The Effects of T-2 Toxin, Deoxynivalenol, and Fumonisin B1 on Oxidative Stress-Related Genes in the Kidneys of Laying Hens
Abstract
1. Introduction
2. Results
2.1. Clinical Observations, Body Weight, and Relative Kidney Weight
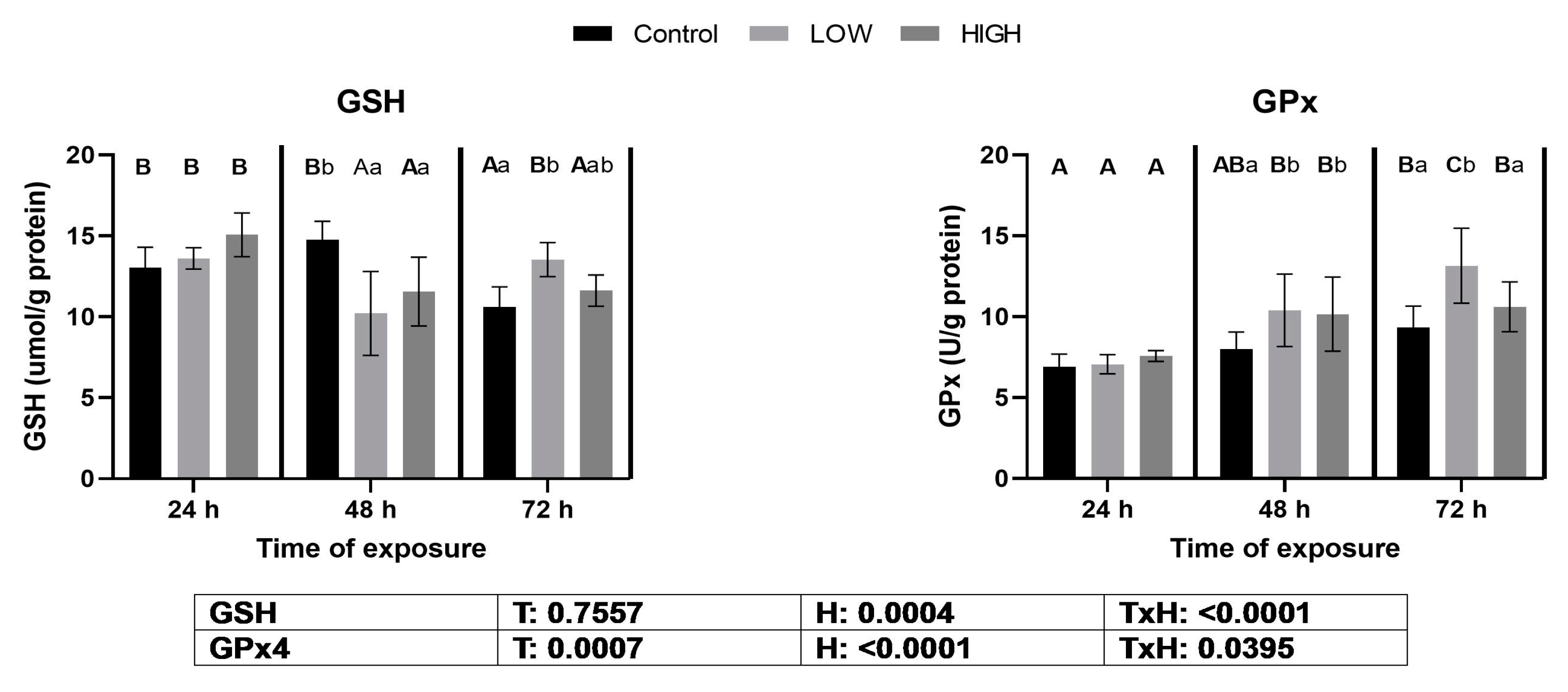
2.2. Glutathione Content and Glutathione Peroxidase Activity
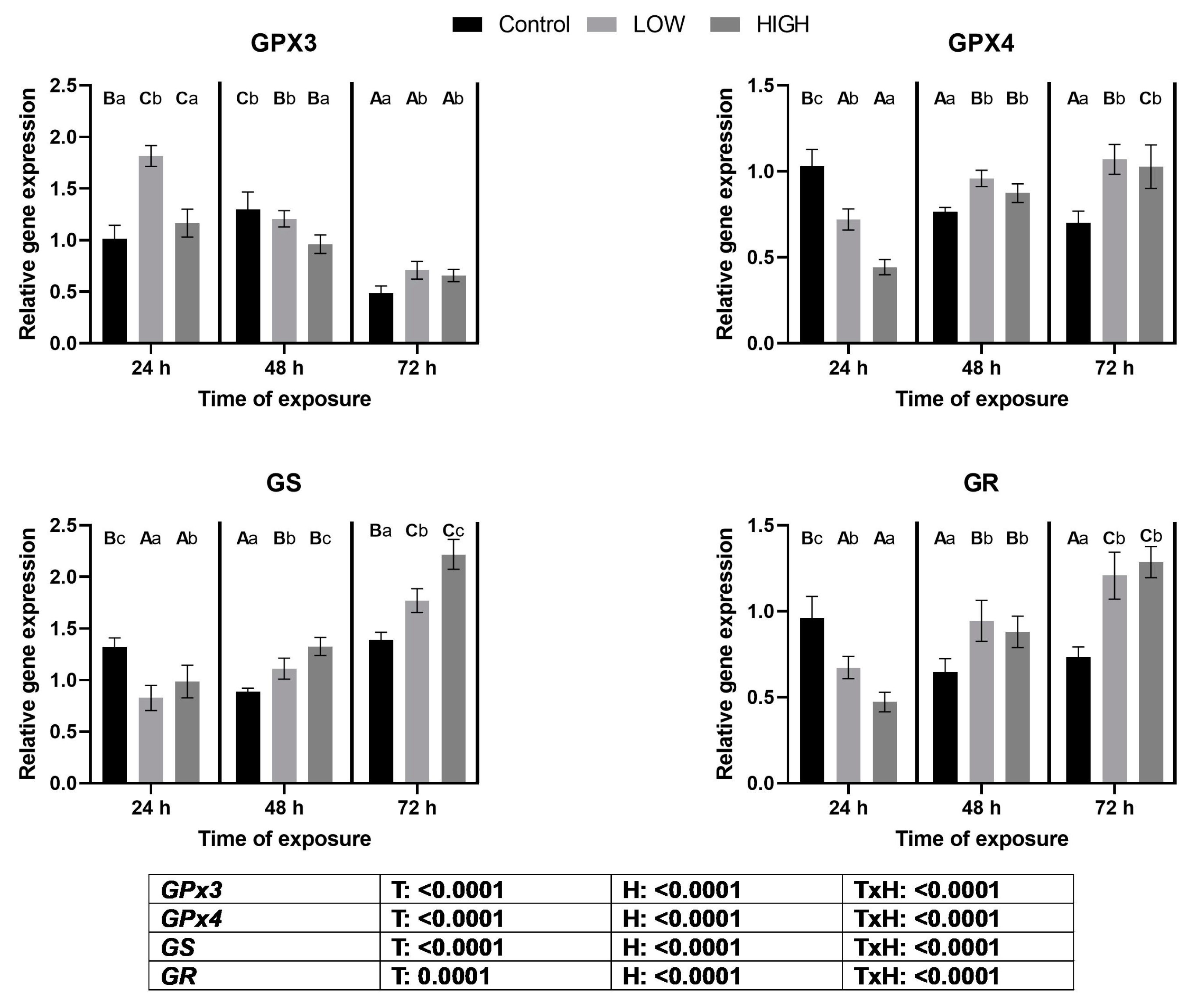
2.3. Relative Expression Levels of GPX3, GPX4, GS, and GR Genes
2.4. Relative Expression Levels of Genes KEAP1 and NRF2
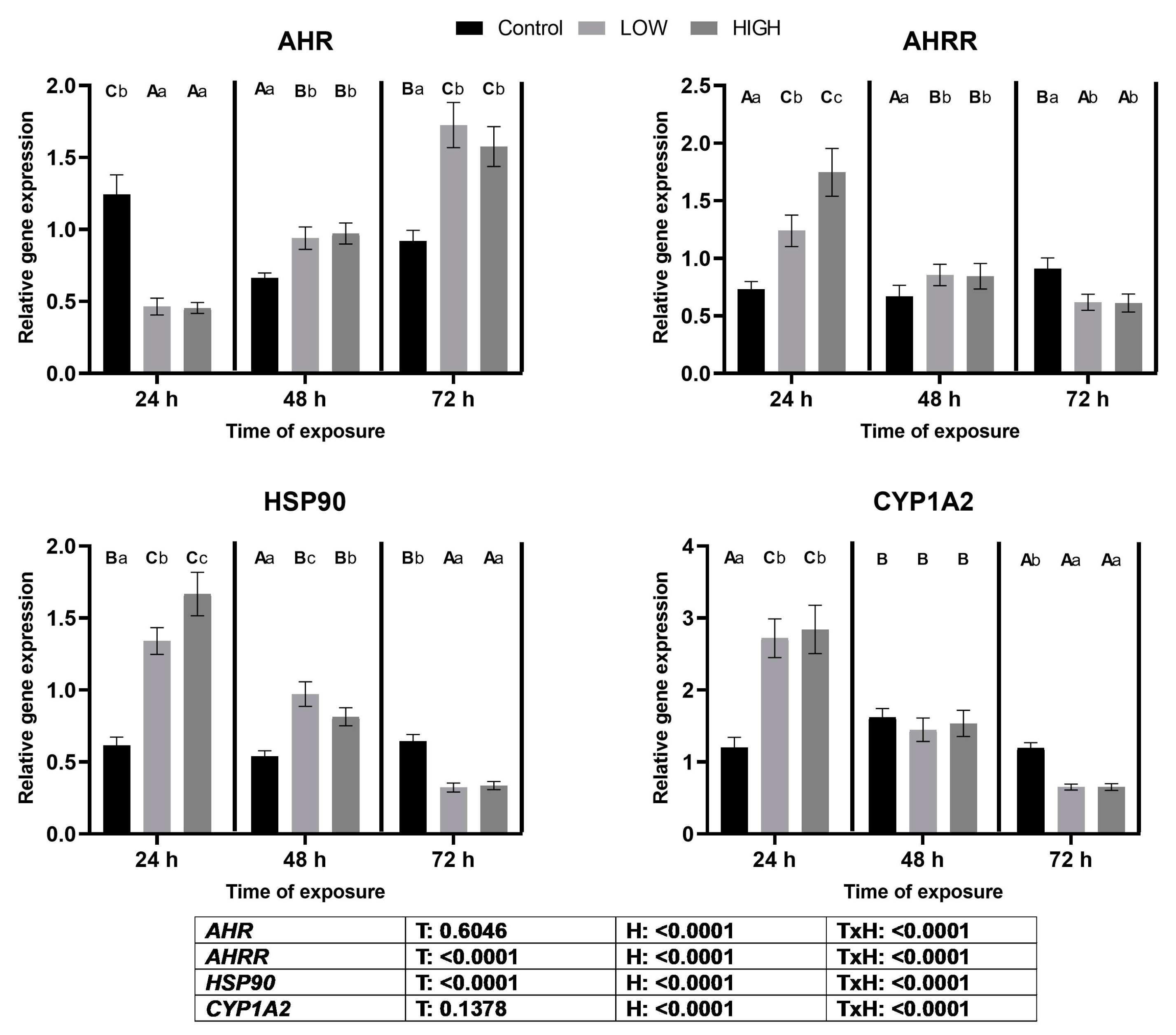
2.5. Relative Expression Levels of AHR, AHRR, HSP90, and CYP1A2 Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Birds and Experimental Design
- Control (CON): hens received a basal diet.
- Low-mix Group (LOW): hens received an experimentally contaminated basal diet. The mycotoxin contents were T-2+HT-2 toxin (0.24 mg), DON+ 3-AcDON+15-AcDON (1.25 mg), and FB1 (20 mg/kg feed).
- High-mix Group (HIGH): hens received an experimentally contaminated basal diet. The mycotoxin contents were T-2+ HT-2 toxin (0.46 mg), DON+3-AcDON+15-AcDON (3.65 mg), and FB1 (40.3 mg/kg feed).
5.2. Production and Determination of Mycotoxins
5.3. Biochemical Analyses
5.4. RNA Extraction and Reverse Transcription
5.5. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ráduly, Z.; Price, R.G.; Dockrell, M.E.C.; Csernoch, L.; Pócsi, I. Urinary Biomarkers of Mycotoxin Induced Nephrotoxicity-Current Status and Expected Future Trends. Toxins 2021, 13, 848. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef]
- Li, S.J.; Zhang, G.; Xue, B.; Ding, Q.; Han, L.; Huang, J.C.; Wu, F.; Li, C.; Yang, C. Toxicity and detoxification of T-2 toxin in poultry. Food Chem. Toxicol. 2022, 169, 113392. [Google Scholar] [CrossRef]
- Manafi, M.; Pirany, N.; Noor Ali, M.; Hedayati, M.; Khalaji, S.; Yari, M. Experimental pathology of T-2 toxicosis and mycoplasma infection on performance and hepatic functions of broiler chickens. Poult. Sci. 2015, 94, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Tso, K.H.; Lumsangkul, C.; Cheng, M.C.; Ju, J.C.; Fan, Y.K.; Chiang, H.I. Differential Effects of Green Tea Powders on the Protection of Brown Tsaiya and Kaiya Ducklings against Trichothecene T-2 Toxin Toxicity. Animals 2021, 11, 2541. [Google Scholar] [CrossRef]
- Hoerr, F.J.; Carlton, W.W.; Yagen, B.; Joffe, A.Z. Mycotoxicosis caused by either T-2 toxin or diacetoxyscirpenol in the diet of broiler chickens. Fundam. Appl. Toxicol. 1982, 2, 121–124. [Google Scholar] [CrossRef]
- Bócsai, A.; Pelyhe, C.; Zándoki, E.; Ancsin, Z.; Szabó-Fodor, J.; Erdélyi, M.; Mézes, M.; Balogh, K. Short-term effects of T-2 toxin exposure on some lipid peroxide and glutathione redox parameters of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Deng, J.; Zuo, Z.; Wang, Y.; Shen, L. Individual and combined effects of deoxynivalenol and zearalenone on mouse kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Zhang, N.; Qi, D. In vitro investigation of individual and combined cytotoxic effects of aflatoxin B1 and other selected mycotoxins on the cell line porcine kidney 15. Exp. Toxicol. Pathol. 2013, 65, 1149–1157. [Google Scholar] [CrossRef]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Mézes, M.; Balogh, K.; Bázár, G.; Kocsó, D.; Ali, O.; Kovács, M. Individual and Combined Effects of Fumonisin B1, Deoxynivalenol and Zearalenone on the Hepatic and Renal Membrane Lipid Integrity of Rats. Toxins 2017, 10, 4. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Z.; Wang, Y.; Long, M.; Wu, W.; Kuca, K. Fumonisin B1: Mechanisms of toxicity and biological detoxification progress in animals. Food Chem. Toxicol. 2021, 149, 111977. [Google Scholar] [CrossRef]
- Guerre, P.; Travel, A.; Tardieu, D. Targeted Analysis of Sphingolipids in Turkeys Fed Fusariotoxins: First Evidence of Key Changes That Could Help Explain Their Relative Resistance to Fumonisin Toxicity. Int. J. Mol. Sci. 2022, 23, 2512. [Google Scholar] [CrossRef]
- Tardieu, D.; Travel, D.; Le Bourhis, C.; Metayer, J.P.; Mika, A.; Cleva, D.; Boissieu, P.; Guerre, P. Fumonisins and zearalenone fed at low levels can persist several days in the liver of turkeys and broiler chickens after exposure to the contaminated diet was stopped. Food Chem. Toxicol. 2021, 148, 111968. [Google Scholar] [CrossRef]
- Laurain, J.; Tardieu, D.; Matard-Mann, M.; Rodriguez, M.A.; Guerre, P. Fumonisin B1 Accumulates in Chicken Tissues over Time and This Accumulation Was Reduced by Feeding Algo-Clay. Toxins 2021, 13, 701. [Google Scholar] [CrossRef]
- Guerre, P.; Matard-Mann, M.; Nyvall Collén, P. Targeted sphingolipid analysis in chickens suggests different mechanisms of fumonisin toxicity in kidney, lung, and brain. Food Chem. Toxicol. 2022, 170, 113467. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.Y.; Kuča, K.; Dohnal, V.; Zhang, X.J.; Yuan, Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Shar, Z.H.; Shar, H.H.; Jatoi, A.; Sherazi, S.T.H.; Mahesar, S.A.; Khan, E.; Phanwar, Q.K. Natural co-occurrence of Fusarium toxins in poultry feed and its ingredients. J. Consum. Prot. Food Saf. 2020, 15, 341–350. [Google Scholar] [CrossRef]
- Silva, E.O.; Bracarense, A.P.F.L.; Oswald, I.P. Mycotoxins and oxidative stress: Where are we? World Mycotoxin J. 2018, 11, 113–134. [Google Scholar] [CrossRef]
- Mavrommatis, A.; Giamouri, E.; Tavrizelou, S.; Zacharioudaki, M.; Danezis, G.; Simitzis, P.E.; Zoidis, E.; Tsiplakou, E.; Pappas, A.C.; Georgiou, C.A.; et al. Impact of mycotoxins on animals’ oxidative status. Antioxidants 2021, 10, 214. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Dano, S.D.; Moukha, S.; Mobio, T.A.; Creppy, E.E. Effects of combinations of Fusarium mycotoxins on the inhibition of macromolecular synthesis, malondialdehyde levels, D.N.A. methylation and fragmentation, and viability in Caco-2 cells. Toxicon 2007, 49, 306–317. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Moukha, S.; Brou, K.; Gnakri, D. Lipid metabolism disorders, lymphocytes cells death, and renal toxicity induced by very low levels of deoxynivalenol and fumonisin B1 alone or in combination following 7 days oral administration to mice. Toxicol. Int. 2013, 20, 218–223. [Google Scholar] [CrossRef]
- Grishanova, A.Y.; Perepechaeva, M.L. Aryl hydrocarbon receptor in oxidative stress as a double agent and its biological and therapeutic significance. Int. J. Mol. Sci. 2022, 23, 6719. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Huertero, F.; Zaragoza-Ojeda, M.; Sánchez-Alarcón, J.; Milić, M.; Segvić Klarić, M.; Montiel-González, J.M.; Valencia-Quintana, R. Involvement of Ahr pathway in toxicity of aflatoxins and other mycotoxins. Front. Microbiol. 2019, 10, 2347. [Google Scholar] [CrossRef]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR signaling pathways and regulatory functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef]
- Sondermann, N.C.; Faßbender, S.; Hartung, F.; Hätälä, A.M.; Rolfes, K.M.; Vogel, C.F.A.; Haarmann-Stemmann, T. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 2023, 208, 115371. [Google Scholar] [CrossRef]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Tox. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.N.; Viktorová, J.; Ruml, T. Mycotoxins: Biotransformation and Bioavailability Assessment Using Caco-2 Cell Monolayer. Toxins 2020, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-mycotoxin occurrence in feed, metabolism and carry-over to animal-derived food products: A review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef] [PubMed]
- European Commission Recommendation. European Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2, HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2007, L 118M, 1111–1113. [Google Scholar]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Kifer, D.; Jakśic, D.; Segviśc Klarić, M. Assessing the effect of mycotoxin combinations: Which mathematical model is (the most) appropriate? Toxins 2020, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wen, J.; Zhu, J.; Zhang, T.; Deng, Y.; Jiang, J. Aromatic hydrocarbon receptor regulates chicken cytochrome P450 1A5 transcription: A novel insight into T-2 toxin-induced gene expression and cytotoxicity in L.M.H. cells. Biochem. Pharmacol. 2019, 168, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Kövesi, B.; Kulcsár, S.; Ancsin, Z.; Erdélyi, M.; Zándoki, E.; Gömbös, P.; Balogh, K.; Mézes, M. Multi-Fusarium mycotoxin exposure activates Nrf2 and Ahr pathway in the liver of laying hens. Tox. Lett. 2024, 391, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I. Glutathione peroxidases in poultry biology: Part 1. Classification and mechanisms of action. World’s Poult. Sci. J. 2018, 74, 185–198. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Kowalska, K.; Piastowska-Ciesielska, A.W. Nrf2: A main responsive element in cells to mycotoxin-induced toxicity. Arch. Toxicol. 2021, 95, 1521–1533. [Google Scholar] [CrossRef]
- Avissar, N.; Ornt, D.B.; Yagil, Y.; Horowitz, S.; Watkins, R.H.; Kerl, E.A.; Takhashi, K.; Palmer, D.S.; Cohen, H.J. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am. J. Physiol. 1994, 266, C367–C375. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular glutathione peroxidase GPx3 and its role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Xiea, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Pelyhe, C.; Kövesi, B.; Zándoki, E.; Kovács, B.; Erdélyi, M.; Kulcsár, S.; Mézes, M.; Balogh, K. Multi-trichothecene mycotoxin exposure activates glutathione-redox system in broiler chicken. Toxicon 2018, 153, 53–57. [Google Scholar] [CrossRef]
- Kulcsár, S.; Kövesi, B.; Balogh, K.; Zándoki, E.; Ancsin, Z.; Erdélyi, M.; Mézes, M. The co-occurrence of T-2 toxin, deoxynivalenol, and fumonisin B1 activated the glutathione redox system in the EU-limiting doses in laying hens. Toxins 2023, 15, 305. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Flohé, L. Glutathione-peroxidase–enzymology and biological aspects. Klin. Wochenschr 1971, 49, 669–683. [Google Scholar] [CrossRef]
- Toppo, S.; Flohé, L.; Ursini, F.; Vanin, S.; Matilde Maiorino, M. Catalytic mechanisms and specificities of glutathione peroxidases: Variations of a basic scheme. Biochim. Biophys. Acta 2009, 1790, 1486–1500. [Google Scholar] [CrossRef]
- Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Canali, E.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; Herskin, M.; et al. Welfare of laying hens on farm. EFSA J. 2023, 21, e07789. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound and non-protein sulfhydryl groups in tissues with Ellmann’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, J.; Deng, F.; Yan, Z.; Xia, Y.; Wang, Z.; Ye, J.; Deng, Y.; Zhang, Z.; Qiao, M.; et al. TqPCR: A touchdown qPCR assay with significantly improved detection sensitivity and amplification efficiency of S.Y.B.R. green qPCR. PLoS ONE 2015, 10, e0132666. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The M.I.Q.E. guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]

| Group | T-2/HT-2 | DON/3-AcDON/15-AcDON | FB1 |
|---|---|---|---|
| Control | <0.01/˂0.01 | <0.02/0.02/<0.02 | 0.2 |
| Low dose | 0.13/0.11 | 0.67/0.57/0.01 | 20.0 |
| High dose | 0.30/0.16 | 2.70/0.89/0.06 | 40.3 |
| Genes | GenBank Accession No. | Primer Sequences, 5′-3′ | Length, bp. | Efficiency, % |
|---|---|---|---|---|
| Internal controls | ||||
| GAPDH | NM_204305.1 | F-TGACCTGCCGTCTGGAGAAA R-TGTGTATCCTAGGATGCCCTTCAG | 98 | 92.64 |
| BAC | NM_205518.2 | F-GACGAGATTGGCATGGCTTTATTT R-TAAGACTGCTGCTGACACCTTC | 92 | 96.29 |
| RPL13 | NM_204999.2 | F-GCTTAAACTGGCGGGCATTAAC R-GGCTTGCAGTGACTCTGTAGAT | 97 | 94.97 |
| Target genes | ||||
| KEAP1 | KU321503.1 | F-CATCGGCATCGCCAACTT R-TGAAGAACTCCTCCTGCTTGGA | 113 | 99.74 |
| NRF2 | NM_205117.1 | F-TTTTCGCAGAGCACAGATAC R-GGAGAAGCCTCATTGTCATC | 110 | 91.74 |
| GPX3 | NM_001163232.2 | F-ATCCCCTTCCGAAAGTACGC R-GACGACAAGTCCATAGGGCC | 129 | 102.51 |
| GPX4 | NM_001346448.1 | F-AGTGCCATCAAGTGGAACTTCAC R-TTCAAGGCAGGCCGTCAT | 203 | 91.03 |
| GS | XM_425692.6 | F-GTACTCACTGGATGTGGGTGAAGA R-CGGCTCGATCTTGTCCATCAG | 196 | 104.84 |
| GR | XM_015276627.2 | F-CCACCAGAAAGGGGATCTACG R-ACAGAGATGGCTTCATCTTCAGTG | 208 | 91.76 |
| AHR | NM_204118.3 | F-GAAGACGGGTGAGAGTGGAA R-CGCTTCCGTAGATGTTCTGC | 171 | 99.20 |
| AHRR | NM_001201387.2 | F-AGAACGGCACCATGAGGAAG R-CAGAGGTCCGGTTCTGCTTT | 73 | 99.86 |
| HSP90 | NM_001109785.2 | F-TGAAACACTGAGGCAGAAGG R-AAAGCCAGAGGACAGGAGAG | 100 | 95.05 |
| CYP1A2 | NM_205146.3 | F-ACGCAGATCCCAAACGAGAA R-GTCAAAGCCTGCTCCAAAGATG | 63 | 102.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kövesi, B.; Kulcsár, S.; Ancsin, Z.; Erdélyi, M.; Zándoki, E.; Gömbös, P.; Balogh, K.; Mézes, M. The Effects of T-2 Toxin, Deoxynivalenol, and Fumonisin B1 on Oxidative Stress-Related Genes in the Kidneys of Laying Hens. Toxins 2024, 16, 154. https://doi.org/10.3390/toxins16030154
Kövesi B, Kulcsár S, Ancsin Z, Erdélyi M, Zándoki E, Gömbös P, Balogh K, Mézes M. The Effects of T-2 Toxin, Deoxynivalenol, and Fumonisin B1 on Oxidative Stress-Related Genes in the Kidneys of Laying Hens. Toxins. 2024; 16(3):154. https://doi.org/10.3390/toxins16030154
Chicago/Turabian StyleKövesi, Benjamin, Szabina Kulcsár, Zsolt Ancsin, Márta Erdélyi, Erika Zándoki, Patrik Gömbös, Krisztián Balogh, and Miklós Mézes. 2024. "The Effects of T-2 Toxin, Deoxynivalenol, and Fumonisin B1 on Oxidative Stress-Related Genes in the Kidneys of Laying Hens" Toxins 16, no. 3: 154. https://doi.org/10.3390/toxins16030154
APA StyleKövesi, B., Kulcsár, S., Ancsin, Z., Erdélyi, M., Zándoki, E., Gömbös, P., Balogh, K., & Mézes, M. (2024). The Effects of T-2 Toxin, Deoxynivalenol, and Fumonisin B1 on Oxidative Stress-Related Genes in the Kidneys of Laying Hens. Toxins, 16(3), 154. https://doi.org/10.3390/toxins16030154







