Botulinum Toxin Injections to the Obliquus Capitis Inferioris Muscle for Dynamic Cervical Dystonia Improves Subjective Patient Outcomes
Abstract
1. Introduction
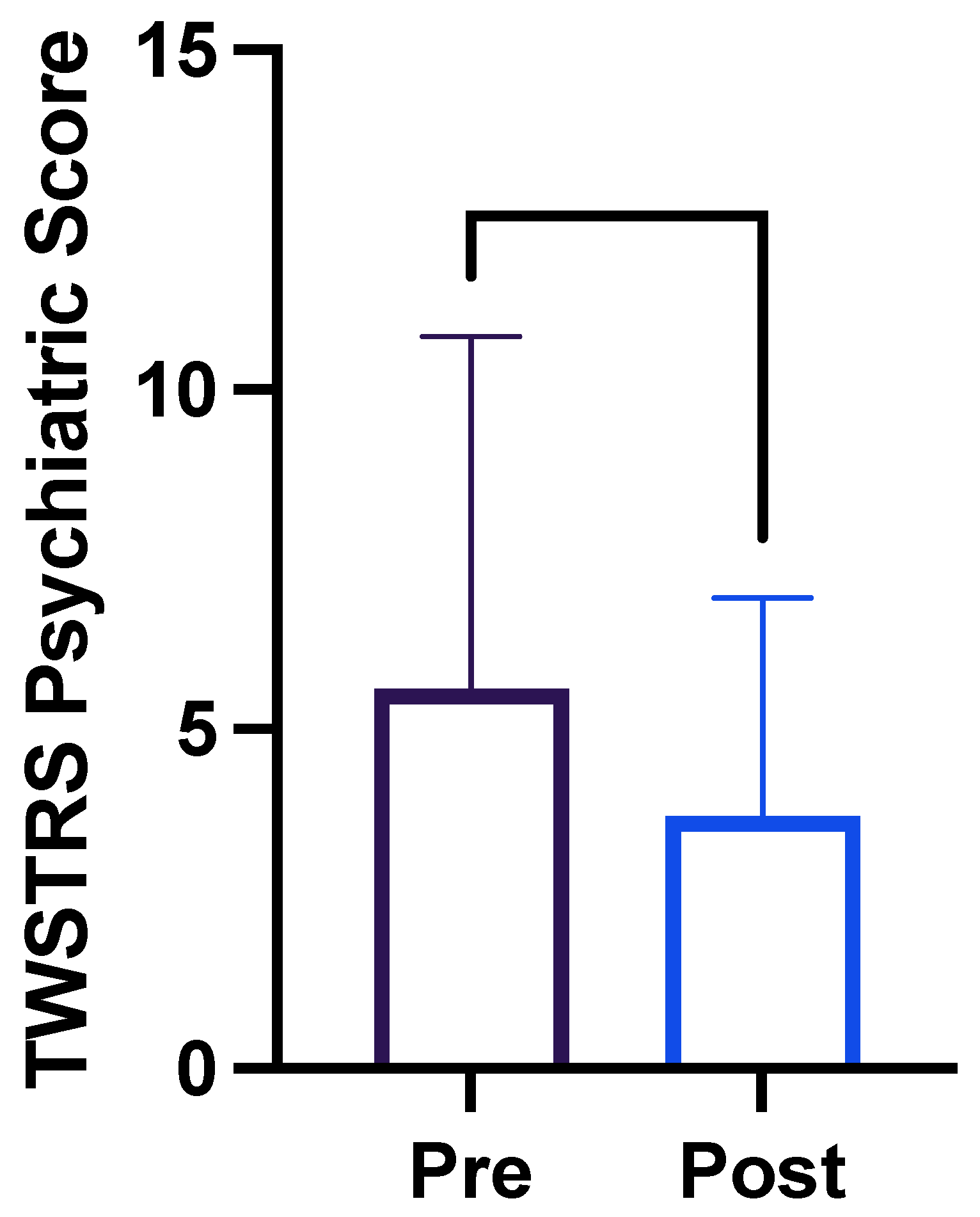
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albanese, A.; Bhatia, K.P.; Cardoso, F.; Comella, C.; Defazio, G.; Fung, V.S.C.; Hallett, M.; Jankovic, J.; Jinnah, H.A.; Kaji, R.; et al. Isolated Cervical Dystonia: Diagnosis and Classification. Mov. Disord. 2023, 38, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Camfield, L.; Ben-Shlomo, Y.; Warner, T.T. Epidemiological Study of Dystonia in Europe Collaborative Group. Impact of cervical dystonia on quality of life. Mov. Disord. 2002, 17, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Sławek, J.; Jost, W.H. Botulinum neurotoxin in cervical dystonia revisited—Recent advances and unanswered questions. Neurol. Neurochir. Pol. 2021, 55, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Vu, J.P.; Lee, H.Y.; Chen, Q.; Cisneros, E.; Barbano, R.L.; Goetz, C.G.; Jankovic, J.; Jinnah, H.A.; Perlmutter, J.S.; Berman, B.D. Head tremor and pain in cervical dystonia. J. Neurol. 2021, 268, 1945–1950. [Google Scholar] [CrossRef] [PubMed]
- Konrad, C.; Vollmer-Haase, J.; Anneken, K.; Knecht, S. Orthopedic and neurological complications of cervical dystonia—Review of the literature. Acta Neurol. Scand. 2004, 109, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Reichel, G. Cervical dystonia: A new phenomenological classification for botulinum toxin therapy. Basal Ganglia 2011, 1, 5–12. [Google Scholar] [CrossRef]
- Jost, W.H. Torticaput versus Torticollis: Clinical Effects with Modified Classification and Muscle Selection. Tremor Other Hyperkinetic Mov. 2019, 9. [Google Scholar] [CrossRef]
- Jost, W.H.; Tatu, L.; Pandey, S.; Sławek, J.; Drużdż, A.; Biering-Sørensen, B.; Altmann, C.F.; Kreisler, A. Frequency of different subtypes of cervical dystonia: A prospective multicenter study according to Col–Cap concept. J. Neural Transm. 2020, 127, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Kreisler, A.; Drużdż, A.; Biering-Sørensen, B.; Sławek, J.; Tatu, L.; Jost, W.H. Tremor in idiopathic cervical dystonia–possible implications for botulinum toxin treatment considering the col-cap classification. Tremor Other Hyperkinetic Mov. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Misra, V.P.; Ehler, E.; Zakine, B.; Maisonobe, P.; Simonetta-Moreau, M.; Group, I.I.C. Factors influencing response to Botulinum toxin type A in patients with idiopathic cervical dystonia: Results from an international observational study. BMJ Open 2012, 2, e000881. [Google Scholar] [CrossRef] [PubMed]
- Schramm, A.; Huber, D.; Möbius, C.; Münchau, A.; Kohl, Z.; Bäumer, T. Involvement of obliquus capitis inferior muscle in dystonic head tremor. Park. Relat. Disord. 2017, 44, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Hu, Y.; Djibo, I.M.; Chen, S.; Pan, Y.; Zhang, X.; Pan, L.; Jin, L.; Teng, F. Pivotal role of obliquus capitis inferior in torticaput revealed by single-photon emission computed tomography. J. Neural Transm. 2022, 129, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Comella, C.L.; Perlmutter, J.S.; Jinnah, H.A.; Waliczek, T.A.; Rosen, A.R.; Galpern, W.R.; Adler, C.A.; Barbano, R.L.; Factor, S.A.; Goetz, C.G. Clinimetric testing of the comprehensive cervical dystonia rating scale. Mov. Disord. 2016, 31, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, R.C.; Andary, M.T.; Wyman, A.J.; Rowan, J.J. A standardized protocol for needle placement in suboccipital muscles. Clin. Anat. 2008, 21, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Walter, U.; Dudesek, A.; Fietzek, U.M. A simplified ultrasonography-guided approach for neurotoxin injection into the obliquus capitis inferior muscle in spasmodic torticollis. J. Neural Transm. 2018, 125, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Fietzek, U.M.; Nene, D.; Schramm, A.; Appel-Cresswell, S.; Kosutzka, Z.; Walter, U.; Wissel, J.; Berweck, S.; Chouinard, S.; Baumer, T. The Role of Ultrasound for the Personalized Botulinum Toxin Treatment of Cervical Dystonia. Toxins 2021, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Dashtipour, K.; Mari, Z.; Jankovic, J.; Adler, C.H.; Schwartz, M.; Brin, M.F. Minimal clinically important change in patients with cervical dystonia: Results from the CD PROBE study. J. Neurol. Sci. 2019, 405, 116413. [Google Scholar] [CrossRef] [PubMed]
- Espay, A.J.; Trosch, R.; Suarez, G.; Johnson, J.; Marchese, D.; Comella, C. Minimal clinically important change in the Toronto Western spasmodic torticollis rating scale. Park. Relat. Disord. 2018, 52, 94–97. [Google Scholar] [CrossRef] [PubMed]


| n | 25 |
|---|---|
| Female n (%) | 22 (88%) |
| Male n (%) | 3 (12%) |
| Average Age (years) | 62 |
| Onabotulinum toxin A n (%) | 10 (40%) |
| Incobotulinum toxin A n (%) | 13 (52%) |
| Abobotulinum toxin A n (%) | 2 (8%) |
| OCI injected at first visit n (%) | 4 (16%) |
| OCI added to injection pattern n (%) | 21 (84%) |
| Descriptive Statistics | Pre-Injection (n = 25) | Post-Injection (n = 25) |
|---|---|---|
| Mean | 10.1 | 7.32 |
| Minimum | 0.0 | 0.0 |
| Maximum | 24.0 | 19.0 |
| Range | 24.0 | 19.0 |
| Standard Deviation | 6.19 | 5.22 |
| Standard Error of Mean | 1.24 | 1.04 |
| Descriptive Statistics | Pre-Injection (n = 25) | Post-Injection (n = 25) |
|---|---|---|
| Mean | 18.8 | 14.2 |
| Minimum | 0.0 | 0.0 |
| Maximum | 40.0 | 33.0 |
| Range | 40.0 | 33.0 |
| Standard Deviation | 12.6 | 10.3 |
| Standard Error of Mean | 2.5 | 2.1 |
| Descriptive Statistics | Pre-Injection (n = 25) | Post-Injection (n = 25) |
|---|---|---|
| Mean | 5.6 | 3.7 |
| Minimum | 0.0 | 0.0 |
| Maximum | 21.0 | 11.0 |
| Range | 21.0 | 21.0 |
| Standard Deviation | 5.2 | 3.7 |
| Standard Error of Mean | 1.0 | 0.6 |
| Descriptive Statistics | Pre-Injection (n = 25) | Post-Injection (n = 25) |
|---|---|---|
| Mean | 7.0 | 4.2 |
| Minimum | 2.0 | 1.0 |
| Maximum | 10.0 | 10.0 |
| Range | 8.0 | 9.0 |
| Standard Deviation | 2.4 | 2.2 |
| Standard Error of Mean | 0.5 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bessemer, R.A.; Jog, M. Botulinum Toxin Injections to the Obliquus Capitis Inferioris Muscle for Dynamic Cervical Dystonia Improves Subjective Patient Outcomes. Toxins 2024, 16, 76. https://doi.org/10.3390/toxins16020076
Bessemer RA, Jog M. Botulinum Toxin Injections to the Obliquus Capitis Inferioris Muscle for Dynamic Cervical Dystonia Improves Subjective Patient Outcomes. Toxins. 2024; 16(2):76. https://doi.org/10.3390/toxins16020076
Chicago/Turabian StyleBessemer, Robin Anne, and Mandar Jog. 2024. "Botulinum Toxin Injections to the Obliquus Capitis Inferioris Muscle for Dynamic Cervical Dystonia Improves Subjective Patient Outcomes" Toxins 16, no. 2: 76. https://doi.org/10.3390/toxins16020076
APA StyleBessemer, R. A., & Jog, M. (2024). Botulinum Toxin Injections to the Obliquus Capitis Inferioris Muscle for Dynamic Cervical Dystonia Improves Subjective Patient Outcomes. Toxins, 16(2), 76. https://doi.org/10.3390/toxins16020076






