Assessing the Risk of Exposure to Aflatoxin B1 through the Consumption of Peanuts among Children Aged 6–59 Months in the Lusaka District, Zambia
Abstract
1. Introduction
2. Results and Discussion
2.1. Contamination and Consumption
2.2. Risk Characterization
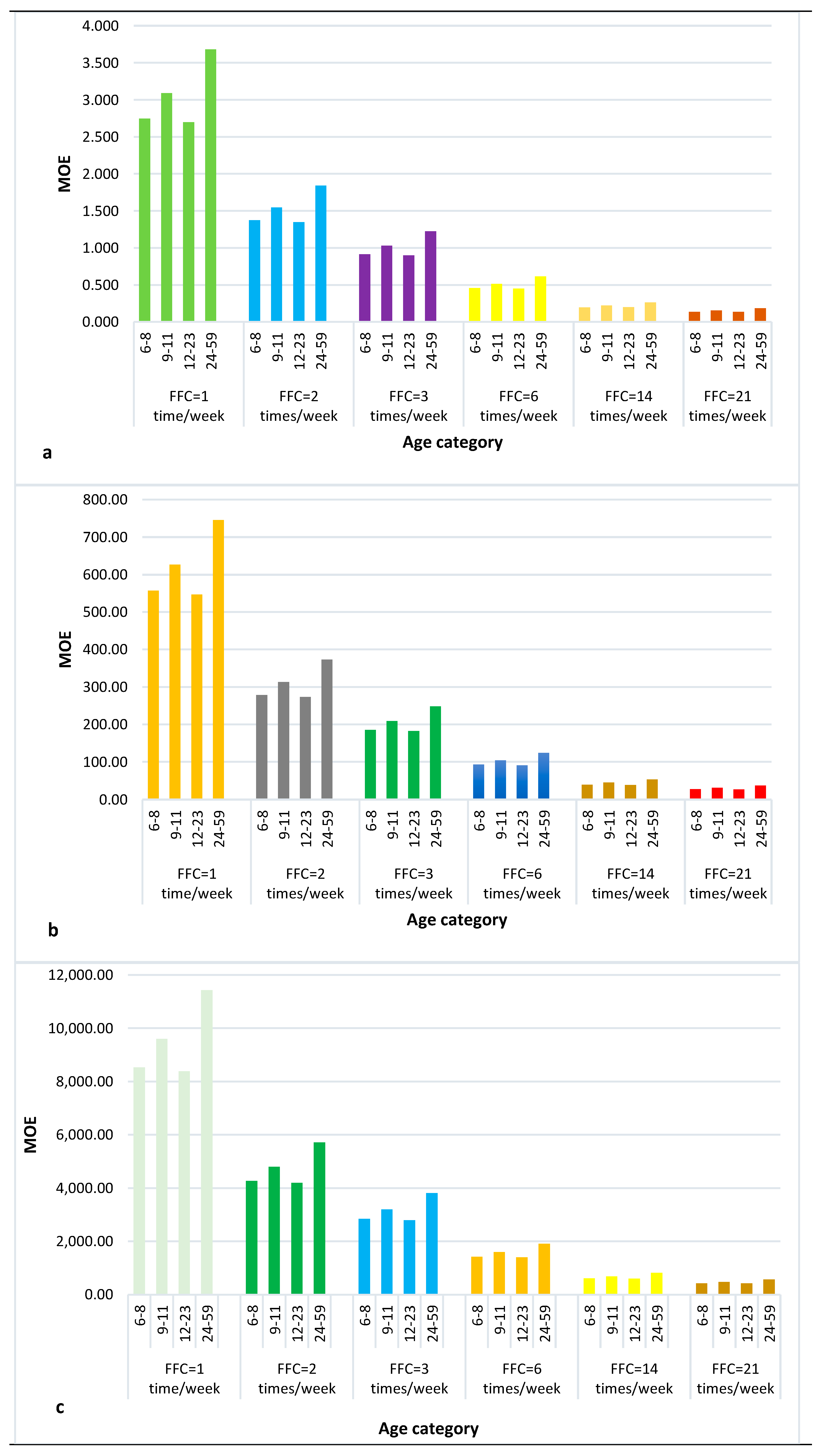
2.2.1. The Margin of Exposure (MOE) Approach
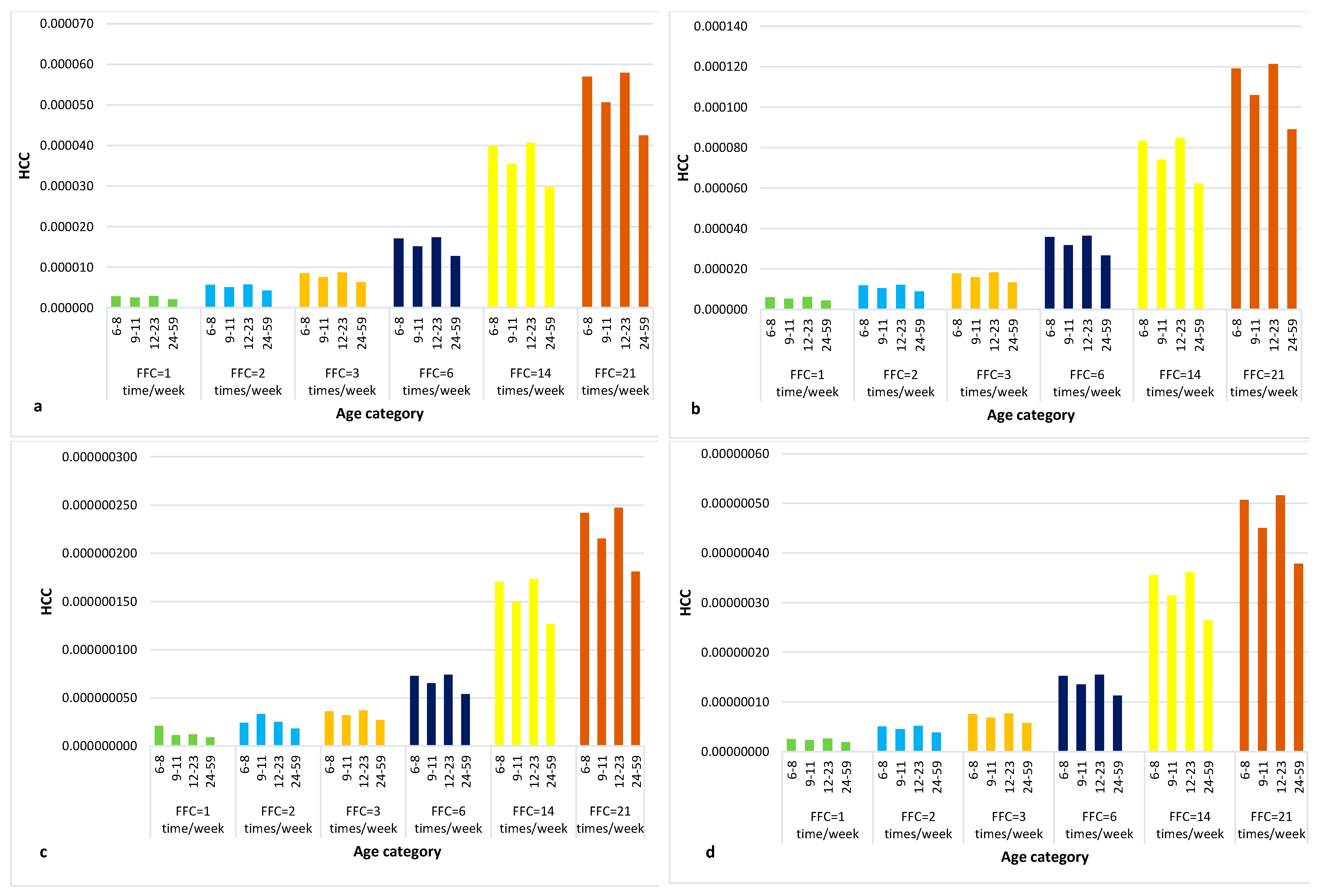
2.2.2. The Quantitative Liver Cancer Risk Approach (HCC)
3. Uncertainty
4. Conclusions and Perspectives
5. Materials and Methods
5.1. Peanuts Sampling
5.2. Aflatoxins Extraction and Determination
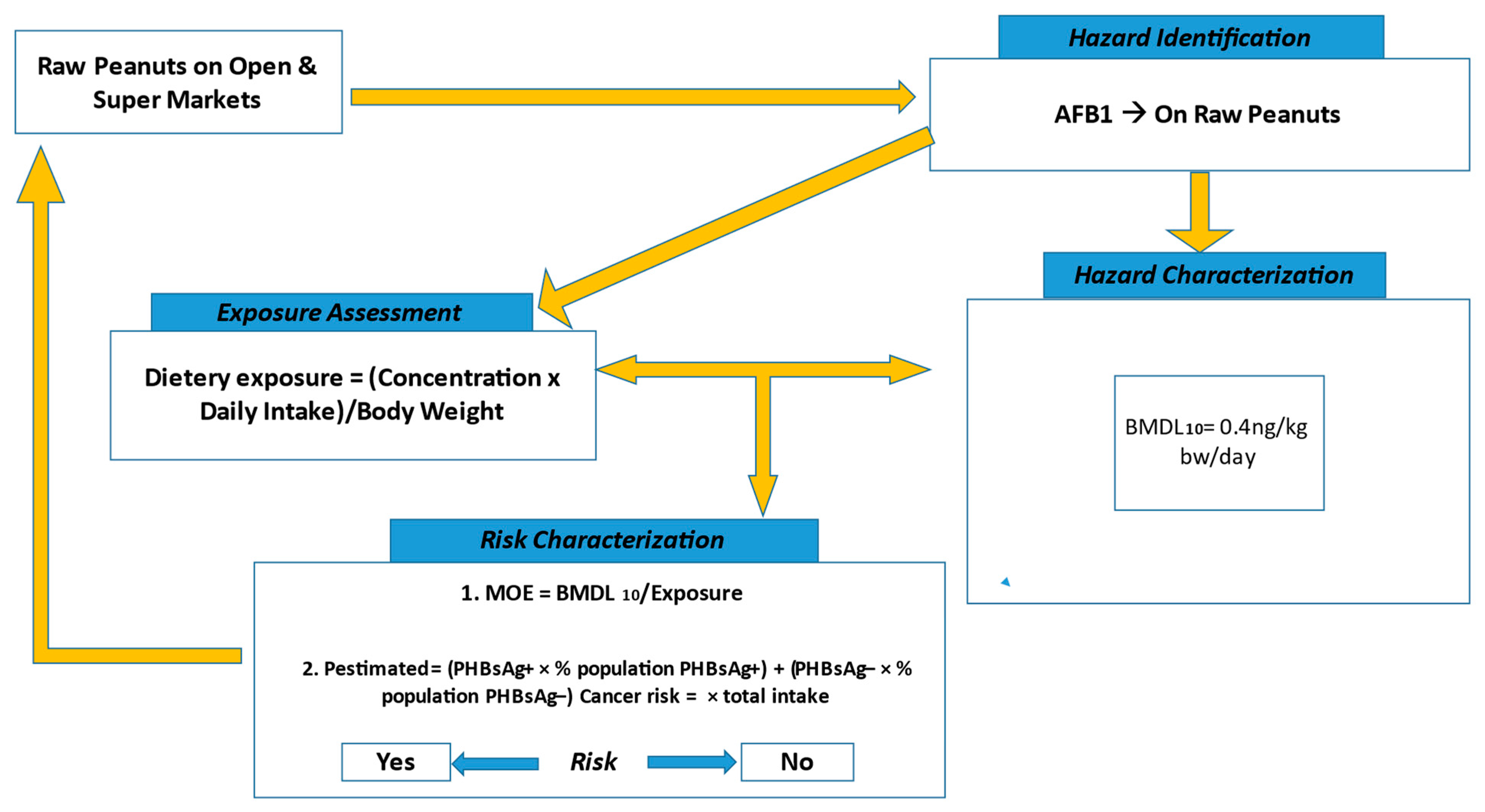
5.3. Determination of the Exposure Risk to AFB1
5.3.1. Hazard Identification and Characterization
5.3.2. Exposure Assessment
5.3.3. Risk Characterization
The Margin of Exposure (MOE)
Quantitative Liver Cancer Risk Approach
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Shamsie, J. The resource-based view of the firm in two environments: The Hollywood film studios from 1936 to 1965. Acad. Manag. J. 1996, 39, 519–543. [Google Scholar] [CrossRef]
- Kachapulula, P.; Akello, J.; Bandyopadhyay, R.; Cotty, P. Aflatoxin contamination of groundnut and maize in Zambia: Observed and potential concentrations. J. Appl. Microbiol. 2017, 122, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.-A.; Vettorazzi, A.; Arbillaga, L.; Pérez, N.; Gil, A.G.; Azqueta, A.; González-Peñas, E.; García-Jalón, J.A.; de Cerain, A.L. Genotoxicity of Aflatoxin B1 and Ochratoxin A after simultaneous application of the in vivo micronucleus and comet assay. Food Chem. Toxicol. 2015, 76, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Vargas, H.; Castelino, J.; Silver, M.J.; Dominguez-Salas, P.; Cros, M.-P.; Durand, G.; Calvez-Kelm, F.L.; Prentice, A.M.; Wild, C.P.; Moore, S.E. Exposure to aflatoxin B1 in utero is associated with DNA methylation in white blood cells of infants in The Gambia. Int. J. Epidemiol. 2015, 44, 1238–1248. [Google Scholar] [CrossRef]
- Marrone, A.K.; Tryndyak, V.; Beland, F.A.; Pogribny, I.P. MicroRNA responses to the genotoxic carcinogens aflatoxin B1 and benzo [a] pyrene in human HepaRG cells. Toxicol. Sci. 2016, 149, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P. The idea and practice of systems thinking and their relevance for capacity development. Maastricht Eur. Cent. Dev. Policy Manag. 2005, 1–34. [Google Scholar]
- Wu, F.; Khlangwiset, P. Health economic impacts and cost-effectiveness of aflatoxin-reduction strategies in Africa: Case studies in biocontrol and post-harvest interventions. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess 2010, 27, 496–509. [Google Scholar] [CrossRef]
- Wild, C.; Turner, P. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 2002, 17, 471–481. [Google Scholar] [CrossRef]
- Bumbangi, N.; Muma, J.; Choongo, K.; Mukanga, M.; Velu, M.; Veldman, F.; Hatloy, A.; Mapatano, M. Occurrence and factors associated with aflatoxin contamination of raw peanuts from Lusaka district’s markets, Zambia. Food Control. 2016, 68, 291–296. [Google Scholar] [CrossRef]
- MoA. Zambia Food-Based Dietary Guidelines Technical Recommendations; Ministry of Agriculture: Lusaka, Zambia; FAO: Rome, Italy, 2021.
- UNICEF. 2021 Situation Analysis of the Status and Well-Being of Children in Zambia; Ministry of Finance: Lusaka, Zambia, 2021.
- Lorán, S.; Bayarri, S.; Conchello, P.; Herrera, A. Risk assessment of PCDD/PCDFs and indicator PCBs contamination in Spanish commercial baby food. Food Chem. Toxicol. 2010, 48, 145–151. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Principles and methods for the risk assessment of chemicals in food. Environ. Health Crit. 2009, 240, 1–52. [Google Scholar]
- Makri, A.; Goveia, M.; Balbus, J.; Parkin, R. Children’s susceptibility to chemicals: A review by developmental stage. J. Toxicol. Environ. Health B Crit. Rev. 2004, 7, 417–435. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006, 95, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Mofya-Mukuka, R.; Kabisa, M. Analysis of indicators and measurement tools used in Zambia to assess impact of agricultural extension programs on gender equity and nutrition outcomes. Work. Pap. 2016. [Google Scholar]
- Alaofè, H.; Taren, D.; Burney, J. Household food insecurity and its determinants in the Kalalé district of Benin (268.1). FASEB J. 2014, 28, 268.1. [Google Scholar] [CrossRef]
- Goma, J. Seroprevalence of Hepatitis b Infection in Children Less than Five Years of Age at the University Teaching Hospitals—Children’s Hospital and Kamwala Health Centre in the Vaccination era in Lusaka, Zambia; University of Zambia: Lusaka, Zambia, 2019. [Google Scholar]
- Chu, Y.-J.; Yang, H.-I.; Wu, H.-C.; Lee, M.-H.; Liu, J.; Wang, L.-Y.; Lu, S.-N.; Jen, C.-L.; You, S.-L.; Santella, R.M. Aflatoxin B1 exposure increases the risk of hepatocellular carcinoma associated with hepatitis C virus infection or alcohol consumption. Eur. J. Cancer 2018, 94, 37–46. [Google Scholar] [CrossRef]
- Garenne, M.; Gakusi, A.E. Vulnerability and resilience: Determinants of under-five mortality changes in Zambia. World Dev. 2006, 34, 1765–1787. [Google Scholar] [CrossRef][Green Version]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- FAO/WHO. Aflatoxins. In Safety Evaluation of Certain Contaminants in Food: Prepared by the Eighty-Third Meeting of the JOINT FAO/WHO Expert Committee on Food Additives (JECFA); Food Additive Series; World Health Organization: Geneva, Switzerland, 2018; Volume 74, pp. 2–280. [Google Scholar]
- EFSA. Panel on Contaminants in the Food Chain. Risk assessment of aflatoxins in food. EFSA J. 2020, 18, e06040. [Google Scholar]
- Ashiq, S.; Hussain, M.; Ahmad, B. Natural occurrence of mycotoxins in medicinal plants: A review. Fungal. Genet. Biol. 2014, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.O. Risk assessment for aflatoxins in foodstuffs. Int. Biodeterior. Biodegrad. 2002, 50, 137–142. [Google Scholar] [CrossRef]
- Cotty, P.J.; Probst, C.; Jaime-Garcia, R. Etiology and management of aflatoxin contamination. In Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade; CABI: Wallingford, UK, 2008; pp. 287–299. [Google Scholar]
- Zhao, Q.; Qiu, Y.; Wang, X.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Inhibitory effects of Eurotium cristatum on growth and aflatoxin B1 biosynthesis in Aspergillus flavus. Front Microbiol. 2020, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- McCullough, A.K.; Lloyd, R.S. Mechanisms underlying aflatoxin-associated mutagenesis–implications in carcinogenesis. DNA Repair 2019, 77, 76–86. [Google Scholar] [CrossRef] [PubMed]
- IARC. A Review of Human Carcinogens. F. In Chemical Agents and Related Occupations: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012. [Google Scholar]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef]
- Wild, C.P.; Montesano, R. A model of interaction: Aflatoxins and hepatitis viruses in liver cancer aetiology and prevention. Cancer Lett. 2009, 286, 22–28. [Google Scholar] [CrossRef]
- Nugraha, A.; Khotimah, K.; Rietjens, I.M. Risk assessment of aflatoxin B1 exposure from maize and peanut consumption in Indonesia using the margin of exposure and liver cancer risk estimation approaches. Food Chem. Toxicol. 2018, 113, 134–144. [Google Scholar] [CrossRef]

| Variables | Positive (%) | Median (ng/kg) | Min (ng/kg) | Max (ng/kg) |
|---|---|---|---|---|
| AFB1 | n = 41(44.6) | 0.00023 | 0.000015 | 0.0466 |
| AFB2 | n = 41(44.6) | 0.000132 | 0.000006 | 0.01317 |
| AFG1 | n = 21(22.8) | 0.000028 | 0.000005 | 0.00051 |
| AFG2 | n = 7(7.6) | 0.000008 | 0.000006 | 0.00004 |
| AF | n = 51(55.) | 0.00023 | 0.000014 | 0.04867 |
| Child’s Age in Months | Frequency of Consumption per Day | Amount of Food ** (Porridge) |
|---|---|---|
| 6–8 | 2–3 | 2–3 tablespoons * |
| 9–11 | 3–4 | 2–3 tablespoons |
| 12–23 | 3–4 | 3–4 tablespoons |
| 24–59 | 4–5 | 3–4 tablespoons |
| Age (Months) | Weight (Kg) | ||
|---|---|---|---|
| Female | Male | Average | |
| 6–8 | 7.3–8.2 | 7.9–8.9 | 8.1 |
| 9–11 | 8.7–9.4 | 9.5–10.2 | 9.4 |
| 12–23 | 9.0–11.3 | 9.7–12.0 | 11.0 |
| 24–59 | 12.1–18.0 | 12.7–18.5 | 15.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musawa, G.; Bumbangi, F.N.; Mumba, C.; Mbunga, B.K.; Phiri, G.; Benhard, V.; Kainga, H.; Banda, M.; Ndaki, E.; Mkandawire, E.; et al. Assessing the Risk of Exposure to Aflatoxin B1 through the Consumption of Peanuts among Children Aged 6–59 Months in the Lusaka District, Zambia. Toxins 2024, 16, 50. https://doi.org/10.3390/toxins16010050
Musawa G, Bumbangi FN, Mumba C, Mbunga BK, Phiri G, Benhard V, Kainga H, Banda M, Ndaki E, Mkandawire E, et al. Assessing the Risk of Exposure to Aflatoxin B1 through the Consumption of Peanuts among Children Aged 6–59 Months in the Lusaka District, Zambia. Toxins. 2024; 16(1):50. https://doi.org/10.3390/toxins16010050
Chicago/Turabian StyleMusawa, Grace, Flavien Nsoni Bumbangi, Chisoni Mumba, Branly Kilola Mbunga, Gladys Phiri, Vistorina Benhard, Henson Kainga, Mkuzi Banda, Enock Ndaki, Ethel Mkandawire, and et al. 2024. "Assessing the Risk of Exposure to Aflatoxin B1 through the Consumption of Peanuts among Children Aged 6–59 Months in the Lusaka District, Zambia" Toxins 16, no. 1: 50. https://doi.org/10.3390/toxins16010050
APA StyleMusawa, G., Bumbangi, F. N., Mumba, C., Mbunga, B. K., Phiri, G., Benhard, V., Kainga, H., Banda, M., Ndaki, E., Mkandawire, E., & Muma, J. B. (2024). Assessing the Risk of Exposure to Aflatoxin B1 through the Consumption of Peanuts among Children Aged 6–59 Months in the Lusaka District, Zambia. Toxins, 16(1), 50. https://doi.org/10.3390/toxins16010050







