An Unprecedented Bloom of Oceanic Dinoflagellates (Karenia spp.) Inside a Fjord within a Highly Dynamic Multifrontal Ecosystem in Chilean Patagonia
Abstract
1. Introduction
Background of Karenia Harmful Events in Patagonia
2. Results
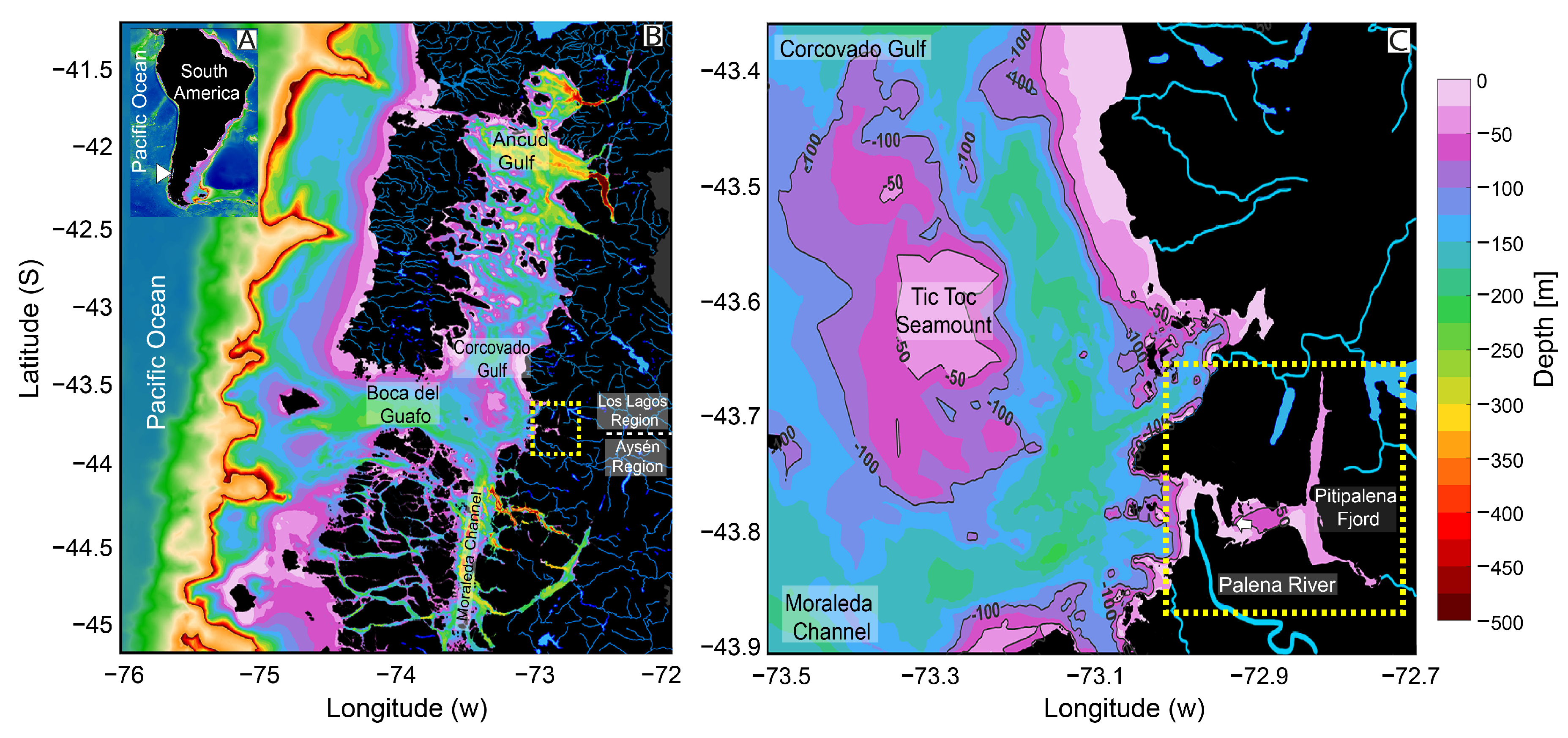
2.1. Meteorological and Hydrographical Conditions
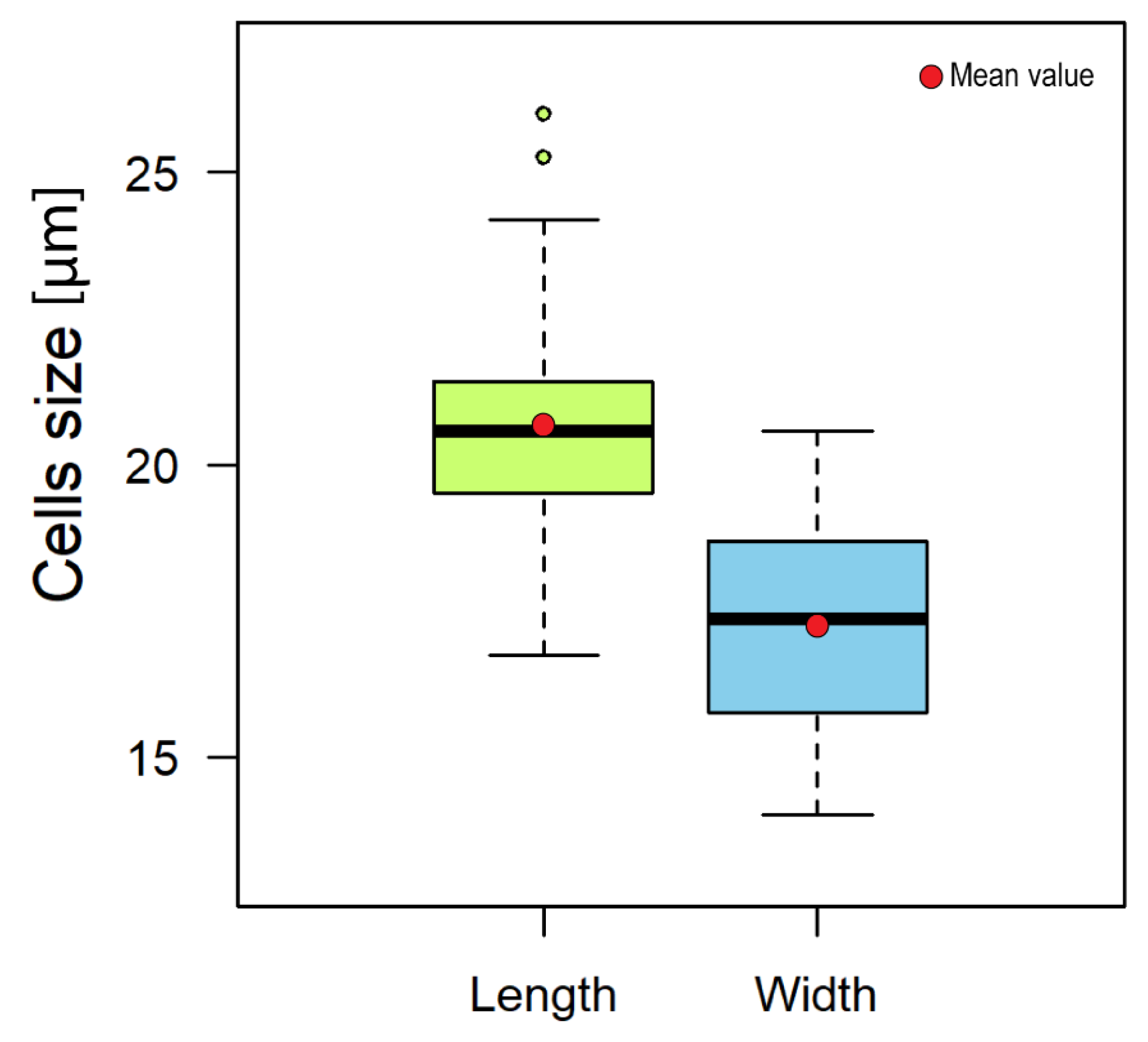
2.2. Karenia Cell Morphology
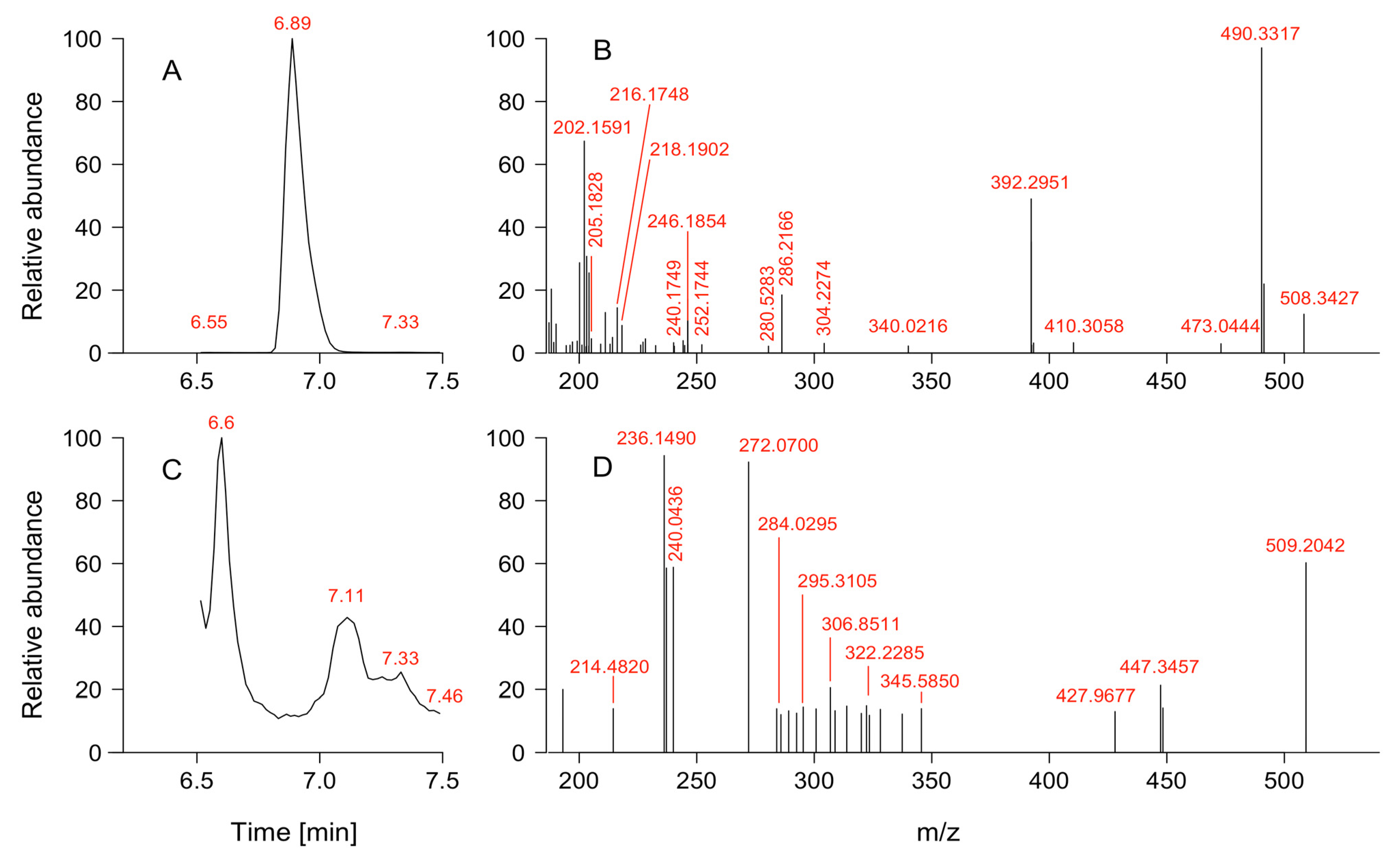
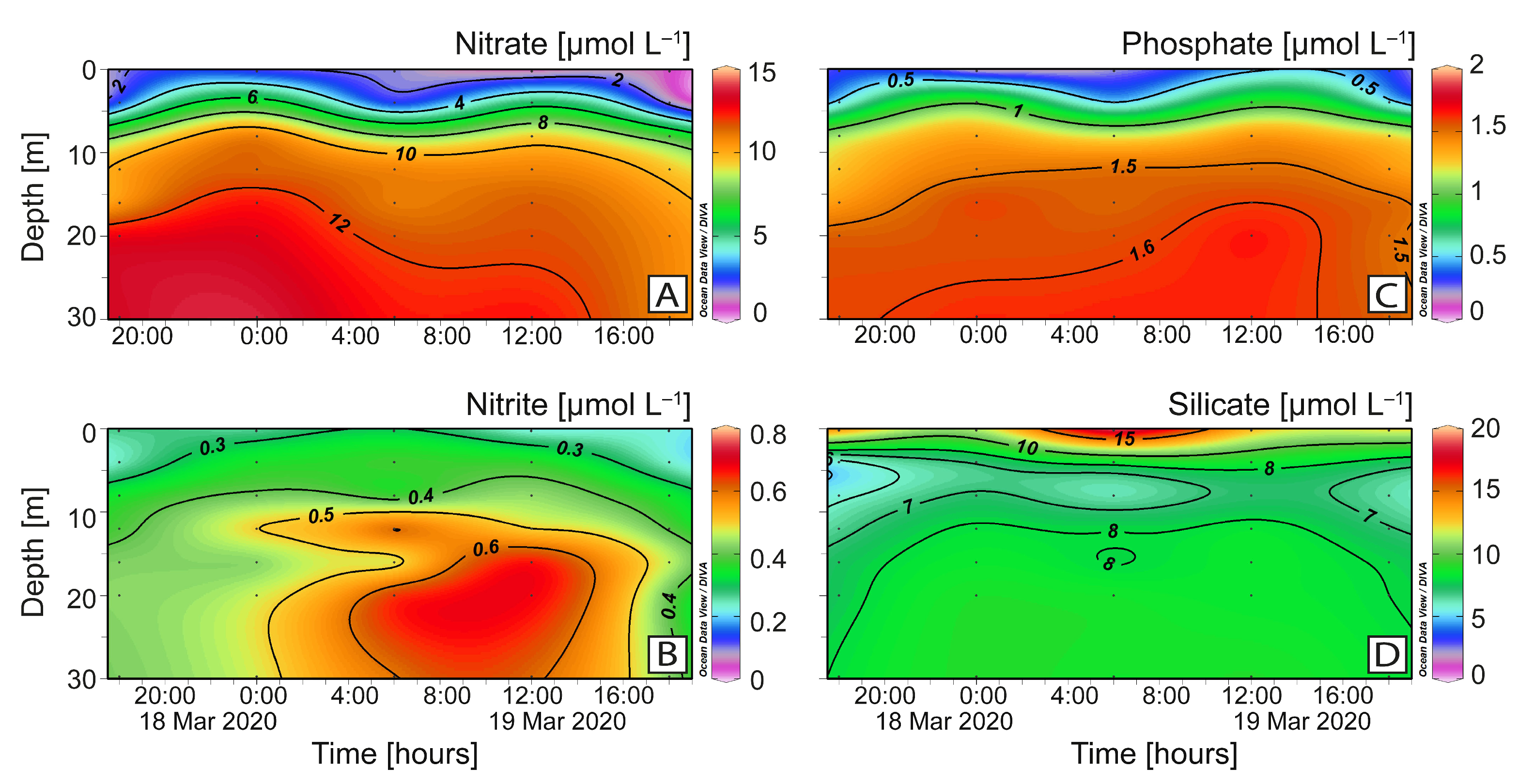
2.3. Vertical Distribution of Karenia sp. and Toxins
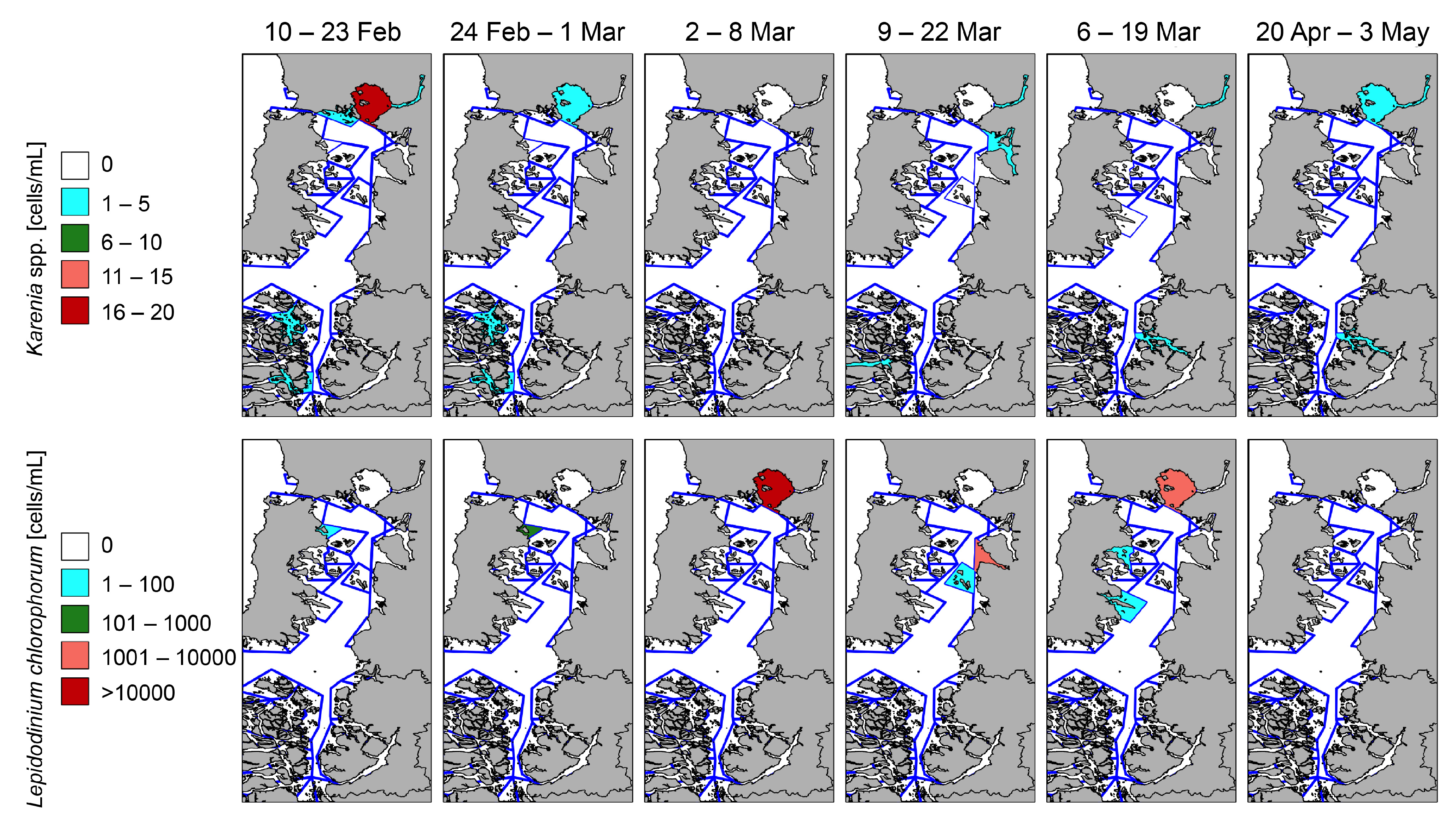
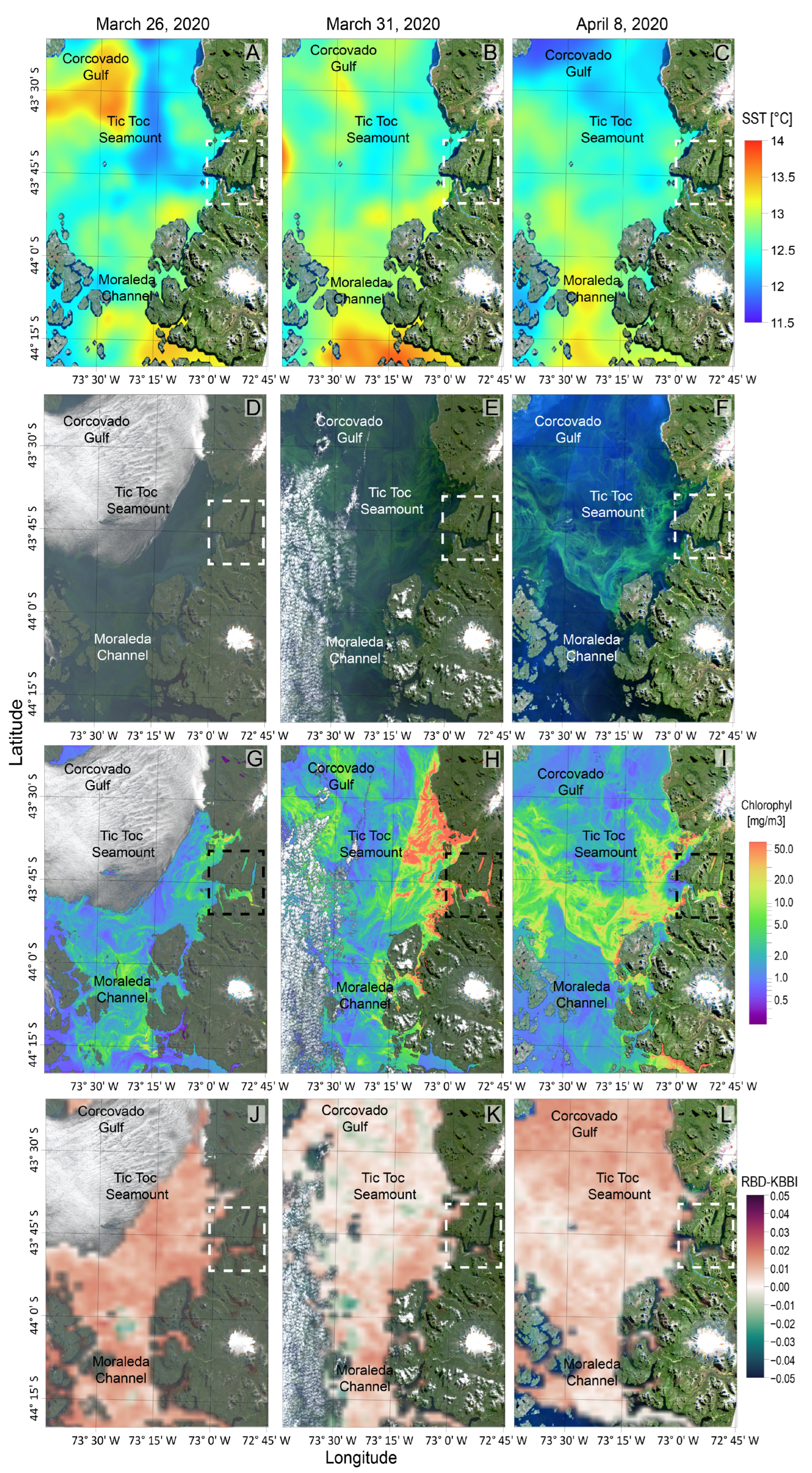
2.4. Satellite Observations
3. Discussion
3.1. The Morphology and Toxic Potential of the Karenia Population in Pitipalena Fjord
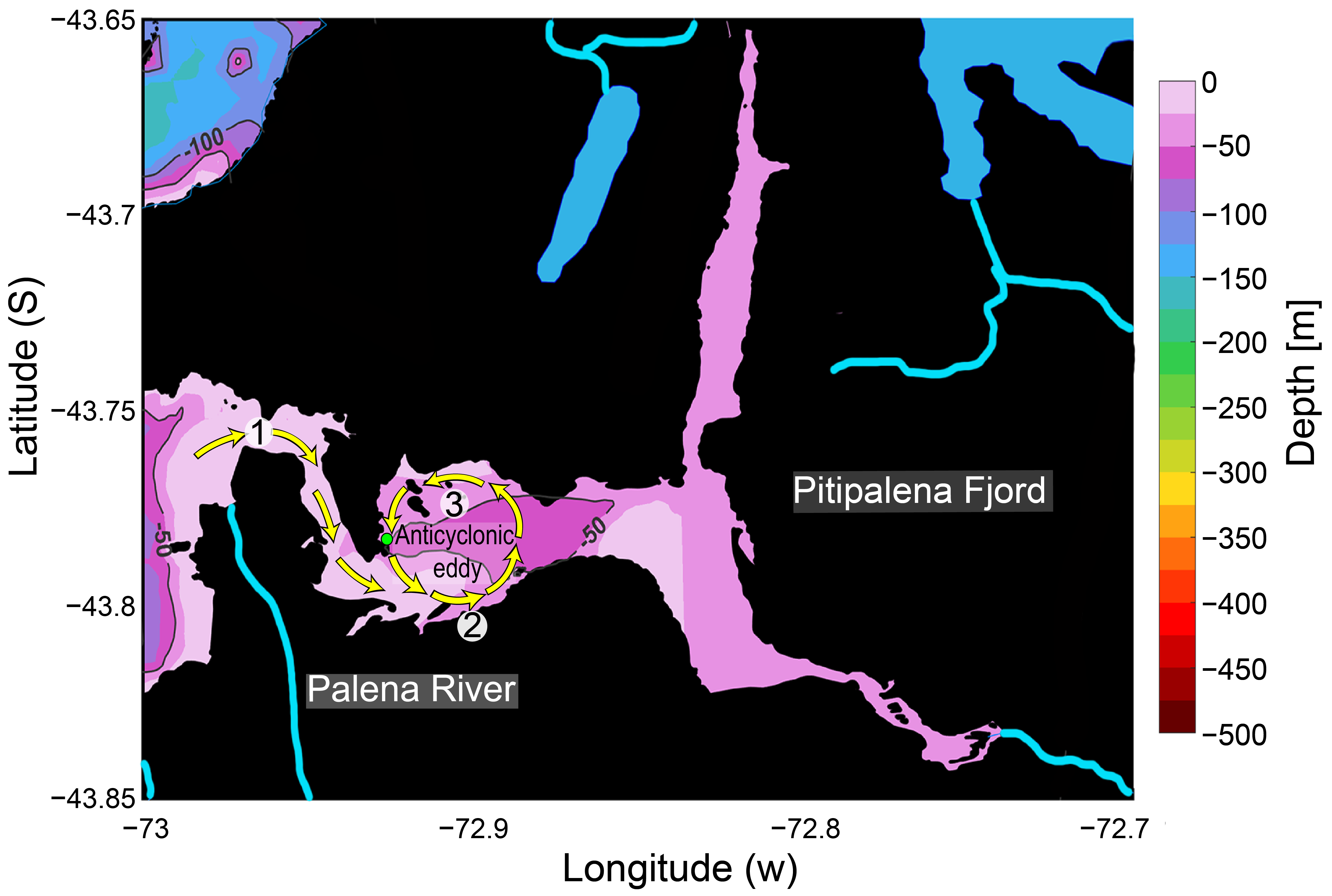
3.2. The Distribution of Karenia Cells and Behaviour
3.3. Climate Variability and Local Hydrodynamics in Northern Patagonia and Karenia spp. Blooms
3.4. Management Considerations
4. Materials and Methods
4.1. Field Sampling of Environmental Conditions and Phytoplankton
4.2. Nutrient Analysis
4.3. Phytoplankton Analysis
4.4. Morphometry
4.5. Toxin Sample Extraction and Analysis
4.6. Satellite Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Leal Filho, W.; Nagy, G.J.; Martinho, F.; Saroar, M.; Erache, M.G.; Primo, A.L.; Pardal, M.A.; Li, C. Influences of climate change and variability on estuarine ecosystems: An impact study in selected European, South American and Asian countries. Int. J. Environ. Res. Public Health 2022, 19, 585. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Berdalet, E.; Montresor, M.; Reguera, B.; Roy, S.; Yamazaki, H.; Cembella, A.; Raine, R. Harmful algal blooms in fjords, coastal embayments, and stratified systems: Recent progress and future research. Oceanography 2017, 30, 46–57. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate change and harmful algal blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef]
- Davidson, K.; Jardine, S.L.; Martino, S.; Myre, G.B.; Peck, L.E.; Raymond, R.N.; West, J.J. The Economic Impacts of Harmful Algal Blooms on Salmon Cage Aquaculture. PICES Sci. Rep. 2020, 59, 84–94. [Google Scholar]
- Lundholm, N.; Churro, C.; Escalera, L.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Larsen, J.; Mertens, K.; Moestrup, Ø.; Tillmann, U.; et al. (Eds.) IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. 2009. Available online: https://www.marinespecies.org/hab (accessed on 20 December 2023).
- Caruana, A.M.N.; Amzil, Z. Microalgae and Toxins. In Microalgae in Health and Disease Prevention; Chapter 3; Ira, A., Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 263–305. [Google Scholar] [CrossRef]
- GlobalHAB. Fish-Killing Marine Algal Blooms: Causative Organisms, Ichthyotoxic Mechanisms, Impacts and Mitigation; IOC Manuals and Guides, 93; Hallegraeff, G.M., Anderson, D.M., Davidson, K., Gianella, F., Hansen, P.J., Hegaret, H., Iwataki, M., Larsen, T.O., Mardones, J., MacKenzie, L., et al., Eds.; UNESCO-IOC/SCOR: Paris, France, 2023; 96p. [Google Scholar] [CrossRef]
- Oda, M. Gymnodinium mikimotoi Miyake et Kominami n. sp. (MS.) no akashiwo to ryusando no koka. (The red tide of Gymnodinium mikimotoi Miyake et Kominami and the influence of copper sulfate on the red tide of November 1972). Zool. Mag. 1935, 47, 35–48. [Google Scholar]
- Davis, C.C. Gymnodinium brevis sp. nov., a cause of discolored water and animal mortality in the Gulf of Mexico. Bot. Gaz. 1948, 109, 358–360. [Google Scholar] [CrossRef]
- Iwataki, M. Gymnodiniales, in IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. 2023. Available online: www.marinespecies.org/hab (accessed on 25 July 2023).
- Iwataki, M.; Lum, W.M.; Kuwata, K.; Takahashi, K.; Arima, D.; Kuribayashi, T.; Kosaka, Y.; Hasegawa, N.; Watanabe, T.; Shikata, T.; et al. Morphological variation and phylogeny of Karenia selliformis (Gymnodiniales, Dinophyceae) in an intensive cold-water algal bloom in eastern Hokkaido, Japan. Harmful Algae 2022, 114, 102204. [Google Scholar] [CrossRef]
- Villac, M.C.; Hoeglund, A.; Tilney, C.; Garrett, M.; Lopez, C.; Hubbard, K.A.; Steidinger, K.A. Ecophysiology and bloom dynamics of Karenia with emphasis on Karenia brevis in Florida waters. In Dinoflagellates: Classification, Evolution, Physiology and Ecological Significance; Marine and Freshwater Biology; Subba Rao, D.V., Ed.; Nova Science Publishers: New York, NY, USA, 2020. [Google Scholar]
- Kim, S.; Cho, M.; Yoo, J.; Park, B.S. Application of a quantitative PCR to investigate the distribution and dynamics of two morphologically similar species, Karenia mikimotoi and K. papilionacea (Dinophyceae) in Korean coastal waters. Toxins 2023, 15, 469. [Google Scholar] [CrossRef]
- Haywood, A.J.; Steidinger, K.A.; Truby, E.W.; Bergquist, P.R.; Bergquist, P.L.; Adamson, J.; Mackenzie, L. Comparative morphology and molecular phylogenetic analysis of three new species of the genus Karenia (Dinophyceae) from New Zealand: Three new species of Karenia. J. Phycol. 2004, 40, 165–179. [Google Scholar] [CrossRef]
- Haywood, A.J.; Scholin, C.A.; Marin III, R.; Steidinger, K.A.; Heil, C.; Ray, J. Molecular detection of the brevetoxin-producing dinoflagellate Karenia brevis and closely related species using rRNA-targeted probes and a semiautomated sandwich hybridization assay J. Phycol. 2007, 43, 1271–1286. [Google Scholar] [CrossRef]
- Villanueva, F.; Cortez, H.; Uribe, C.; Peña, P.; Cassis, D. Mortality of Chilean farmed salmon in wellboats in transit through a Karenia bloom. Harmful Algae News 2017, 57, 4–5. [Google Scholar]
- Brand, L.E.; Campbell, L.; Bresnan, E. Karenia: The biology and ecology of a toxic genus. Harmful Algae 2012, 14, 156–178. [Google Scholar] [CrossRef]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. New analogue of gymnodimine from a Gymnodinium species. J. Agric. Food Chem. 2000, 48, 1373–1376. [Google Scholar] [CrossRef]
- Miles, C.O.; Wilkins, A.L.; Stirling, D.J.; MacKenzie, A.L. Gymnodimine C, an isomer of gymnodimine B, from Karenia selliformis. J. Agric. Food Chem. 2003, 51, 4838–4840. [Google Scholar] [CrossRef]
- Bourdelais, A.J.; Campbell, S.; Jacocks, H.; Naar, J.; Wright, J.L.; Carsi, J.; Baden, D.G. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cell. Mol. Neurobiol. 2004, 24, 553–563. [Google Scholar] [CrossRef]
- Satake, M.; Shoji, M.; Oshima, Y.; Naoki, H.; Fujita, T.; Yasumoto, T. Gymnocin-A, a cytotoxic polyether from the notorious red tide dinoflagellate, Gymnodinium mikimotoi. Tetrahedron Lett. 2002, 43, 5829–5832. [Google Scholar] [CrossRef]
- Mooney, B.D.; Dorantes-Aranda, J.J.; Place, A.R.; Hallegraeff, G.M. Ichthyotoxicity of gymnodinioid dinoflagellates: PUFA and superoxide effects in sheepshead minnow larvae and rainbow trout gill cells. Mar. Ecol. Prog. Ser. 2011, 426, 213–224. [Google Scholar] [CrossRef]
- Mooney, B.D.; Nichols, P.D.; De Salas, M.F.; Hallegraeff, G.M. Lipid, fatty acid and sterol composition of eight species of Kareniaceae (Dinophyta): Chemotaxonomy and putative phycotoxins. J. Phycol. 2007, 43, 101–111. [Google Scholar] [CrossRef]
- Holland, P.T.; Shi, F.; Satake, M.; Hamamoto, Y.; Ito, E.; Beuzenberg, V.; McNabb, P.; Munday, R.; Briggs, L.; Truman, P.; et al. Novel toxins produced by the dinoflagellate Karenia brevisulcata. Harmful Algae 2012, 13, 47–57. [Google Scholar] [CrossRef]
- Mardones, J.I.; Norambuena, L.; Paredes, J.; Fuenzalida, G.; Dorantes-Aranda, J.J.; Chang, K.J.L.; Guzmán, L.; Krock, B.; Hallegraeff, G. Unraveling the Karenia selliformis complex with the description of a non-gymnodimine producing Patagonian phylotype. Harmful Algae 2020, 98, 101892. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I. Screening of Chilean fish-killing microalgae using a gill cell-based assay. Lat. Am. J. Aquat. Res. 2020, 48, 329–335. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hirano, T.; Yoshimatsu, T.; Tanimoto, Y.; Matsumoto, T.; Suzuki, S.; Hayashi, Y.; Urabe, A.; Miyamura, K.; Sakamoto, S.; et al. Occurrence of Karenia papilionacea (Dinophyceae) and its novel sister phylotype in Japanese coastal waters. Harmful Algae 2016, 57, 59–68. [Google Scholar] [CrossRef]
- Mitra, A.; Flynn, K.J.; Tillmann, U.; Raven, J.A.; Caron, D.; Stoecker, D.K.; Not, F.; Hansen, P.J.; Hallegraeff, G.; Sanders, R.; et al. Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: Incorporation of diverse mixotrophic strategies. Protist 2016, 167, 106–120. [Google Scholar] [CrossRef]
- Gentien, P. Bloom dynamics and ecophysiology of the Gymnodinium mikimotoi species complex. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 155–173. [Google Scholar]
- Jeong, H.J.; Park, J.Y.; Nho, J.H.; Park, M.O.; Ha, J.H.; Seong, K.A.; Jeng, C.; Seong, C.N.; Lee, K.Y.; Yih, W.H. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquat. Microb. Ecol. 2005, 41, 131–143. [Google Scholar] [CrossRef]
- Rodríguez, F.; Garrido, J.L.; Llewellyn, C.A. Photosynthetic Pigments in Dinoflagellates. In Dinoflagellates: Classification, Evolution, Physiology and Ecological Significance; Marine and Freshwater Biology; Subba Rao, D.V., Ed.; Nova Science Publishers: New York, NY, USA, 2020. [Google Scholar]
- Stumpf, R.P.; Litaker, R.W.; Lanerolle, L.; Tester, P.A. Hydrodynamic accumulation of Karenia off the west coast of Florida. Cont. Shelf Res. 2008, 28, 189–213. [Google Scholar] [CrossRef]
- Tester, P.A.; Steidinger, K.A. Gymnodinium breve red tide blooms: Initiation, transport, and consequences of surface circulation. Limnol. Oceanogr. 1997, 42, 1039–1051. [Google Scholar] [CrossRef]
- Steidinger, K.A.; Vargo, G.A.; Tester, P.A.; Tomas, C.R. Bloom dynamics and physiology of Gymnodinium breve with emphasis on the Gulf of Mexico. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 133–153. [Google Scholar]
- Steidinger, K.A.; Wolny, J.L.; Haywood, A.J. Identification of Kareniaceae (Dinophyceae) in the Gulf of Mexico. Nova Hedwig. 2008, 133, 269–284. [Google Scholar]
- Pingree, R.D.; Pugh, P.R.; Holligan, P.M.; Forster, G.R. Summer phytoplankton blooms and red tides along tidal fronts in the approaches to the English Channel. Nature 1975, 258, 672–677. [Google Scholar] [CrossRef]
- Raine, R.; O’boyle, S.; O’higgins, T.; White, M.; Patching, J.; Cahill, B.; McMahon, T. A satellite and field portrait of a Karenia mikimotoi bloom off the south coast of Ireland, August 1998. Hydrobiologia 2001, 465, 187–193. [Google Scholar] [CrossRef]
- Koizumi, Y.; Uchida, T.; Honjo, T. Diurnal vertical migration of Gymnodinium mikimotoi during a red tide in Hoketsu Bay, Japan. J. Plankton Res. 1996, 18, 289–294. [Google Scholar] [CrossRef]
- Heil, C.A.; Bronk, D.A.; Mulholland, M.R.; O’Neil, J.M.; Bernhardt, P.W.; Murasko, S.; Havens, J.A.; Vargo, G.A. Influence of daylight surface aggregation behavior on nutrient cycling during a Karenia brevis (Davis) G. Hansen & Ø. Moestrup bloom: Migration to the surface as a nutrient acquisition strategy. Harmful Algae 2014, 38, 86–94. [Google Scholar] [CrossRef]
- Pantoja, S.; Iriarte, J.L.; Daneri, G. Oceanography of the Chilean Patagonia. Cont. Shelf Res. 2011, 31, 149–153. [Google Scholar] [CrossRef]
- Aracena, C.; Lange, C.B.; Iriarte, J.L.; Rebolledo, L.; Pantoja, S. Latitudinal patterns of export production recorded in surface sediments of the Chilean Patagonian fjords (41–55° S) as a response to water column productivity. Cont. Shelf Res. 2011, 31, 340–355. [Google Scholar] [CrossRef]
- Iriarte, J.L.; Pantoja, S.; Daneri, G. Oceanographic processes in Chilean fjords of Patagonia: From small to large-scale studies. Prog. Oceanogr. 2014, 129, 1–7. [Google Scholar] [CrossRef]
- Sauter, T. Revisiting extreme precipitation amounts over southern South America and implications for the Patagonian Icefields. Hydrol. Earth Syst. Sci. 2020, 24, 2003–2016. [Google Scholar] [CrossRef]
- Pickard, G. Some physical oceanographic features of inlets of Chile. J. Fish. Res. Board. Can. 1971, 28, 1077–1106. [Google Scholar] [CrossRef]
- Sievers, H.; Silva, N. Water masses and circulation in austral Chilean channels and fjords. In Progress in the oceanographic knowledge of Chilean interior waters, from Puerto Montt to Cape Horn; Silva, N., Palma, S., Eds.; Comité Oceanográfico Nacional-Pontificia Universidad Católica de Valparaíso: Valparaíso, Chile, 2008; pp. 53–58. [Google Scholar]
- Pérez-Santos, I.; Garcés-Vargas, J.; Schneider, W.; Ross, L.; Parra, S.; Valle-Levinson, A. Double-diffusive layering and mixing in Patagonian fjords. Prog. Oceanogr. 2014, 129, 35–49. [Google Scholar] [CrossRef]
- Schneider, W.; Pérez-Santos, I.; Ross, L.; Bravo, L.; Seguel, R.; Hernández, F. On the hydrography of Puyuhuapi Channel, Chilean Patagonia. Prog. Oceanogr. 2014, 129, 8–18. [Google Scholar] [CrossRef]
- Silva, N.; Calvete, C. Physical and chemical oceanographic features of southern Chilean inlets between Penas Gulf and Magellan Strait (Cimar-Fiordos 2 cruise). Cienc. Tecnol. Mar 2002, 25, 23–28. [Google Scholar]
- Molinet, C.; Díaz, M.; González, A.; Henríquez, J.; Matamala, T.; Boldt, J.; Brito, N.; Espinoza, K.; Lafón, A.; Merino, P.; et al. Exploring biodiversity patterns in a marine and coastal protected area: Implications for management within and outside MCPAs. Estuar. Coast. Shelf Sci. 2023, 294, 108540. [Google Scholar] [CrossRef]
- Díaz, P.A.; Molinet, C.; Cáceres, M.A.; Valle-Levinson, A. Seasonal and intratidal distribution of Dinophysis spp. in a Chilean fjord. Harmful Algae 2011, 10, 155–164. [Google Scholar] [CrossRef]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Díaz, M.; Labra, G.; Figueroa, R. Species diversity and abundance of dinoflagellate resting cysts seven months after a bloom of Alexandrium catenella in two contrasting coastal systems of the Chilean Inland Sea. Eur. J. Phycol. 2018, 53, 410–421. [Google Scholar] [CrossRef]
- Pinilla, E. Determinación de las Escalas de Intercambio de Agua en Fiordos y Canales de la Patagonia Norte; Instituto de Fomento Pesquero: Valparaíso, Chile, 2018; pp. 1–35. [Google Scholar]
- Pinilla, E.; Soto, G.; Soto-Riquelme, C. Determinación de las Escalas de Intercambio de Agua en Fiordos y Canales de la Patagonia sur, Etapa II; Instituto de Fomento Pesquero: Valparaíso, Chile, 2019; pp. 1–52. [Google Scholar]
- Baldrich, Á.M.; Díaz, P.A.; Álvarez, G.; Pérez-Santos, I.; Schwerter, C.; Díaz, M.; Araya, M.; Nieves, M.G.; Rodríguez-Villegas, C.; Barrera, F.; et al. Dinophysis acuminata or Dinophysis acuta: What makes the difference in highly stratified fjords? Mar. Drugs 2023, 21, 64. [Google Scholar] [CrossRef]
- Aguayo, R.; León-Muñoz, J.; Vargas-Baecheler, J.; Montecinos, A.; Garreaud, R.; Urbina, M.; Soto, D.; Iriarte, J.L. The glass half-empty: Climate change drives lower freshwater input in the coastal system of the Chilean Northern Patagonia. Clim. Change 2019, 155, 417–435. [Google Scholar] [CrossRef]
- León-Muñoz, J.; Marcé, R.; Iriarte, J. Influence of hydrological regime of an Andean river on salinity, temperature and oxygen in a Patagonia fjord, Chile. N. Z. J. Mar. Freshw. Res. 2013, 47, 515–528. [Google Scholar] [CrossRef]
- Baldrich, Á.M.; Molinet, C.; Reguera, B.; Espinoza-González, O.; Pizarro, G.; Rodríguez-Villegas, C.; Opazo, D.; Mejías, P.; Díaz, P.A. Interannual variability in mesoscale distribution of Dinophysis acuminata and D. acuta in Northwestern Patagonian fjords. Harmful Algae 2022, 115, 102228. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santos, I.; Díaz, P.A.; Silva, N.; Garreaud, R.; Montero, P.; Henríquez-Castillo, C.; Barrera, F.; Linford, P.; Amaya, C.; Contreras, S.; et al. Oceanography time series reveals annual asynchrony input between oceanic and estuarine waters in Patagonian fjords. Sci. Total Environ. 2021, 798, 149241. [Google Scholar] [CrossRef] [PubMed]
- Montero, P.; Pérez-Santos, I.; Daneri, G.; Gutiérrez, M.; Igor, G.; Seguel, R.; Purdie, D.; Crawford, D. A winter dinoflagellate bloom drives high rates of primary production in a Patagonian fjord ecosystem. Estuar. Coast. Shelf Sci. 2017, 199, 105–116. [Google Scholar] [CrossRef]
- Buchan, S.J.; Rendell, L.E.; Hucke-Gaete, R. Preliminary recordings of blue whale (Balaenoptera musculus) vocalizations in the Gulf of Corcovado, northern Patagonia, Chile. Mar. Mamm. Sci. 2010, 26, 451–459. [Google Scholar] [CrossRef]
- Buchan, S.J.; Stafford, K.M.; Hucke-Gaete, R. Seasonal occurrence of southeast Pacific blue whale songs in southern Chile and the eastern tropical Pacific. Mar. Mamm. Sci. 2014, 31, 440–458. [Google Scholar] [CrossRef]
- Buchan, S.J.; Hucke-Gaete, R.; Stafford, K.M.; Clark, C.W. Occasional acoustic presence of Antarctic blue whales on a feeding ground in southern Chile. Mar. Mamm. Sci. 2017, 34, 220–228. [Google Scholar] [CrossRef]
- Clément, A.; Seguel, M.; Arzul, G.; Guzmán, L.; Alarcon, C. Widespread outbreak of an haemolytic, ichthyotoxic Gymnodinium sp. in southern Chile. In Harmful Algal Blooms 2000; Hallegraeff, G.M., Blackburn, S.I., Bolch, C.J., Lewis, R.J., Eds.; IOC-UNESCO: Paris, France, 2001; pp. 66–69. [Google Scholar]
- Uribe, J.C.; Ruiz, M. Gymnodinium brown tide in the Magellanic fjords, southern Chile. Rev. Biol. Mar. Oceanogr. 2001, 36, 155–164. [Google Scholar] [CrossRef]
- Toro, C.; Alarcón, C.; Pacheco, H.; Salgado, P.; Frangopulos, M.; Rodríguez, F.; Fuenzalida, G.; Raimapo, R.; Pizarro, G.; Guzmán, L. Harmful Algal bloom species associated with massive Atlantic salmon mortalities while transported through the Gulf of Penas, southern Chile. In Harmful Algae 2018—From Ecosystems to Socioecosystems. Proceedings of the 18th International Conference on Harmful Algae, Nantes, France, 21–26 October 2018; Hess, P., Ed.; International Society for the Study of Harmful Algae: Copenhagen, Denmark, 2020; pp. 154–157. [Google Scholar]
- Díaz, P.A.; Figueroa, R.I. Toxic algal bloom recurrence in the era of global change: Lessons from the Chilean Patagonian fjords. Microorganisms 2023, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Clément, A.; Lembeye, G. Phytoplankton monitoring program in the fish farming region of South Chile. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: New York, NY, USA, 1993; pp. 223–228. [Google Scholar]
- Guzmán, L.; Espinoza, O.; Carbonell, P.; Martínez, R.; Pizarro, G.; Salgado, P.; Mardones, J.I.; Fuenzalida, G.; Besoain, V.; Cascales, E.; et al. Programa de Manejo y Monitoreo de las Mareas Rojas en el Sistema de Fiordos y Canals de Chile. Etapa XIII 2019–2020; Informe Final; Ministerio de economía, Fomento y Turismo—Subsecretaría de Economía y EMT: Valparaíso, Chile, 2020; p. 133. [Google Scholar]
- Trefault, N.; Krock, B.; Delherbe, N.; Cembella, A.; Vásquez, M. Latitudinal transects in the southeastern Pacific Ocean reveal a diverse but patchy distribution of phycotoxins. Toxicon 2011, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Shikata, T.; Kitatsuji, S.; Abe, K.; Onitsuka, G.; Matsubara, T.; Nakayama, N.; Yuasa, K.; Nishiyama, Y.; Mizuno, K.-I.; Masuda, T.; et al. Vertical distribution of a harmful red-tide dinoflagellate, Karenia mikimotoi, at the decline stage of blooms. J. Sea Res. 2020, 165, 101960. [Google Scholar] [CrossRef]
- Smayda, T.J. Adaptive ecology, growth strategies and the global bloom expansion of dinoflagellates. J. Oceanogr. 2002, 58, 281–294. [Google Scholar] [CrossRef]
- Smayda, T.J. Adaptations and selection of harmful and other dinoflagellate species in upwelling systems. 2. Motility and migratory behaviour. Prog. Oceanogr. 2010, 85, 71–91. [Google Scholar] [CrossRef]
- Magaña, H.A.; Villareal, T.A. The effect of environmental factors on the growth rate of Karenia brevis (Davis) G. Hansen and Moestrup. Harmful Algae 2006, 5, 192–198. [Google Scholar] [CrossRef]
- Maier-Brown, A.F.; Dortch, Q.; Van Dolah, F.M.; Leighfield, T.A.; Morrison, W.; Thessen, A.E.; Steidinger, K.; Richardson, B.; Moncreiff, C.A.; Pennock, J.R. Effect of salinity on the distribution, growth, and toxicity of Karenia spp. Harmful Algae 2006, 5, 199–212. [Google Scholar] [CrossRef]
- Sourisseau, M.; Jegou, K.; Lunven, M.; Quere, J.; Gohin, F.; Bryere, P. Distribution and dynamics of two species of Dinophyceae producing high biomass blooms over the French Atlantic Shelf. Harmful Algae 2016, 53, 53–63. [Google Scholar] [CrossRef]
- Geesey, M.; Tester, P. Gymnodinium breve: Ubiquitous in Gulf of Mexico waters? In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1993; pp. 251–255. [Google Scholar]
- Raine, R. A review of the biophysical interactions relevant to the promotion of HABs in stratified systems: The case study of Ireland. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2014, 101, 21–31. [Google Scholar] [CrossRef]
- Tewari, K. A review of climate change impact studies on Harmful Algal Blooms. Phycology 2022, 2, 244–253. [Google Scholar] [CrossRef]
- Ruiz, C.; Artal, O.; Pinilla, E.; Sepúlveda, H.H. Stratification and mixing in the Chilean Inland Sea using an operational model. Ocean Model. Online 2021, 158, 101750. [Google Scholar] [CrossRef]
- Narváez, D.A.; Vargas, C.A.; Cuevas, L.A.; García-Loyola, S.A.; Lara, C.; Segura, C.; Tapia, F.J.; Broitman, B.R. Dominant scales of subtidal variability in coastal hydrography of the Northern Chilean Patagonia. J. Mar. Syst. 2019, 193, 59–73. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Seguel, R.; Schneider, W.; Linford, P.; Donoso, D.; Navarro, E.; Amaya-Cárcamo, C.; Pinilla, E.; Daneri, G. Synoptic-scale variability of surface winds and ocean response to atmospheric forcing in the eastern austral Pacific Ocean. Ocean Sci. 2019, 15, 1247–1266. [Google Scholar] [CrossRef]
- Aguirre, C.; Pizarro, Ó.; Strub, P.T.; Garreaud, R.; Barth, J.A. Seasonal dynamics of the near-surface alongshore flow off central Chile. J. Geophys. Res. 2012, 117. [Google Scholar] [CrossRef]
- Linford, P.; Pérez-Santos, I.; Montes, I.; Dewitte, B.; Buchan, S.; Narváez, D.; Saldías, G.; Pinilla, E.; Garreaud, R.; Díaz, P.; et al. Recent deoxygenation of Patagonian fjord subsurface waters connected to the Peru–Chile undercurrent and equatorial subsurface water variability. Glob. Biogeochem. Cycles 2023, 37, e2022GB007688. [Google Scholar] [CrossRef]
- Pinilla, E.; Castillo, M.; Pérez-Santos, I.; Venegas, O.; Valle-Levinson, A. Water age variability in a Patagonian fjord. J. Mar. Syst. 2020, 210, 103376. [Google Scholar] [CrossRef]
- Rodríguez-Benito, C.V.; Navarro, G.; Caballero, I. Using Copernicus Sentinel-2 and Sentinel-3 data to monitor harmful algal blooms in Southern Chile during the COVID-19 lockdown. Mar. Pollut. Bull. 2020, 161, 111722. [Google Scholar] [CrossRef] [PubMed]
- Morozov, E.; Pozdnyakov, D.; Smyth, T.; Sychev, V.; Grassl, H. Space-borne study of seasonal, multi-year, and decadal phytoplankton dynamics in the Bay of Biscay. Int. J. Remote Sens. 2013, 34, 1297–1331. [Google Scholar] [CrossRef]
- Napoléon, C.; Fiant, L.; Raimbault, V.; Riou, P.; Claquin, P. Dynamics of phytoplankton diversity structure and primary productivity in the English Channel. Mar. Ecol. Prog. Ser. 2014, 505, 49–64. [Google Scholar] [CrossRef]
- Garreaud, R. Record-breaking climate anomalies lead to severe drought and environmental disruption in western Patagonia in 2016. Clim. Res. 2018, 74, 217–229. [Google Scholar] [CrossRef]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef]
- Muñoz, A.A.; González-Reyes, A.; Lara, A.; Sauchyn, D.; Christie, D.; Puchi, P.; Urrutia-Jalabert, R.; Toledo-Guerrero, I.; Aguilera-Betti, I.; Mundo, I.; et al. Streamflow variability in the Chilean Temperate-Mediterranean climate transition (35°S–42°S) during the last 400 years inferred from tree-ring records. Clim. Dyn. 2016, 47, 4051–4066. [Google Scholar] [CrossRef]
- Schlitzer, R. Ocean Data View. 2019. Available online: https://odv.awi.de.
- Lovegrove, T. An improved form of sedimentation apparatus for use with an inverted microscope. ICES J. Mar. Sci. 1960, 25, 279–284. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; Verlag Chemie Weinhein: New York, NY, USA, 1983. [Google Scholar]
- Kattner, G.; Becker, H. Nutrients and organic nitrogenous compounds in the marginal ice zone of the Fram Strait. J. Mar. Syst. 1991, 2, 385–394. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Theor. Angew. Limnol. Mitteilungen 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Regueiro, J.; Rossignoli, A.E.; Álvarez, G.; Blanco, J. Automated on-line solid-phase extraction coupled to liquid chromatography–tandem mass spectrometry for determination of lipophilic marine toxins in shellfish. Food Chem. 2011, 129, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mishra, D.R. Normalized difference chlorophyll index: A novel model for remote estimation of chlorophyll-a concentration in turbid productive waters. Remote Sens. Environ. 2012, 117, 394–406. [Google Scholar] [CrossRef]
- O’Reilly, J.E.; Werdell, P.J. Chlorophyll algorithms for ocean color sensors-OC4, OC5 & OC6. Remote Sens. Environ. 2019, 229, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Pahlevan, N.; Smith, B.; Schalles, J.; Binding, C.; Cao, Z.; Ma, R.; Alikas, K.; Kangro, K.; Gurlin, D.; Hà, N.; et al. Seamless retrievals of chlorophyll-a from Sentinel-2 (MSI) and Sentinel-3 (OLCI) in inland and coastal waters: A machine-learning approach. Remote Sens. Environ. 2020, 240, 111604. [Google Scholar] [CrossRef]
- Soto, I.M.; Cannizzaro, J.; Muller-Karger, F.E.; Hu, C.; Wolny, J.; Goldgof, D. Evaluation and optimization of remote sensing techniques for detection of Karenia brevis blooms on the West Florida Shelf. Remote Sens. Environ. 2015, 170, 239–254. [Google Scholar] [CrossRef]
- Amin, R.; Zhou, J.; Gilerson, A.; Gross, B.; Moshary, F.; Ahmed, S. Novel optical techniques for detecting and classifying toxic dinoflagellate Karenia brevis blooms using satellite imagery. Opt. Express 2009, 17, 9126–9144. [Google Scholar] [CrossRef]
- Beggs, H.; Karagali, I.; Castro, S. GHRSST Project Office: Sea Surface Temperature: An Introduction to Users on the Set of GHRSST Formatted Products (1/2023); Zenodo: Geneva, Switzerland, 2023. [Google Scholar]
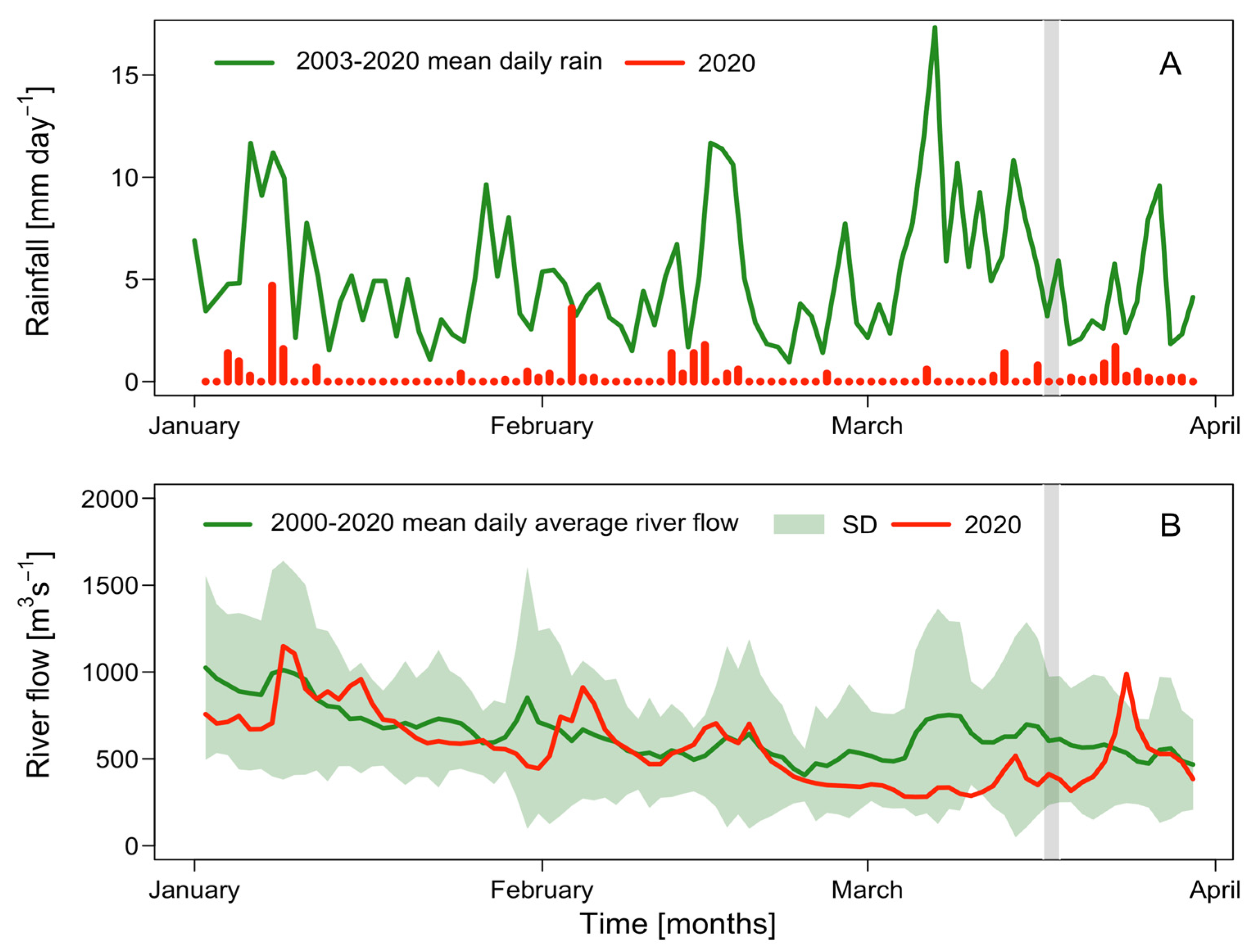


| Rainfall (mm/month−1) | River Discharge (m3s−1/month−1) | |||||
|---|---|---|---|---|---|---|
| Year | 2003–2019 mean | 2020 | Anomaly | 2003–2019 mean | 2020 | Anomaly |
| January | 155.6 ± 92.4 | 10.9 | −144.7 | 817.1 ± 242.5 | 717.6 | −99.5 |
| February | 124.5 ± 102.1 | 10.8 | −113.7 | 547.5 ± 170.8 | 539.9 | −7.6 |
| March | 172.4 ± 91.61 | 7.7 | −164.7 | 586.8 ± 221.8 | 418.6 | −168.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldrich, Á.M.; Díaz, P.A.; Rosales, S.A.; Rodríguez-Villegas, C.; Álvarez, G.; Pérez-Santos, I.; Díaz, M.; Schwerter, C.; Araya, M.; Reguera, B. An Unprecedented Bloom of Oceanic Dinoflagellates (Karenia spp.) Inside a Fjord within a Highly Dynamic Multifrontal Ecosystem in Chilean Patagonia. Toxins 2024, 16, 77. https://doi.org/10.3390/toxins16020077
Baldrich ÁM, Díaz PA, Rosales SA, Rodríguez-Villegas C, Álvarez G, Pérez-Santos I, Díaz M, Schwerter C, Araya M, Reguera B. An Unprecedented Bloom of Oceanic Dinoflagellates (Karenia spp.) Inside a Fjord within a Highly Dynamic Multifrontal Ecosystem in Chilean Patagonia. Toxins. 2024; 16(2):77. https://doi.org/10.3390/toxins16020077
Chicago/Turabian StyleBaldrich, Ángela M., Patricio A. Díaz, Sergio A. Rosales, Camilo Rodríguez-Villegas, Gonzalo Álvarez, Iván Pérez-Santos, Manuel Díaz, Camila Schwerter, Michael Araya, and Beatriz Reguera. 2024. "An Unprecedented Bloom of Oceanic Dinoflagellates (Karenia spp.) Inside a Fjord within a Highly Dynamic Multifrontal Ecosystem in Chilean Patagonia" Toxins 16, no. 2: 77. https://doi.org/10.3390/toxins16020077
APA StyleBaldrich, Á. M., Díaz, P. A., Rosales, S. A., Rodríguez-Villegas, C., Álvarez, G., Pérez-Santos, I., Díaz, M., Schwerter, C., Araya, M., & Reguera, B. (2024). An Unprecedented Bloom of Oceanic Dinoflagellates (Karenia spp.) Inside a Fjord within a Highly Dynamic Multifrontal Ecosystem in Chilean Patagonia. Toxins, 16(2), 77. https://doi.org/10.3390/toxins16020077







