Botulinum Toxin for Pain Relief in Cancer Patients: A Systematic Review of Randomized Controlled Trials
Abstract
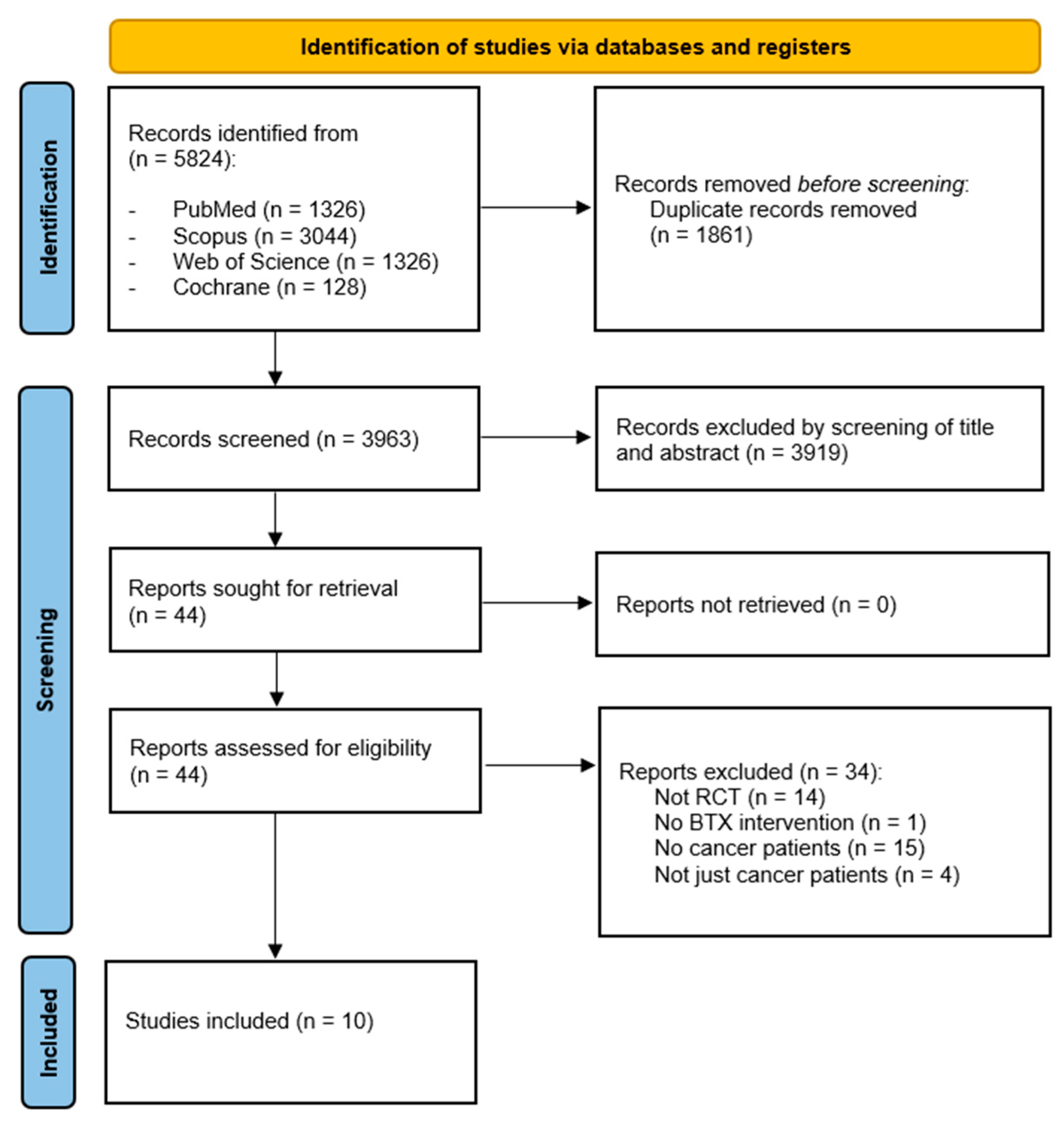
1. Introduction
2. Results
2.1. Study Characteristics
2.2. Intervention Characteristics
2.2.1. Intervention Characteristics Based on Cancer Type
- −
- Breast cancer was assessed in four studies [27,28,29,30]. More in detail, in the studies conducted by De Groef et al. [27,28], the intervention was characterized by single BoNT-A infiltration combined with a standard physical therapy program. Intramuscular injection of BONT-A (100 units, OnabotulinumtoxinA; BOTOX, Allergan, Inc., Irvine, CA, USA) was administered in the pectoralis major muscle. Moreover, in the study by Gabriel et al. [29], a single injection of 40 units (20 units/mL) of onabotulinumtoxinA, was administered during surgery, in the pectoralis major muscle. In addition, a single injection of 100 units of botulinum toxin combined with 10 mL of normal saline throughout the pectoralis major muscle was performed and assessed in the study by Lo et al. [30].
- −
- Head and Neck cancer was assessed in four studies [31,32,34]. More in detail, in the study conducted by Nieri et al. [31], the intervention was characterized by injection of 1 mL of onabotulinumtoxinA (50 units) in each submandibular gland. The injections were prepared by mixing 100 units of onabotulinumtoxinA in 2 mL of sterile saline in a syringe. Moreover, in the study by Teymoortash et al. [32], 15 U onabotulinumtoxinA was injected into the submandibular glands. In this study, there were two different intervention groups. In IG1, the BoNT-A was injected in the right gland, and NaCl (0.9%) in the left gland, while in the IG2, the BoNT-A was injected in the left gland, and NaCl (0.9%) in the right gland.
- −
- Esophageal cancer was assessed in two studies [33,35]. More in detail, in the study conducted by Wen et al. [33], the intervention was characterized by a single session of BoNT-A (lanbotulinumtoxinA, Lanzhou Institute of Biological Products, Lanzhou, China) injections that was undertaken immediately after endoscopic submucosal dissection. A total of 100 units of BoNT-A was combined with 5 mL of saline solution (20 units/mL). The BoNT-A solution was injected in 0.5 mL increments into 10 separate points equally spaced along the circumference of the defect. Moreover, in the study by Zhou [35], lanbotulinumtoxinA was injected in 5 mL increments into 10 separate points at the level of the muscularis propria equally spaced along the circumference of the defect, immediately after the endoscopic submucosal dissection procedure. A total of 100 units of BoNT-A was combined with 5 mL of saline solution (20 units/mL).
- −
- Thoracic and gastric esophageal cancer was assessed in one study [3]. More in detail, in the study conducted by Bagheri et al. [26], the intervention was characterized by a single injection of botulinum toxin into the pyloric sphincter muscle, immediately after surgery. OnabotulinumtoxinA was injected (200 units of toxin combined with 5 mL of 0.9% saline solution) with a 21 G needle at the upper and lower sections of the pyloric muscle in a transmural manner.
2.2.2. Control Characteristics
- −
- Placebo infiltrations were assessed in five studies [27,28,29,30,31]. In the two studies conducted by De Groef [27,28], the CG was treated with placebo (saline) infiltration. In particular, the placebo infiltration consisted of 50 mL saline (Mini-Plasco 20 mL B. Braun NaCl 0.9%). In the study by Gabriel et al. [29], the CG was treated with a single injection of 2 mL of NaCl during surgery, in the pectoralis major muscle. Moreover, in the study conducted by Nieri et al. [31], the CG was treated with a single injection of 1 mL of saline in each submandibular gland.
- −
- A combination of BoNT-A and BoNT-B was assessed in one study [32]. In this study, control groups were treated with 15 U of onabotulinumtoxinA and 750 U BoNT-B (rimabotulinumtoxinB, Eisai Manufacturing Knowledge Centre, United Kingdom), injected into the submandibular glands. In particular, one control group was treated with BoNT-A and B in the right gland, and NaCl (0.9%) in the left gland, while the second control group was treated with BoNT-A and B in the left gland, and NaCl (0.9%) in the right gland.
- −
- A high dose of BoNT was assessed by Wittekindt et al. [34]. This study assessed the effects of a low dose of onabotulinumtoxinA that was reconstituted in saline to a concentration of 10 mouse units (MU)/0.1 mL saline (intervention group). The control group was treated with a high dose of BoNT. In particular, BoNT-A was reconstituted in saline to a concentration of 20 MU/0.1 mL saline (high-dose group).
- −
- Triamcinolone acetonide (TA) was assessed in the study conducted by Zhou et al. [35]. In particular, TA was combined with 0.9% NaCl to a final concentration of 4 mg/mL. A total of 40 mg (10 mL) TA was injected into the deep submucosa of the ulcer base at 10 sites, with a 1 mL dose at each site, immediately after the ESD procedure.
- −
2.3. Main Findings
2.3.1. Pain Intensity
2.3.2. Quality of Life
2.3.3. Physical Functioning
2.3.4. Dysphagia and Salivary Outcomes
2.3.5. Esophageal Strictures and Bougie Dilatations
2.3.6. Gastric Emptying
2.3.7. Main Findings in Terms of Expansions
| Authors and Year, Journal | Population | Intervention | Comparator | Protocol Duration | Outcomes | Main Findings | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | Age (years) | BMI (kg/m2) | Male/Female | Cancer | Cancer Characteristics | Cancer Treatment | ||||||
| Bagheri et al., 2013, Asian Cardiovascular & Thoracic Annals, Iran [26] | N = 60 IG = 30 CG = 30 | N = 61 ± 10.7 (range 41–89) | NR | N = 33 M (55%)/27 F (45%) IG: 19 M (63.3%)/11 F (36.7%) CG: 14 M (46.6%)/16 F (53.4%) | Thoracic and gastric esophageal cancer | Esophageal cancer in the middle and lower third parts | Esophagectomy | After the surgery, a single injection of botulinum toxin was administered into the pyloric sphincter muscle. The injection consisted of 200 units of toxin combined with 5 mL of 0.9% saline solution, which was delivered using a 21 G needle. The injection was given transmurally, targeting both the upper and lower sections of the pyloric muscle. | No BoNT treatment | 3 weeks follow-up) |
| This study reports about 60 patients with thoracic and gastric esophageal cancer (mean age: 61 ± 10.7; 55% male). Immediately after surgery, they were treated with single injection of botulinum toxin into the pyloric sphincter muscle. The study duration was 3 weeks (follow-up). The main finding is represented by no significant difference between the 2 groups in terms of gastric emptying at 7 days (p = 0.446) and no significant differences after 3 weeks (p = 0.355). The success rate of BoNT injection was 90%, and there were no negative effects. |
| De Groef et al., 2018, Archives of physical medicine and rehabilitation, Belgium [27] | N = 50 IG = 25 CG = 25 | IG: 53.4 ± 10.0 CG: 56.6 ± 10.0 | IG: 24.8 ± 3.6 CG: 28.1 ± 5.0 | N = 50 F (100%) | Breast cancer | Primary breast cancer |
| Single Botulinum Toxin A (BoNT-A) infiltration + standard physical therapy program Intramuscular injection of BoNT-A (100 units, Allergan Botox) in the pectoralis major muscle. Standard physical therapy program of 12 weeks (one 30 min session per week). | Placebo (saline) infiltration + standard physical therapy program 50 mL saline (Mini-Plasco 20 mL B. Braun NaCl 0.9%). Standard physical therapy program of 12 weeks (one 30 min session per week). | 6 months follow-up |
| This study reports about 50 breast cancer patients (mean age IG: 53.4 ± 10.0, CG: 56.6 ± 10.0; mean BMI IG: IG: 24.8 ± 3.6, CG: 28.1 ± 5.0; 100% female) They were treated with single injection of Botulinum Toxin A and standard physical therapy program. BoNT-A was injected into the pectoralis major muscle. The study duration was 6 months (follow-up). The main finding is represented by upper limb pain intensity. With a mean difference in change of 16 points on the VAS scale (0–100), there was a significant difference in upper limb pain intensity between the groups from baseline to 6 months, favoring the intervention group (p = 0.040; 95% CI: 1 to 31). For pain quality, no differences between groups were found. For pressure hypersensitivity only for the serratus anterior muscle a significantly different change was found (0.61 kg/cm2; 95% CI: 0.07 to 1.15) after 1 month (p = 0.028). There were no differences in upper limb function across the groups. There was a tendency to a significant difference between the two groups, with the intervention group showing a higher prevalence rate of reduced shoulder function at one month (74% versus 96%, p = 0.096). A marginally significant outcome (p = 0.049) in favor of the control group was obtained for the mental functioning domain in terms of quality of life. |
| De Groef et al., 2020, European Journal of Cancer Care, Belgium [28] | N = 50 IG = 25 CG = 25 | IG: 53.4 ± 10.0 CG: 56.6 ± 10.0 | IG: 24.8 ± 3.6 CG: 28.1 ± 5.0 | N = 50 F (100%) | Breast cancer | Primary breast cancer |
| Single Botulinum Toxin A (BoNT-A) infiltration + standard physical therapy program Intramuscular injection of BoNT-A (100 units, Allergan Botox) in the pectoralis major muscle. Standard physical therapy program of 12 weeks (one 30 min session per week). | Placebo (saline) infiltration + standard physical therapy program 50 mL saline (Mini-Plasco 20 mL B. Braun NaCl 0.9%). Standard physical therapy program of 12 weeks (one 30 min session per week). | 6 months follow-up |
| This study reports about 50 breast cancer patients (mean age IG: 53.4 ± 10.0, CG: 56.6 ± 10.0; mean BMI IG: IG: 24.8 ± 3.6, CG: 28.1 ± 5.0; 100% female) They were treated with single injection of Botulinum Toxin A and standard physical therapy program. BoNT-A was injected into the pectoralis major muscle. The study duration was 6 months (follow-up). There has only been a significant difference (p = 0.042) in the change between groups from baseline to three months for scapular upward rotation during maximal range motion (>135°) of the upper limb (mean difference in change of 9°; 95% CI: 0 to 9). However, both groups had overall improvements in shoulder function and range of motion with the physical therapy for active forward flexion. |
| Gabriel et al., 2015, Aesthetic Surgery Journal, USA [29] | N = 30 IG = 15 CG = 15 | N = 44.5 (range 27–64) | NR | N = 30 F (100%) | Breast cancer | NR | Unilateral or bilateral tissue expander Breast reconstruction following therapeutic skin-sparing or nipple-sparing mastectomy | Single injection of 40 units (20 units/mL) of neurotoxin (OnabotulinumtoxinA; BOTOX, Allergan, Inc., Irvine, CA, USA), during surgery, in the pectoralis major muscle | Single injection of 40 units (20 units/mL) of placebo (0.9% NaCl), during surgery, in the pectoralis major muscle | 12–36 months follow-up |
| This study reports about 30 breast cancer patients (mean age: 44.5 [range 27–64]; 100% female) They were treated with single injection of Botulinum Toxin A, during surgery. BoNT-A was injected into the pectoralis major muscle. The study duration was 12–36 months (follow-up). The VAS score showed a significant difference (p < 0.05) between the two groups, indicating reduced pain in the IG. Furthermore, a noteworthy rise in the volume of expansion each visit was seen in the intervention group vs. to the placebo group (p < 0.0001). The usage of the neurotoxic did not result in any adverse effects. |
| Lo et al., 2015, Annals of Plastic Surgery, USA [30] | N = 23 | N = 46.5 (range 28–68) | NR | N = 23 F (100%) | Breast cancer | NR | Bilateral mastectomy | 100 units of botulinum toxin were injected in a fan-like manner throughout the pectoralis major muscle after tion with 10 mL of normal saline. | 100 units of normal saline placebo were injected throughout the pectoralis major muscle | 12 weeks follow-up |
| This study reports about 23 breast cancer patients (mean age 46.5 [range 28–68]; 100% female) They were treated with single injection of Botulinum Toxin A. BoNT was injected into the in the pectoralis major muscle. The study duration was 12 weeks (follow-up). At every stage of the postoperative period, there were no statistically significant improvements in the mean change in pain levels between the botulinum toxin and placebo groups (all p > 0.05). The complication rate did not differ significantly between the groups (p = 0.53). |
| Nieri et al., 2023, Head & Neck, USA [31] | N = 20 IG = 10 CG = 10 | IG: 55.1 ± 10.2 CG: 66.3 ± 10.2 | NR | N = 33 M (55%)/27 F (45%) IG: 7 M (70%)/3 F (30%) CG: 8 M (80%)/2 F (20%) | Head and neck cancer | Stage III or IV head and neck squamous cell carcinoma | Radiation or chemoradiation | Injection of 1 mL of BoNT (50 units) in each submandibular gland (100 units of BoNT were mixed in 2 mL of sterile saline in a syringe) | Injection of 1 mL of 1 mL of saline in each submandibular gland (2 mL of sterile saline in a syringe) | 6 weeks follow-up |
| This study reports about 20 head and neck cancer patients (mean age IG: 55.1 ± 10.2, CG: 66.3 ± 10.2; 55% male) They were treated with single injection of Botulinum Toxin A. BoNT-A was injected in each submandibular gland. The study duration was 6 months (follow-up). The main finding is that both the control and BoNT groups resulted in a significant improvement in quality of life from V1 (one week prior to radiation therapy) to V2 (one week following radiation therapy) (IG: p = 0.049; CG: p = 0.034). The OHIP-14 did not significantly improve in either group from V1 to V3 or from V2 to V3 (6 weeks after radiation therapy) (p > 0.05). Salivary flow rate was significantly reduced in both groups in V2 (one week following radiation therapy) compared to V1 (one week prior to radiation therapy) (p < 0.05). Six weeks following radiation therapy, there was no statistically significant difference in the salivary flow rates between V2 and V3 in either group (p > 0.05). The control group resulted in a significant decrease in salivary flow rate from V1 to V3 (p < 0.05), but the group that received BoNT treatment did not experience this same reduction (p > 0.05). At V3, the BoNT group’s level of the neutrophil chemoattractant CXCL-1 (GRO) was lower than that of the control group. |
| Teymoortash et al., 2016, PLoS One, Germany [32] | N = 12 IG1 = 3 IG2 = 3 IG3 = 3 IG4 = 3 | N = 55.4 (range 45–66) | NR | N = 10 M (83%)/2 F (17%) | Head and neck cancer | Stage III or IV head and neck squamous cell carcinoma | Chemoradiation | 15 U BoNT/A (Allergan Pharmaceuticals, Ireland) was injected into the submandibular glands. IG1: BoNT/A injected in the right gland, NaCl (0.9%) in the left gland. IG2: BoNT/A injected in the left gland, NaCl (0.9%) in the right gland | 15 U BoNT/A and 750 U BoNT/B (Eisai Manufacturing Knowledge Centre, United Kingdom) were injected into the submandibular glands. IG3: BoNT/A and B in the right gland, NaCl (0.9%) in the left gland IG4: BoNT/A and B in the left gland, NaCl (0.9%) in the right gland | 6 months follow-up |
| This study reports about 12 head and neck cancer patients (mean age: 55.4 [range 45–66]; 83% male) The study duration was 6 months (follow-up). The main finding is represented by significant improvements in the scintigraphic uptake difference. The scintigraphic uptake difference between BoNT and placebo was not significantly different, according to the analysis of the scintigraphic data (BoNT/A: p = 0.84 and BoNT/A-B: p = 0.56 for BoNT/A vs. placebo and BoNT/A-B vs. placebo, respectively). Additionally, the salivary excretion fraction did not significantly differ between the BoNT and placebo groups (BoNT/A: p = 0.44; BoNT/A-B: p = 0.44). The BoNT/A and B were well tolerated. |
| Wen et al., 2016, Gastrointestinal Endoscopy, China [33] | N = 67 IG = 33 CG = 34 | N = 60.4 ± 11.4 IG = 61.8 ± 7.9 CG = 60.1 ± 7.3 | NR | N = 46 M (68.7%)/21 F (31.3%) IG: 23 M (69.7%)/10 F (30.3%) CG: 23 M (67.6%)/11 F (32.4%) | Esophageal carcinoma | Esophageal cancer in the upper, middle, and lower third parts | Endoscopic submucosal dissection | BoNT-A injections were administered after ESD. 100 units of BoNT-A were combined with 5 mL of saline solution and injected into 10 points around the circumference of the defect in 0.5 mL increments. | No BoNT treatment | 12 weeks |
| This study reports about 67 esophageal cancer patients (mean age IG: 61.8 ± 7.9; CG = 60.1 ± 7.3; 68.7% male) They were treated with single injection of Botulinum Toxin A. BoNT-A was injected into 10 separate points equally spaced along the circumference of the defect immediately after endoscopic submucosal dissection. The study duration was 12 weeks (follow-up). The main finding is represented by significant improvements in the Quality of Life. The Group B had a mean EORTC QLQ-OES18 score of 30.5 ± 7.2, while group A had a mean score of 25.8 ± 6.2. This difference was statistically significant (p < 0.05). The Atkinson assessment of dysphagia showed a significant difference (p = 0.02) between the two groups, in favor of BoNT group. In the intervention group, there were significantly less patients diagnosed with esophageal stricture (PP analysis, 6.1%, 2/33; ITT analysis, 11.4%, 4/35) than in the control group (PP analysis, 32.4%, 11/34; ITT analysis, 37.8%, 14/37) (p < 0.05). Moreover, there was a statistically significant difference in the number of bougie dilation procedures between the intervention group (mean, 1.5; range, 0–2) and control group (mean, 2.8; range, 0–5) (p < 0.05). |
| Wittekindt et al., 2006, Laryngoscope, Germany [34] | N = 23 IG1 = 13 IG2 = 10 | N = 60.4 ± 11.4 | NR | N = 21 M (91.3%)/ 2 F (8.7%) | Head and neck cancer | Stage II–III squamous cell carcinoma of the upper aerodigestive tract | Radical cervical neck dissection | BoNT-A formulation Dysport (Ipsen Pharma, Ettlingen, Germany). BoNT-A was reconstituted in saline to a concentration of 10 mouse units (MU) per 0.1 mL saline (low-dose group). | BoNT-A formulation Dysport (Ipsen Pharma, Ettlingen, Germany). BoNT-A was reconstituted in saline to a concentration of 20 MU/0.1 mL saline (high-dose group). | 28 days |
| This study reports about 23 head and neck cancer patients (mean age 60.4 ± 11.4; 91.3% male) They were treated with single injection of Botulinum Toxin A. BoNT-A was injected into the submandibular glands. The study duration was 28 days (follow-up). The main finding is represented by significant improvements in pain. Patients in the low-dose group showed a statistically significant decrease in pain (VAS) from 4.3 on day 0 to 3.0 on day 28 (p < 0.05). The high-dose group’s pain VAS scores did not significantly improve. In the low-dose group, there was a trend in global quality of life, but not a statistically significant increase (p = 0.15). There were no adverse events observed. |
| Zhou et al., 2021, Journal of Cancer, China [35] | N = 78 IG1 = 26 IG2 = 16 CG = 36 | IG1: 65.15 ± 7.23 IG2: 65.06 ± 7.88 CG: 65.28 ± 8.11 | IG1: 23.35 ± 2.72 IG2: 23.47 ± 3.04 CG: 23.83 ± 3.03 | IG1: 65.4% M/34.6% F IG2: 62.5% M/37.5 F CG: 72.2% M/17.8% F | Esophageal cancer | Endoscopic submucosal dissection | IG1: BoNT-A solution was injected into 10 points at the muscularis propria level immediately after the ESD procedure. Each injection was done in 5-mL increments, and a total of 100 units of BoNT-A were used. The solution was combined with 5 mL of saline solution, resulting in a concentration of 20 units/mL. | IG2: TA mixed with NaCl was injected into the submucosa of the ulcer base at 10 sites (1 mL per site) after the ESD procedure, totaling 40 mg (10 mL). CG: No BoNT treatment | 12 weeks |
| This study reports about 78 esophageal cancer patients (mean age IG1: 65.15 ± 7.23, IG2: 65.06 ± 7.88, CG: 65.28 ± 8.11; IG1: 65.4% male, IG2: 62.5% male, CG: 72.2% male) They were treated with single injection of Botulinum Toxin A. BoNT-A was injected into 10 separate points at the level of the muscularis propria equally spaced along the circumference of the defect, immediately after ESD procedure. The study duration was 12 weeks (follow-up). The main finding is represented by significant improvements in esophageal stricture development. In the BoNT-A group, the percentage of patients developing stricture was 30.00% (intention to treat analysis, 9/30) and 26.92% (per protocol analysis, 7/26). In the TA group, it was 40.90% (intention to treat analysis, 9/22) and 43.75% (per protocol analysis, 7/16). In the control group, the percentage was 84.21% (intention to treat analysis, 32/38) and 83.33% (per protocol analysis, 30/36) (p < 0.001). Subsequent analysis comparing the two groups revealed that the incidence of esophageal stricture was reduced in the BoNT-A group (p < 0.001) and in the TA group (p = 0.004). In addition, the esophageal stricture in the BoNT-A group was significantly lower compared to the TA group in the entire circumference mucosal defect subgroup (33.3% vs. 100%, p = 0.0454). | |
2.4. Meta-Analysis
2.5. Quality Assessment and Risk of Bias
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Registration
5.2. Search Strategy
5.3. Selection Criteria
- −
- (P) Participants: patients over 18 years old with cancer.
- −
- (I) Intervention: pre-operative, intra-operative, or post-operative treatment with botulinum.
- −
- (C) Comparator: any comparator.
- −
- (O) Outcome: The primary outcome was pain intensity. Secondary outcomes were physical functioning, fatigue, and quality of life.
5.4. Data Extraction and Synthesis
5.5. Quality Assessment and Risk of Bias
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Sire, A.; Lippi, L.; Ammendolia, A.; Cisari, C.; Venetis, K.; Sajjadi, E.; Fusco, N.; Invernizzi, M. Physical exercise with or without whole-body vibration in breast cancer patients suffering from aromatase inhibitor—Induced musculoskeletal symptoms: A pilot randomized clinical study. J. Pers. Med. 2021, 11, 1369. [Google Scholar] [CrossRef]
- Caraceni, A.; Shkodra, M. Cancer pain assessment and classification. Cancers 2019, 11, 510. [Google Scholar] [CrossRef]
- de Sire, A.; Losco, L.; Cisari, C.; Gennari, A.; Boldorini, R.; Fusco, N.; Cigna, E.; Invernizzi, M. Axillary web syndrome in women after breast cancer surgery referred to an Oncological Rehabilitation Unit: Which are the main risk factors? A retrospective case-control study. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8028–8035. [Google Scholar] [CrossRef]
- Talbot, K.; Madden, V.; Jones, S.; Moseley, G. The sensory and affective components of pain: Are they differentially modifiable dimensions or inseparable aspects of a unitary experience? A systematic review. Br. J. Anaesth. 2019, 123, e263–e272. [Google Scholar] [CrossRef] [PubMed]
- De Laurentis, M.; Rossana, B.; Andrea, B.; Riccardo, T.; Valentina, I. The impact of social-emotional context in chronic cancer pain: Patient-caregiver reverberations: Social-emotional context in chronic cancer pain. Support. Care Cancer 2019, 27, 705–713. [Google Scholar] [CrossRef]
- Scholz, J.; Finnerup, N.B.; Attal, N.; Aziz, Q.; Baron, R.; Bennett, M.I.; Benoliel, R.; Cohen, M.; Cruccu, G.; Davis, K.D. The IASP classification of chronic pain for ICD-11: Chronic neuropathic pain. Pain 2019, 160, 53–59. [Google Scholar] [CrossRef]
- Bennett, M.I.; Kaasa, S.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.-D. The IASP classification of chronic pain for ICD-11: Chronic cancer-related pain. Pain 2019, 160, 38–44. [Google Scholar] [CrossRef]
- Invernizzi, M.; Lippi, L.; Folli, A.; Turco, A.; Zattoni, L.; Maconi, A.; de Sire, A.; Fusco, N. Integrating molecular biomarkers in breast cancer rehabilitation. What is the current evidence? A systematic review of randomized controlled trials. Front. Mol. Biosci. 2022, 9, 930361. [Google Scholar] [CrossRef]
- Lippi, L.; de Sire, A.; Folli, A.; Maconi, A.; Polverelli, M.; Vecchio, C.; Fusco, N.; Invernizzi, M. Effects of Ultrasound-Guided Injection Combined with a Targeted Therapeutic Exercise in Breast Cancer Women with Subacromial Pain Syndrome: A Randomized Clinical Study. J. Pers. Med. 2022, 12, 1833. [Google Scholar] [CrossRef]
- Invernizzi, M.; Runza, L.; De Sire, A.; Lippi, L.; Blundo, C.; Gambini, D.; Boldorini, R.; Ferrero, S.; Fusco, N. Integrating Augmented Reality Tools in Breast Cancer Related Lymphedema Prognostication and Diagnosis. J. Vis. Exp. 2020, 156, e60093. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Liu, S.; Li, B.; Song, Z.; Han, Q.; Wang, C.; Wang, Y.; Yu, Y.; Xia, H.; et al. Acupuncture for cancer pain: A scoping review of systematic reviews and meta-analyses. Front. Oncol. 2023, 13, 1169458. [Google Scholar] [CrossRef]
- Cleeland, C.S. Pain assessment in cancer. In Effect of Cancer on Quality of Life; CRC Press: Boca Raton, FL, USA, 2021; pp. 293–305. [Google Scholar]
- Check, D.K.; Avecilla, R.A.V.; Mills, C.; Dinan, M.A.; Kamal, A.H.; Murphy, B.; Rezk, S.; Winn, A.; Oeffinger, K.C. Opioid Prescribing and Use Among Cancer Survivors: A Mapping Review of Observational and Intervention Studies. J. Pain Symptom Manag. 2022, 63, e397–e417. [Google Scholar] [CrossRef] [PubMed]
- Virgen, C.G.; Kelkar, N.; Tran, A.; Rosa, C.M.; Cruz-Topete, D.; Amatya, S.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kaye, A.D. Pharmacological management of cancer pain: Novel therapeutics. Biomed. Pharmacother. 2022, 156, 113871. [Google Scholar] [CrossRef]
- Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr. Oncol. 2023, 30, 6838–6858. [Google Scholar] [CrossRef]
- Lippi, L.; de Sire, A.; Folli, A.; D’abrosca, F.; Grana, E.; Baricich, A.; Carda, S.; Invernizzi, M. Multidimensional effectiveness of botulinum toxin in neuropathic pain: A systematic review of randomized clinical trials. Toxins 2022, 14, 308. [Google Scholar] [CrossRef]
- Baricich, A.; Picelli, A.; Santamato, A.; Carda, S.; de Sire, A.; Smania, N.; Cisari, C.; Invernizzi, M. Safety profile of high-dose botulinum toxin type A in post-stroke spasticity treatment. Clin. Drug Investig. 2018, 38, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Carda, S.; Invernizzi, M.; Baricich, A.; Cisari, C. Casting, taping or stretching after botulinum toxin type A for spastic equinus foot: A single-blind randomized trial on adult stroke patients. Clin. Rehabil. 2011, 25, 1119–1127. [Google Scholar] [CrossRef]
- Wang, J.; Rieder, E.A. A systematic review of patient-reported outcomes for cosmetic indications of botulinum toxin treatment. Dermatol. Surg. 2019, 45, 668–688. [Google Scholar] [CrossRef] [PubMed]
- Domoto, R.; Sekiguchi, F.; Tsubota, M.; Kawabata, A. Macrophage as a peripheral pain regulator. Cells 2021, 10, 1881. [Google Scholar] [CrossRef]
- Siqueira, S.R.D.T.D.; Alves, B.; Malpartida, H.; Teixeira, M.J.; Siqueira, J.T.T.D. Abnormal expression of voltage-gated sodium channels Nav1. 7, Nav1. 3 and Nav1. 8 in trigeminal neuralgia. Neuroscience 2009, 164, 573–577. [Google Scholar] [CrossRef]
- Mittal, S.O.; Jabbari, B. Botulinum neurotoxins and cancer—A review of the literature. Toxins 2020, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.I.; Choi, Y. Botulinum toxin injection for intractable pain in cancer patients with psoas muscle invasion. J. Pain Symptom Manag. 2022, 63, e441–e444. [Google Scholar] [CrossRef] [PubMed]
- Rostami, R.; Mittal, S.O.; Radmand, R.; Jabbari, B. Incobotulinum toxin-A improves post-surgical and post-radiation pain in cancer patients. Toxins 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Grenda, T.; Grenda, A.; Krawczyk, P.; Kwiatek, K. Botulinum toxin in cancer therapy—Current perspectives and limitations. Appl. Microbiol. Biotechnol. 2022, 106, 485–495. [Google Scholar] [CrossRef]
- Bagheri, R.; Fattahi, S.H.; Haghi, S.Z.; Aryana, K.; Aryanniya, A.; Akhlaghi, S.; Riyabi, F.N.; Sheibani, S. Botulinum toxin for prevention of delayed gastric emptying after esophagectomy. Asian Cardiovasc. Thorac. Ann. 2013, 21, 689–692. [Google Scholar] [CrossRef]
- De Groef, A.; Devoogdt, N.; Van Kampen, M.; Nevelsteen, I.; Smeets, A.; Neven, P.; Geraerts, I.; Dams, L.; Van der Gucht, E.; Debeer, P. Effectiveness of Botulinum Toxin A for Persistent Upper Limb Pain After Breast Cancer Treatment: A Double-Blinded Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2018, 99, 1342–1351. [Google Scholar] [CrossRef]
- De Groef, A.; Devoogdt, N.; Van Kampen, M.; De Hertogh, L.; Vergote, M.; Geraerts, I.; Dams, L.; Van der Gucht, E.; Debeer, P. The effectiveness of Botulinum Toxin A for treatment of upper limb impairments and dysfunctions in breast cancer survivors: A randomised controlled trial. Eur. J. Cancer Care 2020, 29, e13175. [Google Scholar] [CrossRef]
- Gabriel, A.; Champaneria, M.C.; Maxwell, G.P. The efficacy of botulinum toxin A in post-mastectomy breast reconstruction: A pilot study. Aesthetic Surg. J. 2015, 35, 402–409. [Google Scholar] [CrossRef]
- Lo, K.K.; Aycock, J.K. A blinded randomized controlled trial to evaluate the use of botulinum toxin for pain control in breast reconstruction with tissue expanders. Ann. Plast. Surg. 2015, 74, 281–283. [Google Scholar] [CrossRef]
- Nieri, C.A.; Benaim, E.H.; Zhang, Y.H.; Garcia-Godoy, F.; Herr, M.J.; Zhang, W.; Schwartz, D.; Coca, K.K.; Gleysteen, J.P.; Gillespie, M.B. Botox for the prevention of radiation-induced Sialadenitis and xerostomia in head and neck cancer patients: A pilot study. Head Neck 2023, 45, 2198–2206. [Google Scholar] [CrossRef]
- Teymoortash, A.; Pfestroff, A.; Wittig, A.; Franke, N.; Hoch, S.; Harnisch, S.; Schade-Brittinger, C.; Hoeffken, H.; Engenhart-Cabillic, R.; Brugger, M.; et al. Safety and Efficacy of Botulinum Toxin to Preserve Gland Function after Radiotherapy in Patients with Head and Neck Cancer: A Prospective, Randomized, Placebo-Controlled, Double-Blinded Phase I Clinical Trial. PLoS ONE 2016, 11, e0151316. [Google Scholar] [CrossRef]
- Wen, J.; Lu, Z.; Linghu, E.; Yang, Y.; Yang, J.; Wang, S.; Yan, B.; Song, J.; Zhou, X.; Wang, X.; et al. Prevention of esophageal strictures after endoscopic submucosal dissection with the injection of botulinum toxin type A. Gastrointest. Endosc. 2016, 84, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, C.; Liu, W.C.; Preuss, S.F.; Guntinas-Lichius, O. Botulinum toxin A for neuropathic pain after neck dissection: A dose-finding study. Laryngoscope 2006, 116, 1168–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, H.; Chen, M.; Ding, C.; Zhang, G.; Si, X. Comparison of endoscopic injection of botulinum toxin and steroids immediately after endoscopic submucosal dissection to prevent esophageal stricture: A prospective cohort study. J. Cancer 2021, 12, 5789–5796. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Dekhne, A.; Goklani, H.D.; Doshi, N.; Baskara Salian, R.; Gandhi, S.K.; Patel, P. Effectiveness of Botulinum Toxin in the Treatment of Neuropathic Pain: A Literature Review. Cureus 2023, 15, e46848. [Google Scholar] [CrossRef] [PubMed]
- Flynn, T.C. Botulinum toxin: Examining duration of effect in facial aesthetic applications. Am. J. Clin. Dermatol. 2010, 11, 183–199. [Google Scholar] [CrossRef]
- Money, S.; Garber, B. Management of cancer pain. Curr. Emerg. Hosp. Med. Rep. 2018, 6, 141–146. [Google Scholar] [CrossRef]
- de Sire, A.; Ferrillo, M.; Gennari, A.; Cisari, C.; Pasqua, S.; Foglio Bonda, P.L.; Invernizzi, M.; Migliario, M. Bone health, vitamin D status and oral hygiene screening in breast cancer women before starting osteoporosis treatment: A cross-sectional study. J. Biol. Regul. Homeost. Agents 2021, 35, 397–402. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Lippi, L.; Antonelli, A.; Calafiore, D.; Ammendolia, V.; Fortunato, L.; Renò, F.; Giudice, A.; et al. Oral Health in Breast Cancer Women with Vitamin D Deficiency: A Machine Learning Study. J. Clin. Med. 2022, 11, 4662. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, N.J.; Elnahal, S.M.; Alvarez, R.H. Cancer pain: A review of epidemiology, clinical quality and value impact. Future Oncol. 2017, 13, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.L.; Kehlet, H.; Belfer, I.; Edwards, R.R. Predicting, preventing and managing persistent pain after breast cancer surgery: The importance of psychosocial factors. Pain Manag. 2014, 4, 445–459. [Google Scholar] [CrossRef]
- Suraj, D.; Zhang, A.; Appelbaum, T.; Ahmed, N.; Shih, S.; Gofman, J.; Kalenja, K.; Abrigo, J.N.; Shaporova, V.; Mannan, A.; et al. Clinical Presentation and Management of Malignant Psoas Syndrome: A Scoping Review of Case Reports and Case Series. Cureus 2023, 15, e41522. [Google Scholar] [CrossRef]
- Awadeen, A.; Fareed, M.; Elameen, A.M. The Impact of Botulinum Toxin Injection on the Outcomes of Breast Surgeries: A Systematic Review and Meta-Analysis. Aesthetic Plast. Surg. 2023, 47, 1771–1784. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. AMIA Symp. 2006, 2006, 359–363. [Google Scholar] [PubMed]


| Articles | Domain | Score | ||||
|---|---|---|---|---|---|---|
| Random Sequence Generation | Appropriate Randomization | Blinding of Participants or Personnel | Blinding of Outcome Assessors | Withdrawals and Dropouts | ||
| Bagheri et al. 2013 [26] | 1 | 0 | 0 | 0 | 1 | 2 |
| De Groef et al. 2018 [27] | 1 | 1 | 1 | 1 | 1 | 5 |
| De Groef et al. 2020 [28] | 1 | 1 | 1 | 1 | 1 | 5 |
| Gabriel et al. 2015 [29] | 1 | 1 | 0 | 0 | 1 | 3 |
| Lo et al. 2015 [30] | 1 | 1 | 1 | 1 | 1 | 5 |
| Nieri et al. 2023 [31] | 1 | 1 | 1 | 1 | 1 | 5 |
| Teymoortash et al. 2016 [32] | 1 | 1 | 1 | 1 | 1 | 5 |
| Wen et al. 2016 [33] | 1 | 1 | 1 | 1 | 1 | 5 |
| Wittekindt et al. 2006 [34] | 1 | 0 | 1 | 1 | 1 | 4 |
| Zhou et al. 2021 [35] | 1 | 1 | 0 | 0 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lippi, L.; de Sire, A.; Turco, A.; Ferrillo, M.; Kesikburun, S.; Baricich, A.; Carda, S.; Invernizzi, M. Botulinum Toxin for Pain Relief in Cancer Patients: A Systematic Review of Randomized Controlled Trials. Toxins 2024, 16, 153. https://doi.org/10.3390/toxins16030153
Lippi L, de Sire A, Turco A, Ferrillo M, Kesikburun S, Baricich A, Carda S, Invernizzi M. Botulinum Toxin for Pain Relief in Cancer Patients: A Systematic Review of Randomized Controlled Trials. Toxins. 2024; 16(3):153. https://doi.org/10.3390/toxins16030153
Chicago/Turabian StyleLippi, Lorenzo, Alessandro de Sire, Alessio Turco, Martina Ferrillo, Serdar Kesikburun, Alessio Baricich, Stefano Carda, and Marco Invernizzi. 2024. "Botulinum Toxin for Pain Relief in Cancer Patients: A Systematic Review of Randomized Controlled Trials" Toxins 16, no. 3: 153. https://doi.org/10.3390/toxins16030153
APA StyleLippi, L., de Sire, A., Turco, A., Ferrillo, M., Kesikburun, S., Baricich, A., Carda, S., & Invernizzi, M. (2024). Botulinum Toxin for Pain Relief in Cancer Patients: A Systematic Review of Randomized Controlled Trials. Toxins, 16(3), 153. https://doi.org/10.3390/toxins16030153












