining health and contributing to diseases. Cell Commun. Signal. 2023, 21, 348. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, S.; Houdou, M.; Cascalho, A.; Eggermont, J.; Vangheluwe, P. Polyamines in Parkinson’s Disease: Balancing Between Neurotoxicity and Neuroprotection. Annu. Rev. Biochem. 2023, 92, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Hisada, M.; Satake, H.; Masuda, K.; Aoyama, M.; Murata, K.; Shinada, T.; Iwashita, T.; Ohfune, Y.; Nakajima, T. Molecular components and toxicity of the venom of the solitary wasp, Anoplius samariensis. Biochem. Biophys. Res. Commun. 2005, 330, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Igarashi, K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol. Ther. 2013, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Haenisch, B.; von Kügelgen, I.; Bönisch, H.; Göthert, M.; Sauerbruch, T.; Schepke, M.; Marklein, G.; Höfling, K.; Schröder, D.; Molderings, G.J. Regulatory mechanisms underlying agmatine homeostasis in humans. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G1104–G1110. [Google Scholar] [CrossRef] [PubMed]
- Damiani, E.; Wallace, H.M. Polyamines and Cancer. Methods Mol. Biol. 2018, 1694, 469–488. [Google Scholar] [CrossRef] [PubMed]
- Ramani, D.; De Bandt, J.P.; Cynober, L. Aliphatic polyamines in physiology and diseases. Clin. Nutr. 2014, 33, 14–22. [Google Scholar] [CrossRef]
- Uzbay, T.I. The pharmacological importance of agmatine in the brain. Neurosci. Biobehav. Rev. 2012, 36, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Williams, K. Interactions of polyamines with ion channels. Biochem. J. 1997, 325 Pt 2, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.; Baukrowitz, T.; Fakler, B. Polyamines as gating molecules of inward-rectifier K+ channels. Eur. J. Biochem. 2000, 267, 5824–5829. [Google Scholar] [CrossRef] [PubMed]
- Bowie, D. Ionotropic glutamate receptors & CNS disorders. CNS Neurol. Disor. Drug Targets 2008, 7, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Baroli, G.; Sanchez, J.R.; Agostinelli, E.; Mariottini, P.; Cervelli, M. Polyamines: The possible missing link between mental disorders and epilepsy (Review). Int. J. Mol. Med. 2020, 45, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, L.; Dodd, P.R.; Hynd, M.R. Role of Ionotropic Glutamate Receptors in Neurodegenerative and Other Disorders. In Handbook of Neurotoxicity; Kostrzewa, R., Ed.; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Aird, S.D.; Villar Briones, A.; Roy, M.C.; Mikheyev, A.S. Polyamines as Snake Toxins and Their Probable Pharmacological Functions in Envenomation. Toxins 2016, 8, 279. [Google Scholar] [CrossRef] [PubMed]
- Bowie, D. Polyamine-mediated channel block of ionotropic glutamate receptors and its regulation by auxiliary proteins. J. Biol. Chem. 2018, 293, 18789–18802. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Chemistry and Functions of Imported Fire Ant Venom. Toxins 2023, 15, 489. [Google Scholar] [CrossRef] [PubMed]
- Numata, A.; Ibuka, T. Chapter 6 Alkaloids from Ants and Other Insects. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; Academic Press: Cambridge, MA, USA, 1987; Volume 31, pp. 193–315. [Google Scholar]
- Rádis-Baptista, G.; Dodou, H.V.; Prieto-da-Silva, Á.R.B.; Zaharenko, A.J.; Kazuma, K.; Nihei, K.I.; Inagaki, H.; Mori-Yasumoto, K.; Konno, K. Comprehensive analysis of peptides and low molecular weight components of the giant ant Dinoponera quadriceps venom. Biol. Chem. 2020, 401, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, E.F.; Mourão, C.B.; Moreira, K.G.; Camargos, T.S.; Mortari, M.R. Arthropod venoms: A vast arsenal of insecticidal neuropeptides. Biopolymers 2012, 98, 385–405. [Google Scholar] [CrossRef] [PubMed]
- Eldefrawi, A.T.; Eldefrawi, M.E.; Konno, K.; Mansour, N.A.; Nakanishi, K.; Oltz, E.; Usherwood, P.N. Structure and synthesis of a potent glutamate receptor antagonist in wasp venom. Proc. Natl. Acad. Sci. 1988, 85, 4910–4913. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Nentwig, L.; Stöcklin, R.; Nentwig, W. Venom Composition and Strategies in Spiders: Is Everything Possible? In Advances in Insect Physiology; Casas, J., Ed.; Academic Press: Cambridge, MA, USA, 2011; Volume 40, pp. 1–86. [Google Scholar]
- Klupczynska, A.; Plewa, S.; Dereziński, P.; Garrett, T.J.; Rubio, V.Y.; Kokot, Z.J.; Matysiak, J. Identification and quantification of honeybee venom constituents by multiplatform metabolomics. Sci. Rep. 2020, 10, 21645. [Google Scholar] [CrossRef] [PubMed]
- Estrada, G.; Villegas, E.; Corzo, G. Spider venoms: A rich source of acylpolyamines and peptides as new leads for CNS drugs. Nat. Prod. Rep. 2007, 24, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Vassilevski, A.A.; Grishin, E.V. Novel active principles from spider venom. Acta Chim. Slov. 2011, 58, 717–723. [Google Scholar] [PubMed]
- Langenegger, N.; Nentwig, W.; Kuhn-Nentwig, L. Spider Venom: Components, Modes of Action, and Novel Strategies in Transcriptomic and Proteomic Analyses. Toxins 2019, 11, 611. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Kazuma, K.; Nihei, K. Peptide Toxins in Solitary Wasp Venoms. Toxins 2016, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.D.; Wilson, I.D. 8.05—Insect Hormones and Insect Chemical Ecology. In Comprehensive Natural Products Chemistry; Barton, S.D., Nakanishi, K., Meth-Cohn, O., Eds.; Pergamon: Oxford, UK, 1999; pp. 263–375. [Google Scholar]
- Barygin, O.I.; Grishin, E.V.; Tikhonov, D.B. Argiotoxin in the closed AMPA receptor channel: Experimental and modeling study. Biochemistry 2011, 50, 8213–8220. [Google Scholar] [CrossRef] [PubMed]
- Forster, Y.M.; Reusser, S.; Forster, F.; Bienz, S.; Bigler, L. VenoMS-A Website for the Low Molecular Mass Compounds in Spider Venoms. Metabolites 2020, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Chesnov, S.; Bigler, L.; Hesse, M. The Acylpolyamines from the Venom of the Spider Agelenopsis aperta. Helv. Chim. Acta 2001, 84, 2178–2197. [Google Scholar] [CrossRef]
- Usherwood, P.N.; Blagbrough, I.S. Spider toxins affecting glutamate receptors: Polyamines in therapeutic neurochemistry. Pharmacol. Ther. 1991, 52, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Antonov, S.M.; Grishin, E.V.; Magazanik, L.G.; Shupliakov, O.V.; Vesselkin, N.P.; Volkova, T.M. Argiopin blocks the glutamate responses and sensorimotor transmission in motoneurones of isolated frog spinal cord. Neurosci. Lett. 1987, 83, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, M.H.; Andersen, J.; Christensen, R.; Hansen, K.B.; Traynelis, S.F.; Strømgaard, K.; Kristensen, A.S. Binding of ArgTX-636 in the NMDA receptor ion channel. J. Mol. Biol. 2015, 427, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.; Bukownik, R.; Eldefrawi, A.T.; Eldefrawi, M.E.; Goodnow, R., Jr.; Kallimopoulos, T.; Konno, K.; Nakanishi, K.; Niwa, M.; Usherwood, P.N. Structure-activity relationships of analogues of the wasp toxin philanthotoxin: Non-competitive antagonists of quisqualate receptors. Toxicon Off. J. Int. Soc. Toxinology 1990, 28, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Krzystanek, M. Spider Neurotoxins as Modulators of NMDA Receptor Signaling. NeuroMolecular Med. 2022, 24, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.F.; Poulsen, M.H.; Hussein, R.A.; Nørager, N.G.; Strømgaard, K. Structure-activity relationship study of spider polyamine toxins as inhibitors of ionotropic glutamate receptors. ChemMedChem 2014, 9, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Kachel, H.S.; Patel, R.N.; Franzyk, H.; Mellor, I.R. Block of nicotinic acetylcholine receptors by philanthotoxins is strongly dependent on their subunit composition. Sci. Rep. 2016, 6, 38116. [Google Scholar] [CrossRef] [PubMed]
- Bixel, M.G.; Krauss, M.; Weise, C.; Bolognesi, M.L.; Rosini, M.; Usherwood, P.N.; Melchiorre, C.; Hucho, F. Binding of polyamine-containing toxins in the vestibule of the nicotinic acetylcholine receptor ion channel. Farm. (Soc. Chim. Ital. 1989) 2001, 56, 133–135. [Google Scholar] [CrossRef]
- Kachel, H.S.; Buckingham, S.D.; Sattelle, D.B. Insect toxins-selective pharmacological tools and drug/chemical leads. Curr. Opin. Insect Sci. 2018, 30, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nakazawa, K.; Inoue, K.; Ohno, Y. Potent and voltage-dependent block by philanthotoxin-343 of neuronal nicotinic receptor/channels in PC12 cells. Br. J. Pharmacol. 1997, 122, 379–385. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.D.; Meinwald, J. Neurotoxic acylpolyamines from spider venoms. J. Chem. Ecol. 1993, 19, 2411–2451. [Google Scholar] [CrossRef] [PubMed]
- Balázs, R.; Bridges, R.J.; Cotman, C.W.; Balazs, R.; Bridges, R.J.; Cotman, C.W.; Cotman, C.A. Glutamate and Glutamate Receptors in Neurological Diseases. In Excitatory Amino Acid Transmission in Health and Disease; Oxford University Press: Oxford, UK, 2005; pp. 269–308. [Google Scholar]
- Mutluay, S.U.; Karataş, H. A Review of Glutamate and Its Receptors: Their Roles in Brain Physiology and Pathology. Acta Medica 2022, 53, 99–109. [Google Scholar] [CrossRef]
- Olsen, C.A.; Kristensen, A.S.; Strømgaard, K. Small molecules from spiders used as chemical probes. Angew. Chem. (Int. Ed. Engl.) 2011, 50, 11296–11311. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Denholm, I. Nicotinic acetylcholine receptors: Targets for commercially important insecticides. Invertebr. Neurosci. IN 2007, 7, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, M.; Casida, J.E. Structure and diversity of insect nicotinic acetylcholine receptors. Pest Manag. Sci. 2001, 57, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Liu, Z.; Fan, X.; Zhang, X.; Qiao, X.; Huang, J. Nicotinic acetylcholine receptor modulator insecticides act on diverse receptor subtypes with distinct subunit compositions. PLoS Genet. 2022, 18, e1009920. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, M.; Jiang, L.; Tang, X.; Liu, Z.; Zhou, Z.; Hu, W.; Duan, Z.; Liang, S. Structural Foundation for Insect-Selective Activity of Acylpolyamine Toxins from Spider Araneus ventricosus. Chem. Res. Toxicol. 2019, 32, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Strømgaard, K.; Jensen, L.S.; Vogensen, S.B. Polyamine toxins: Development of selective ligands for ionotropic receptors. Toxicon Off. J. Int. Soc. Toxinology 2005, 45, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.A.; Kaufmann, L.; Hue, B.; Pelhate, M.; Schürmann, F.; Gsell, L.; Piek, T. The physiological action of analogues of philanthotoxin-4.3.3 at insect nicotinic acetylcholine receptors. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1993, 105, 303–310. [Google Scholar] [CrossRef]
- Nihei, K.; Kato, M.J.; Yamane, T.; Palma, M.S.; Konno, K. An efficient and versatile synthesis of acylpolyamine spider toxins. Bioorganic Med. Chem. Lett. 2002, 12, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Pauli, D.; Bienz, S. Regioselective solid-phase synthesis of N-mono-hydroxylated and N-mono-methylated acylpolyamine spider toxins using an 2-(ortho-nitrophenyl)ethanal-modified resin. Org. Biomol. Chem. 2015, 13, 4473–4485. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Horikawa, M.; Corzo, G.; Naoki, H.; Haupt, J.; Nakajima, T.; Iwashita, T. Structure and enantioselective synthesis of polyamine toxin MG30 from the venom of the spider Macrothele gigas. Tetrahedron Lett. 2004, 45, 5371–5373. [Google Scholar] [CrossRef]
- Grishin, E.V.; Volkova, T.M.; Arseniev, A.S. Isolation and structure analysis of components from venom of the spider Argiope lobata. Toxicon Off. J. Int. Soc. Toxinology 1989, 27, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Antonov, S.M.; Dudel, J.; Franke, C.; Hatt, H. Argiopine blocks glutamate-activated single-channel currents on crayfish muscle by two mechanisms. J. Physiol. 1989, 419, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.S.; Baganoff, M.P.; Grishin, E.V.; Lanthorn, T.H.; Volkova, T.M.; Watson, G.B.; Wiegand, R.C. Polyamine spider toxins are potent un-competitive antagonists of rat cortex excitatory amino acid receptors. Eur. J. Pharmacol. 1992, 227, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Nørager, N.G.; Poulsen, M.H.; Jensen, A.G.; Jeppesen, N.S.; Kristensen, A.S.; Strømgaard, K. Structure-activity relationship studies of N-methylated and N-hydroxylated spider polyamine toxins as inhibitors of ionotropic glutamate receptors. J. Med. Chem. 2014, 57, 4940–4949. [Google Scholar] [CrossRef] [PubMed]
- Ojomoko, L.O.; Kryukova, E.V.; Egorova, N.S.; Salikhov, A.I.; Epifanova, L.A.; Denisova, D.A.; Khomutov, A.R.; Sukhov, D.A.; Vassilevski, A.A.; Khomutov, M.A.; et al. Inhibition of nicotinic acetylcholine receptors by oligoarginine peptides and polyamine-related compounds. Front. Pharmacol. 2023, 14, 1327603. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, Y.; Yasuhara, T.; Shimazaki, K.; Kawai, N.; Nakajima, T. Chemical structure of Joro spider toxin (JSTX). Biomed. Res. 1987, 8, 241–245. [Google Scholar] [CrossRef]
- Itagaki, Y.; Nakajima, T. Acylpolyamines: Mass spectrometric analytical methods for Araneidae spider acylpolyamines. J. Toxicol. Toxin Rev. 2000, 19, 23–52. [Google Scholar] [CrossRef]
- Tzouros, M.; Chesnov, S.; Bigler, L.; Bienz, S. A template approach for the characterization of linear polyamines and derivatives in spider venom. Eur. J. Mass Spectrom. 2013, 19, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Poulsen, M.H.; Nørager, N.G.; Barslund, A.F.; Bach, T.B.; Kristensen, A.S.; Strømgaard, K. General synthesis of β-alanine-containing spider polyamine toxins and discovery of nephila polyamine toxins 1 and 8 as highly potent inhibitors of ionotropic glutamate receptors. J. Med. Chem. 2012, 55, 10297–10301. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.F.; Tikhonov, D.B.; Bølcho, U.; Bolshakov, K.; Nelson, J.K.; Pluteanu, F.; Mellor, I.R.; Egebjerg, J.; Strømgaard, K. Uncompetitive antagonism of AMPA receptors: Mechanistic insights from studies of polyamine toxin derivatives. J. Med. Chem. 2006, 49, 5414–5423. [Google Scholar] [CrossRef] [PubMed]
- Brier, T.J.; Mellor, I.R.; Tikhonov, D.B.; Neagoe, I.; Shao, Z.; Brierley, M.J.; Strømgaard, K.; Jaroszewski, J.W.; Krogsgaard-Larsen, P.; Usherwood, P.N. Contrasting actions of philanthotoxin-343 and philanthotoxin-(12) on human muscle nicotinic acetylcholine receptors. Mol. Pharmacol. 2003, 64, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Bähring, R.; Mayer, M.L. An analysis of philanthotoxin block for recombinant rat GluR6(Q) glutamate receptor channels. J. Physiol. 1998, 509 Pt 3, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Piek, T.; Hue, B. Philanthotoxins, a new class of neuroactive polyamines, block nicotinic transmission in the insect CNS. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1989, 93, 403–406. [Google Scholar] [CrossRef]
- Huang, D.; Jiang, H.; Nakanishi, K.; Usherwood, P. Synthesis and pharmacological activity of philanthotoxin-343 analogs: Antagonists of ionotropic glutamate receptors. Tetrahedron 1997, 53, 12391–12404. [Google Scholar] [CrossRef]
- Frølund, S.; Bella, A.; Kristensen, A.S.; Ziegler, H.L.; Witt, M.; Olsen, C.A.; Strømgaard, K.; Franzyk, H.; Jaroszewski, J.W. Assessment of structurally diverse philanthotoxin analogues for inhibitory activity on ionotropic glutamate receptor subtypes: Discovery of nanomolar, nonselective, and use-dependent antagonists. J. Med. Chem. 2010, 53, 7441–7451. [Google Scholar] [CrossRef] [PubMed]
- Kachel, H.S.; Franzyk, H.; Mellor, I.R. Philanthotoxin Analogues That Selectively Inhibit Ganglionic Nicotinic Acetylcholine Receptors with Exceptional Potency. J. Med. Chem. 2019, 62, 6214–6222. [Google Scholar] [CrossRef] [PubMed]
- Baslé, A.; Delcour, A.H. Effect of two polyamine toxins on the bacterial porin OmpF. Biochem. Biophys. Res. Commun. 2001, 285, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, T.; Swartz, K.J. An Inhibitor of TRPV1 Channels Isolated from Funnel Web Spider Venom. Biochemistry 2005, 44, 15544–15549. [Google Scholar] [CrossRef] [PubMed]
- Grishin, E.; Volkova, T.M.; Arseniev, A.; Reshetova, O.S.; Onoprienko, V.V.; Antonov, S. Structure-functional characterisation of argiopin - an ion channel blocker from the venom of spider Argiope lobata. Bioorganicheskaia Khimiia 1986, 12, 1121–1124. [Google Scholar] [PubMed]
- Budd, T.; Clinton, P.; Dell, A.; Duce, I.R.; Johnson, S.J.; Quicke, D.L.J.; Taylor, G.W.; Usherwood, P.N.R.; Usoh, G. Isolation and characterisation of glutamate receptor antagonists from venoms of orb-web spiders. Brain Res. 1988, 448, 30–39. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.D.; Kobayashi, K.; Goldin, S.M.; Reddy, N.L.; Meinwald, J. Characterization and synthesis of a new calcium antagonist from the venom of a fishing spider. Tetrahedron 1993, 49, 11155–11168. [Google Scholar] [CrossRef]
- Kawai, N.; Miwa, A.; Shimazaki, K.; Sahara, Y.; Robinson, H.P.; Nakajima, T. Spider toxin and the glutamate receptors. Comp. Biochem. Physiol. C Comp. Pharmacol. Toxicol. 1991, 98, 87–95. [Google Scholar] [PubMed]
- Teshima, T.; Matsumoto, T.; Wakamiya, T.; Shiba, T.; Nakajima, T.; Kawai, N. Structure-activity relationship of NSTX-3, spider toxin of nephila maculata. Tetrahedron 1990, 46, 3813–3818. [Google Scholar] [CrossRef]
- Melchiorre, C.; Bolognesi, M.L.; Minarini, A.; Rosini, M.; Tumiatti, V. Polyamines in drug discovery: From the universal template approach to the multitarget-directed ligand design strategy. J. Med. Chem. 2010, 53, 5906–5914. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, M.D.; Amunugama, H.; Boncher, T.D.; Casero, R.A.; Woster, P.M. Design of polyamine-based therapeutic agents: New targets and new directions. Essays Biochem. 2009, 46, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Phanstiel, O.; Archer, J. Design of Polyamine Transport Inhibitors as Therapeutics; RSC Publishing: Cambridge, UK, 2012; pp. 162–187. [Google Scholar]
- Kirby, B.P.; Ryder, S.A.; Seiler, N.; Renault, J.; Shaw, G.G. N1-dansyl-spermine: A potent polyamine antagonist. Brain Res. 2004, 1011, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, Z.; Maimaiti, B.; Meng, Q.; Meng, H. The Potential Role of Polyamines in Epilepsy and Epilepsy-Related Pathophysiological Changes. Biomolecules 2022, 12, 1596. [Google Scholar] [CrossRef] [PubMed]
- Forster, Y.M.; Green, J.L.; Khatiwada, A.; Liberato, J.L.; Narayana Reddy, P.A.; Salvino, J.M.; Bienz, S.; Bigler, L.; Dos Santos, W.F.; Karklin Fontana, A.C. Elucidation of the Structure and Synthesis of Neuroprotective Low Molecular Mass Components of the Parawixia bistriata Spider Venom. ACS Chem. Neurosci. 2020, 11, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Alkhzem, A.H.; Li, S.; Wonfor, T.; Woodman, T.J.; Laabei, M.; Blagbrough, I.S. Practical Synthesis of Antimicrobial Long Linear Polyamine Succinamides. ACS Bio Med. Chem. Au 2022, 2, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Inclán, M.; Torres Hernández, N.; Martínez Serra, A.; Torrijos Jabón, G.; Blasco, S.; Andreu, C.; del Olmo, M.L.; Jávega, B.; O’Connor, J.-E.; García-España, E. Antimicrobial Properties of New Polyamines Conjugated with Oxygen-Containing Aromatic Functional Groups. Molecules 2023, 28, 7678. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.G.; da Silva, P.I.; Galhardo, C.S.; Nassar, R.; Daffre, S.; Sato, M.N.; Borges, M.M. The spider acylpolyamine Mygalin is a potent modulator of innate immune responses. Cell. Immunol. 2012, 275, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Culupú, A.; Mendes, E.; Vitorino, H.A.; da Silva, P.I., Jr.; Borges, M.M. Mygalin: An Acylpolyamine With Bactericidal Activity. Front. Microbiol. 2019, 10, 2928. [Google Scholar] [CrossRef]
- Razvi, S.; Choudhry, H.; Moselhy, S.; Kumosani, T.; Hasan, M.; Zamzami, M.; Abualnaja, K.; Al-Malki, A.; Alhosin, M.; Asami, T. Synthesis, screening and pro-apoptotic activity of novel acyl spermidine derivatives on human cancer cell lines. Biomed. Pharmacother. 2017, 93, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Vassileiou, C.; Kalantzi, S.; Vachlioti, E.; Athanassopoulos, C.M.; Koutsakis, C.; Piperigkou, Z.; Karamanos, N.; Stivarou, T.; Lymberi, P.; Avgoustakis, K.; et al. New Analogs of Polyamine Toxins from Spiders and Wasps: Liquid Phase Fragment Synthesis and Evaluation of Antiproliferative Activity. Molecules 2022, 27, 447. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Boyle, G.M.; McIntyre, L.; Nolan, M.J.; Parsons, P.G.; Smith, J.J.; Tribolet, L.; Loukas, A.; Liddell, M.J.; Rash, L.D.; et al. The Aromatic Head Group of Spider Toxin Polyamines Influences Toxicity to Cancer Cells. Toxins 2017, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.S.; Silva, P.I., Jr.; Miranda, M.T.; Almeida, I.C.; Naoki, H.; Konno, K.; Daffre, S. Structural and biological characterization of one antibacterial acylpolyamine isolated from the hemocytes of the spider Acanthocurria gomesiana. Biochem. Biophys. Res. Commun. 2007, 352, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Verdoni, M.; Roudaut, H.; De Pomyers, H.; Gigmes, D.; Bertin, D.; Luis, J.; Bengeloune, A.H.; Mabrouk, K. ArgTX-636, a polyamine isolated from spider venom: A novel class of melanogenesis inhibitors. Bioorganic Med. Chem. 2016, 24, 5685–5692. [Google Scholar] [CrossRef] [PubMed]
- Sudan, H.L.; Kerry, C.J.; Mellor, I.R.; Choi, S.K.; Huang, D.; Nakanishi, K.; Usherwood, P.N.R. The action of philanthotoxin-343 and photolabile analogues on locust (Schistocerca gregaria) muscle. Invertebr. Neurosci. 1995, 1, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Nishimaru, T.; Sano, M.; Yamaguchi, Y.; Wakamiya, T. Syntheses and biological activities of fluorescent-labeled analogs of acylpolyamine toxin NPTX-594 isolated from the venom of Madagascar Joro spider. Bioorganic Med. Chem. 2009, 17, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Nørager, N.G.; Jensen, C.B.; Rathje, M.; Andersen, J.; Madsen, K.L.; Kristensen, A.S.; Strømgaard, K. Development of Potent Fluorescent Polyamine Toxins and Application in Labeling of Ionotropic Glutamate Receptors in Hippocampal Neurons. ACS Chem. Biol. 2013, 8, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Shinada, T.; Nakagawa, Y.; Hayashi, K.; Corzo, G.; Nakajima, T.; Ohfune, Y. Synthesis and paralytic activities of squaryl amino acid-containing polyamine toxins. Amino Acids 2003, 24, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Luck, V.L.; Richards, D.P.; Shaikh, A.Y.; Franzyk, H.; Mellor, I.R. The Effects of Structural Alterations in the Polyamine and Amino Acid Moieties of Philanthotoxins on Nicotinic Acetylcholine Receptor Inhibition in the Locust, Schistocerca gregaria. Molecules 2021, 26, 7007. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Sakaue, S.; Nagasaki, Y. Redox-active injectable gel using polyion complex to achieve sustained release of exenatide and enhance therapeutic efficacy for the treatment of type 2 diabetes. J. Biomed. Mater. Res. Part A 2019, 107, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Rioux, B.; Pinon, A.; Gamond, A.; Martin, F.; Laurent, A.; Champavier, Y.; Barette, C.; Liagre, B.; Fagnère, C.; Sol, V.; et al. Synthesis and biological evaluation of chalcone-polyamine conjugates as novel vectorized agents in colorectal and prostate cancer chemotherapy. Eur. J. Med. Chem. 2021, 222, 113586. [Google Scholar] [CrossRef] [PubMed]
- Basagni, F.; Marotta, G.; Rosini, M.; Minarini, A. Polyamine-Drug Conjugates: Do They Boost Drug Activity? Molecules 2023, 28, 4518. [Google Scholar] [CrossRef] [PubMed]
- Houdou, M.; Jacobs, N.; Coene, J.; Azfar, M.; Vanhoutte, R.; Haute, C.V.d.; Eggermont, J.; Daniëls, V.; Verhelst, S.H.L.; Vangheluwe, P. Novel green fluorescent polyamines to analyze ATP13A2 and ATP13A3 activity in the mammalian polyamine transport system. bioRxiv 2022, 13, 337. [Google Scholar] [CrossRef]
- Hashimoto, M.; Liu, Y.; Fang, K.; Li, H.Y.; Campiani, G.; Nakanishi, K. Preparation and biological properties of biotinylated PhTX derivatives. Bioorganic Med. Chem. 1999, 7, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.B.; Lee, J.S. Natural-product-based fluorescent probes: Recent advances and applications. RSC Med. Chem. 2023, 14, 412–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Marton, J.; Ametamey, S.M.; Cumming, P. A Review of Molecular Imaging of Glutamate Receptors. Molecules 2020, 25, 4749. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mao, J.; Tang, J.; Li, G.; Fang, F.; Tang, Y.; Ding, J. Surface spermidine functionalized PEGylated poly(lactide-co-glycolide) nanoparticles for tumor-targeted drug delivery. RSC Adv. 2017, 7, 22954–22963. [Google Scholar] [CrossRef]
- Song, X.; Han, X.; Yu, F.; Zhang, X.; Chen, L.; Lv, C. Polyamine-Targeting Gefitinib Prodrug and its Near-Infrared Fluorescent Theranostic Derivative for Monitoring Drug Delivery and Lung Cancer Therapy. Theranostics 2018, 8, 2217–2228. [Google Scholar] [CrossRef] [PubMed]
- Holbert, C.E.; Foley, J.R.; Yu, A.; Murray Stewart, T.; Phanstiel, O.T.; Oupicky, D.; Casero, R.A., Jr. Polyamine-Based Nanostructures Share Polyamine Transport Mechanisms with Native Polyamines and Their Analogues: Significance for Polyamine-Targeted Therapy. Med. Sci. 2022, 10, 44. [Google Scholar] [CrossRef] [PubMed]

| Organism | Common Name | Acylpolyamine | Membrane Receptor | Ref.: |
|---|---|---|---|---|
| Spider | ||||
| Agelenopsis aperta | Desert grass spider | AG-489 | TRPV1 channel ** | [74] |
| Araneus ventricosus | Nocturnal orb-weaver spider | AVTX-622 | Nav ion channel ¶ | [51] |
| Argiope lobata | Argiope spider (orb-weaver spider) | ARG-636 | iGluR (AMPA) ¶, **; nAChRs ** | [31,35,61,75,76] |
| Dolomedes okefinokensis | Fishing spider | CNS-2130 | Cav ion channel ** (L- and R-type) | [44,77] |
| Nephila clavata | Orb-weaver spider (Joro spider) | JSTX-3 | iGluR (AMPA) ** | [78] |
| NPTX-1 | iGluR (KA) ** | [65] | ||
| NPTX-8 | iGluR (KA) ** | [65] | ||
| Nephila maculata | Papua New Guinean orb-web spider | NSXT-3 | iGluR ¶ | [79] |
| Wasp | ||||
| Philanthus triangulum | Egyptian digger wasp | PhTX-433 | nAChR ¶ iGluR (NMDA) ¶ | [53] [23,69] |
| PhTX-343 | iGluR (AMPA) ¶, ** iGluR (NMDA) ¶, ** nAChR ¶, ** | [40,43,72] |
| (Acyl-)polyamine Analogs and Derivates | Application | Ref.: |
|---|---|---|
| Pharmacological leads | ||
| N1-dansyl-spermine | Antagonist of the CNS effects of spermine | [83] |
| ArgTX-636 | Inhibitor of neuronal nAchR and potential analgesic to reduce neuropathic pain | [61] |
| Parawixin (Pwtx)-1, 2, and -10 | Inhibition of seizures and neurodegeneration; neuroprotective and anticonvulsant | [85] |
| Long linear polyamines derivatives | Antimicrobial agent | [86] |
| Polyamine-drug conjugates | Antimicrobial agent | [87] |
| Mygalin (bis-acylpolyamine spermidine) | Antimicrobial and modulator of innate immune responses; anticancer | [88,89,93] |
| Acylspermidine derivatives | Antiproliferative (anticancer) and pro-apoptotic | [90] |
| Agel 416, HO-416b and JSTX-3 analogs | Antiproliferative (anticancer) agent | [91] |
| PA-366 and PA386 | Cytotoxic agent for specific lines of cancer cells | [92] |
| ArgTX-636 | Inhibition of melanogenesis | [94] |
| Probes | ||
| Photolabile analogs of PhTx-343 | Mapping sensitive receptors | [70,95] |
| Photolabile analogs of PhTx-433 | Mapping ligand-binding sites on receptors | [41] |
| Fluorescent analogs of NPTX-594 | Visualization of acylpolyamine toxin interactions with iGluRs | [96] |
| Fluorescent analogs of ArgTX-636 | Imaging of iGlu receptors in neurons | [97] |
| Insecticides | ||
| Glu-type squaryl-NPTX derivatives | Paralysis on insects, glutamatergic signaling disruptor | [98] |
| AVTX-636 | Paralysis on insects, inhibition of Nav ion channels | [51] |
| Cyclohexylalanine-PhTX-343 | Paralysis on insects, inhibition of locust nAchR | [99] |
| Carriers | ||
| Polyamine polyion complexes | Delivery of antidiabetic peptide | [100] |
| Chalcone-polyamine conjugates | Anticancer therapy acting via the upregulated polyamine transport system | [101] |
| Polyamine-drug conjugates | Delivery of bioactive payloads through the polyamine transporter system | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rádis-Baptista, G.; Konno, K. Spider and Wasp Acylpolyamines: Venom Components and Versatile Pharmacological Leads, Probes, and Insecticidal Agents. Toxins 2024, 16, 234. https://doi.org/10.3390/toxins16060234
Rádis-Baptista G, Konno K. Spider and Wasp Acylpolyamines: Venom Components and Versatile Pharmacological Leads, Probes, and Insecticidal Agents. Toxins. 2024; 16(6):234. https://doi.org/10.3390/toxins16060234
Chicago/Turabian StyleRádis-Baptista, Gandhi, and Katsuhiro Konno. 2024. "Spider and Wasp Acylpolyamines: Venom Components and Versatile Pharmacological Leads, Probes, and Insecticidal Agents" Toxins 16, no. 6: 234. https://doi.org/10.3390/toxins16060234
APA StyleRádis-Baptista, G., & Konno, K. (2024). Spider and Wasp Acylpolyamines: Venom Components and Versatile Pharmacological Leads, Probes, and Insecticidal Agents. Toxins, 16(6), 234. https://doi.org/10.3390/toxins16060234






