TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA
Abstract
1. Introduction
2. Results
2.1. Case History
2.2. Cyanobacterial Toxin BMAA Exposure
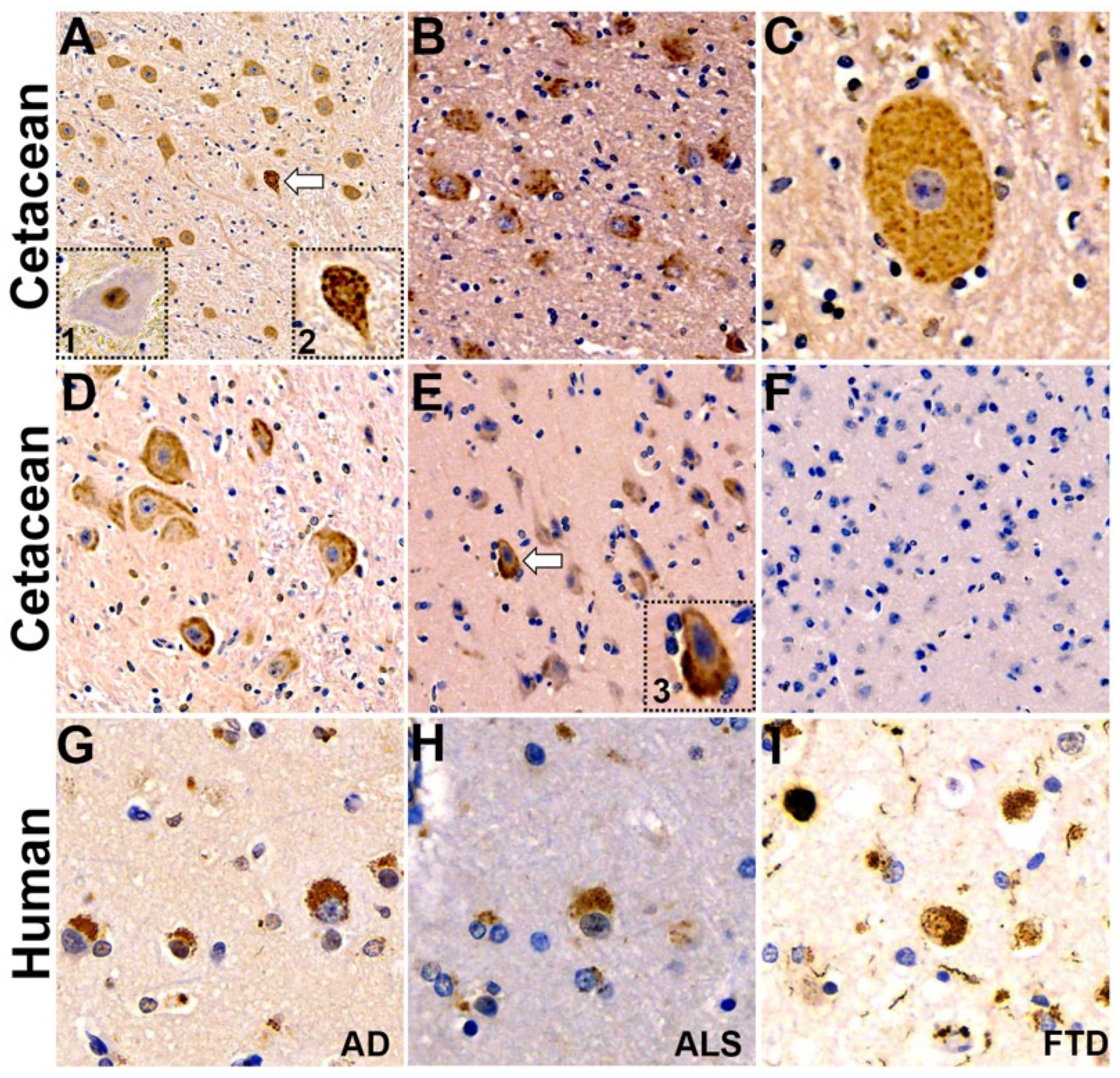
2.3. Expression of Genes Implicated in Alzheimer’s Disease and TDP-43 Proteinopathy
| Gene Probes | Neurological Diseases | Associated Neuropathology | Harbor Porpoise (qPCR) | Human (BioGPS) | ||
|---|---|---|---|---|---|---|
| PcV1:CE | Fc:CE | Pc:CE | PreFc:CE | |||
| APP [34] | AD | Aβ+ plaques | 1.41 ± 0.34 | 2.88 ± 0.56 p < 0.0001 | 1.73 | 4.20 |
| PSEN1 [35] | AD | Aβ+ plaques | 1.23 ± 0.02 | 1.33 ± 0.03 | 7.48 | 7.56 |
| PSEN2 [36] | AD | Aβ+ plaques | 0.66 ± 0.03 | 0.79 ± 0.01 | 1.30 | 1.46 |
| GRN [37] | FTLD | progranulin loss | 1.38 ± 0.02 | 1.96 ± 0.04 | 1.24 | 1.42 |
| MAPT [38] | Tauopathies | NFTs | 1.52 ± 0.07 | 2.40 ± 0.14 p = 0.0134 | 4.08 | 7.24 |
| TARBDP [39] | TDP-43 proteinopathies | IC | 1.21 ± 0.01 | 1.95 ± 0.04 | 1.40 | 2.28 |
| C9orf72 [39] | ALS and FTD | IC | 1.22 ± 0.09 | 1.66 ± 0.09 | 1.03 | 0.96 |
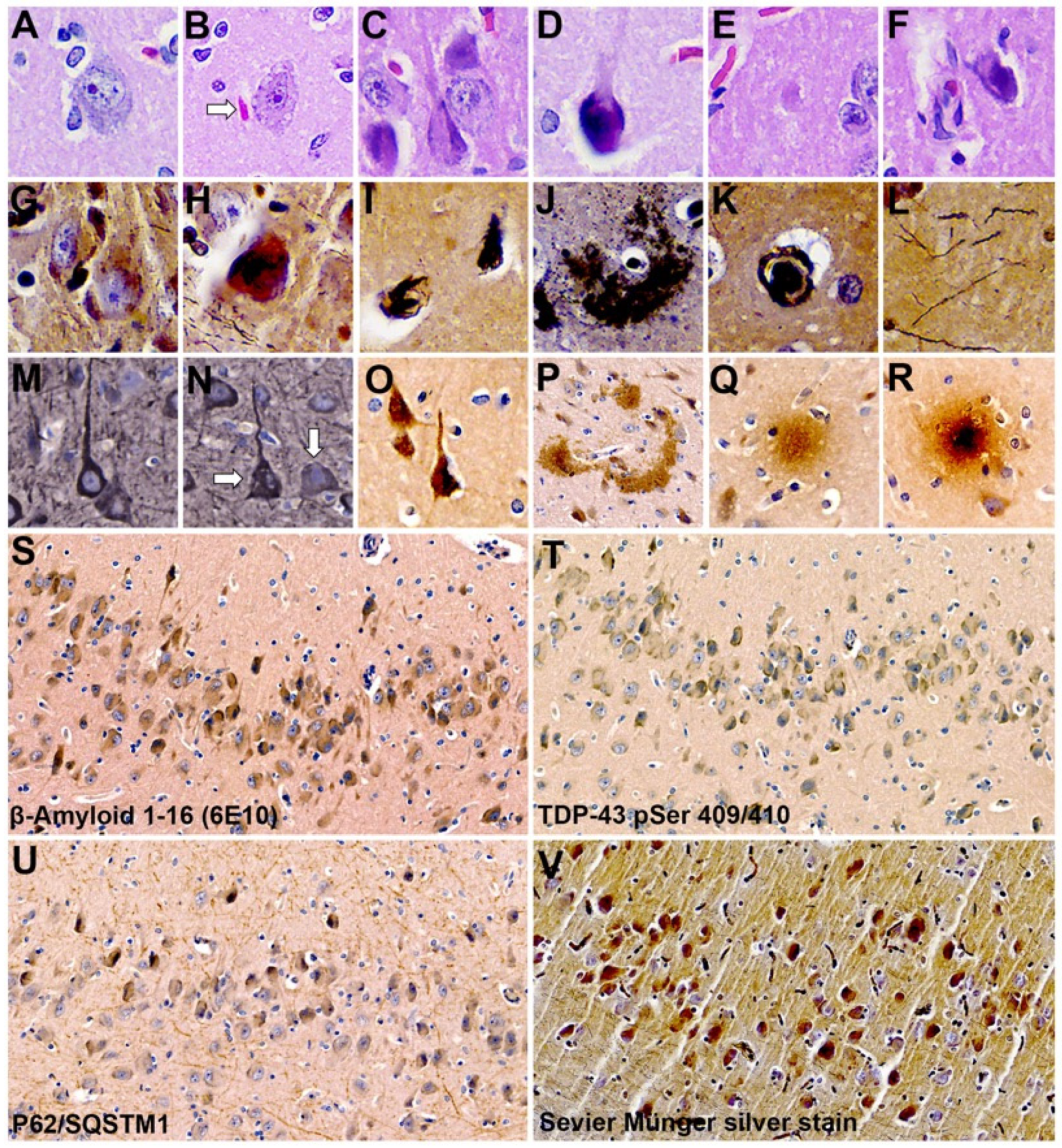
2.4. Neuropathology
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Harbor Porpoise Brain
5.2. HPLC/FD
5.3. qPCR Analysis
5.4. Immunohistochemistry and Digital Pathology
5.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruck, J.N. Decades-long social memory in bottlenose dolphins. Proc. Biol. Sci. 2013, 280, 20131726. [Google Scholar] [CrossRef] [PubMed]
- Cairomicron, O. External measures of cognition. Front. Hum. Neurosci. 2011, 5, 108. [Google Scholar] [CrossRef]
- Hof, P.R.; Chanis, R.; Marino, L. Cortical complexity in cetacean brains. Anat. Rec. Discov. Mol. Cell Evol. Biol. 2005, 287, 1142–1152. [Google Scholar] [CrossRef]
- Dell, L.A.; Patzke, N.; Spocter, M.A.; Siegel, J.M.; Manger, P.R. Organization of the sleep-related neural systems in the brain of the harbour porpoise (Phocoena phocoena). J. Comp. Neurol. 2016, 524, 1999–2017. [Google Scholar] [CrossRef]
- Kruger, L. Edward Tyson’s 1680 account of the ‘porpess’ brain and its place in the history of comparative neurology. J. Hist. Neurosci. 2003, 12, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Garamszegi, S.P.; Banack, S.A.; Dooley, P.D.; Coyne, T.M.; McLean, D.W.; Rotstein, D.S.; Mash, D.C.; Cox, P.A. BMAA, Methylmercury, and Mechanisms of Neurodegeneration in Dolphins: A Natural Model of Toxin Exposure. Toxins 2021, 13, 697. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef]
- Pintore, M.D.; Mignone, W.; Di Guardo, G.; Mazzariol, S.; Ballardini, M.; Florio, C.L.; Goria, M.; Romano, A.; Caracappa, S.; Giorda, F.; et al. Neuropathologic Findings in Cetaceans Stranded in Italy (2002–2014). J. Wildl. Dis. 2018, 54, 295–303. [Google Scholar] [CrossRef]
- Sips, G.J.; Chesik, D.; Glazenburg, L.; Wilschut, J.; De Keyser, J.; Wilczak, N. Involvement of morbilliviruses in the pathogenesis of demyelinating disease. Rev. Med. Virol. 2007, 17, 223–244. [Google Scholar] [CrossRef]
- Vacher, M.C.; Durrant, C.S.; Rose, J.; Hall, A.J.; Spires-Jones, T.L.; Gunn-Moore, F.; Dagleish, M.P. Alzheimer’s disease-like neuropathology in three species of oceanic dolphin. Eur. J. Neurosci. 2023, 57, 1161–1179. [Google Scholar] [CrossRef]
- Di Guardo, G. Alzheimer’s disease, cellular prion protein, and dolphins. Alzheimers Dement. 2018, 14, 259–260. [Google Scholar] [CrossRef]
- Reif, J.S.; Schaefer, A.M.; Bossart, G.D. Atlantic Bottlenose Dolphins (Tursiops truncatus) as A Sentinel for Exposure to Mercury in Humans: Closing the Loop. Vet. Sci. 2015, 2, 407–422. [Google Scholar] [CrossRef]
- Tilbury, K.L.; Stein, J.E.; Meador, J.P.; Krone, C.A.; Chan, S.L. Chemical contaminants in harbor porpoise (Phocoena phocoena) from the north Atlantic coast: Tissue concentrations and intra- and inter-organ distribution. Chemosphere 1997, 34, 2159–2181. [Google Scholar] [CrossRef]
- Van de Vijver, K.I.; Holsbeek, L.; Das, K.; Blust, R.; Joiris, C.; De Coen, W. Occurrence of perfluorooctane sulfonate and other perfluorinated alkylated substances in harbor porpoises from the Black Sea. Environ. Sci. Technol. 2007, 41, 315–320. [Google Scholar] [CrossRef]
- Banack, S.A.; Johnson, H.E.; Cheng, R.; Cox, P.A. Production of the neurotoxin BMAA by a marine cyanobacterium. Mar. Drugs 2007, 5, 180–196. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef]
- Cox, P.A.; Kostrzewa, R.M.; Guillemin, G.J. BMAA and Neurodegenerative Illness. Neurotox. Res. 2018, 33, 178–183. [Google Scholar] [CrossRef]
- Cox, P.A.; Sacks, O.W. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology 2002, 58, 956–959. [Google Scholar] [CrossRef]
- Banack, S.A.; Murch, S.J.; Cox, P.A. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J. Ethnopharmacol. 2006, 106, 97–104. [Google Scholar] [CrossRef]
- Murch, S.J.; Cox, P.A.; Banack, S.A.; Steele, J.C.; Sacks, O.W. Occurrence of beta-methylamino-l-alanine (BMAA) in ALS/PDC patients from Guam. Acta Neurol. Scand. 2004, 110, 267–269. [Google Scholar] [CrossRef]
- Mimuro, M.; Yoshida, M.; Kuzuhara, S.; Kokubo, Y. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Hohara focus of the Kii Peninsula: A multiple proteinopathy? Neuropathology 2018, 38, 98–107. [Google Scholar] [CrossRef]
- Geser, F.; Winton, M.J.; Kwong, L.K.; Xu, Y.; Xie, S.X.; Igaz, L.M.; Garruto, R.M.; Perl, D.P.; Galasko, D.; Lee, V.M.; et al. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2008, 115, 133–145. [Google Scholar] [CrossRef]
- Davis, D.A.; Cox, P.A.; Banack, S.A.; Lecusay, P.D.; Garamszegi, S.P.; Hagan, M.J.; Powell, J.T.; Metcalf, J.S.; Palmour, R.M.; Beierschmitt, A.; et al. l-Serine Reduces Spinal Cord Pathology in a Vervet Model of Preclinical ALS/MND. J. Neuropathol. Exp. Neurol. 2020, 79, 393–406. [Google Scholar] [CrossRef]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. Biol. Sci. 2016, 283, 20152397. [Google Scholar] [CrossRef]
- Yin, H.Z.; Yu, S.; Hsu, C.I.; Liu, J.; Acab, A.; Wu, R.; Tao, A.; Chiang, B.J.; Weiss, J.H. Intrathecal infusion of BMAA induces selective motor neuron damage and astrogliosis in the ventral horn of the spinal cord. Exp. Neurol. 2014, 261, 1–9. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Davis, D.A.; Mondo, K.; Seely, M.S.; Murch, S.J.; Glover, W.B.; Divoll, T.; Evers, D.C.; Mash, D.C. Cyanobacterial Neurotoxin BMAA and Mercury in Sharks. Toxins 2016, 8, 238. [Google Scholar] [CrossRef]
- Mondo, K.; Hammerschlag, N.; Basile, M.; Pablo, J.; Banack, S.A.; Mash, D.C. Cyanobacterial neurotoxin beta-N-methylamino-L-alanine (BMAA) in shark fins. Mar. Drugs 2012, 10, 509–520. [Google Scholar] [CrossRef]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial blooms and the occurrence of the neurotoxin beta-N-methylamino-L-alanine (BMAA) in South Florida aquatic food webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef]
- Pablo, J.; Banack, S.A.; Cox, P.A.; Johnson, T.E.; Papapetropoulos, S.; Bradley, W.G.; Buck, A.; Mash, D.C. Cyanobacterial neurotoxin BMAA in ALS and Alzheimer’s disease. Acta Neurol. Scand. 2009, 120, 216–225. [Google Scholar] [CrossRef]
- Garamszegi, S.P.; Banack, S.A.; Duque, L.L.; Metcalf, J.S.; Stommel, E.W.; Cox, P.A.; Davis, D.A. Detection of beta-N-methylamino-l-alanine in postmortem olfactory bulbs of Alzheimer’s disease patients using UHPLC-MS/MS: An autopsy case-series study. Toxicol. Rep. 2023, 10, 87–96. [Google Scholar] [CrossRef]
- Berntzon, L.; Ronnevi, L.O.; Bergman, B.; Eriksson, J. Detection of BMAA in the human central nervous system. Neuroscience 2015, 292, 137–147. [Google Scholar] [CrossRef]
- Meneely, J.P.; Chevallier, O.P.; Graham, S.; Greer, B.; Green, B.D.; Elliott, C.T. beta-methylamino-L-alanine (BMAA) is not found in the brains of patients with confirmed Alzheimer’s disease. Sci. Rep. 2016, 6, 36363. [Google Scholar] [CrossRef]
- Montine, T.J.; Li, K.; Perl, D.P.; Galasko, D. Lack of beta-methylamino-l-alanine in brain from controls, AD, or Chamorros with PDC. Neurology 2005, 65, 768–769. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Kelleher, R.J., 3rd; Shen, J. Presenilin-1 mutations and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2017, 114, 629–631. [Google Scholar] [CrossRef]
- Cai, Y.; An, S.S.; Kim, S. Mutations in presenilin 2 and its implications in Alzheimer’s disease and other dementia-associated disorders. Clin. Interv. Aging 2015, 10, 1163–1172. [Google Scholar] [CrossRef]
- Kuang, L.; Hashimoto, K.; Huang, E.J.; Gentry, M.S.; Zhu, H. Frontotemporal dementia non-sense mutation of progranulin rescued by aminoglycosides. Hum. Mol. Genet. 2020, 29, 624–634. [Google Scholar] [CrossRef]
- Strang, K.H.; Golde, T.E.; Giasson, B.I. MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab. Investig. 2019, 99, 912–928. [Google Scholar] [CrossRef]
- Gendron, T.F.; Rademakers, R.; Petrucelli, L. TARDBP mutation analysis in TDP-43 proteinopathies and deciphering the toxicity of mutant TDP-43. J. Alzheimers Dis. 2013, 33 (Suppl. S1), S35–S45. [Google Scholar] [CrossRef]
- Mitake, S.; Ojika, K.; Hirano, A. Hirano bodies and Alzheimer’s disease. Kaohsiung J. Med. Sci. 1997, 13, 10–18. [Google Scholar]
- Yamazaki, Y.; Matsubara, T.; Takahashi, T.; Kurashige, T.; Dohi, E.; Hiji, M.; Nagano, Y.; Yamawaki, T.; Matsumoto, M. Granulovacuolar degenerations appear in relation to hippocampal phosphorylated tau accumulation in various neurodegenerative disorders. PLoS ONE 2011, 6, e26996. [Google Scholar] [CrossRef] [PubMed]
- Moloney, C.M.; Lowe, V.J.; Murray, M.E. Visualization of neurofibrillary tangle maturity in Alzheimer’s disease: A clinicopathologic perspective for biomarker research. Alzheimers Dement. 2021, 17, 1554–1574. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate Change and Harmful Algal Blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef] [PubMed]
- Heisler, J.; Glibert, P.; Burkholder, J.; Anderson, D.; Cochlan, W.; Dennison, W.; Gobler, C.; Dortch, Q.; Heil, C.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Van den Heuvel-Greve, M.J.; van den Brink, A.M.; Kotterman, M.J.J.; Kwadijk, C.; Geelhoed, S.C.V.; Murphy, S.; van den Broek, J.; Heesterbeek, H.; Grone, A.; LL, I.J. Polluted porpoises: Generational transfer of organic contaminants in harbour porpoises from the southern North Sea. Sci. Total Environ. 2021, 796, 148936. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.; Daoust, P.Y.; Forzan, M.J.; Vanderstichel, R.V.; Ford, J.K.; Spaven, L.; Lair, S.; Raverty, S. Causes of mortality of harbor porpoises Phocoena phocoena along the Atlantic and Pacific coasts of Canada. Dis. Aquat. Organ. 2017, 122, 171–183. [Google Scholar] [CrossRef]
- Schneider, T.; Simpson, C.; Desai, P.; Tucker, M.; Lobner, D. Neurotoxicity of isomers of the environmental toxin L-BMAA. Toxicon 2020, 184, 175–179. [Google Scholar] [CrossRef]
- Martin, R.M.; Stallrich, J.; Bereman, M.S. Mixture designs to investigate adverse effects upon co-exposure to environmental cyanotoxins. Toxicology 2019, 421, 74–83. [Google Scholar] [CrossRef]
- Rush, T.; Liu, X.; Lobner, D. Synergistic toxicity of the environmental neurotoxins methylmercury and beta-N-methylamino-L-alanine. Neuroreport 2012, 23, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Newell, M.E.; Adhikari, S.; Halden, R.U. Systematic and state-of the science review of the role of environmental factors in Amyotrophic Lateral Sclerosis (ALS) or Lou Gehrig’s Disease. Sci. Total Environ. 2022, 817, 152504. [Google Scholar] [CrossRef]
- Meneses, A.; Koga, S.; O’Leary, J.; Dickson, D.W.; Bu, G.; Zhao, N. TDP-43 Pathology in Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.C.; Dugger, B.N.; Dickson, D.W.; Wang, D.S. TDP-43 in aging and Alzheimer’s disease—A review. Int. J. Clin. Exp. Pathol. 2011, 4, 147–155. [Google Scholar] [PubMed]
- Nelson, P.T.; Dickson, D.W.; Trojanowski, J.Q.; Jack, C.R.; Boyle, P.A.; Arfanakis, K.; Rademakers, R.; Alafuzoff, I.; Attems, J.; Brayne, C.; et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019, 142, 1503–1527. [Google Scholar] [CrossRef]
- De Boer, E.M.J.; Orie, V.K.; Williams, T.; Baker, M.R.; De Oliveira, H.M.; Polvikoski, T.; Silsby, M.; Menon, P.; van den Bos, M.; Halliday, G.M.; et al. TDP-43 proteinopathies: A new wave of neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 2020, 92, 86–95. [Google Scholar] [CrossRef]
- Hasegawa, M.; Arai, T.; Akiyama, H.; Nonaka, T.; Mori, H.; Hashimoto, T.; Yamazaki, M.; Oyanagi, K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain 2007, 130, 1386–1394. [Google Scholar] [CrossRef]
- Kuusisto, E.; Salminen, A.; Alafuzoff, I. Early accumulation of p62 in neurofibrillary tangles in Alzheimer’s disease: Possible role in tangle formation. Neuropathol. Appl. Neurobiol. 2002, 28, 228–237. [Google Scholar] [CrossRef]
- Fecto, F.; Yan, J.; Vemula, S.P.; Liu, E.; Yang, Y.; Chen, W.; Zheng, J.G.; Shi, Y.; Siddique, N.; Arrat, H.; et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch. Neurol. 2011, 68, 1440–1446. [Google Scholar] [CrossRef]
- Chevaleyre, V.; Piskorowski, R.A. Hippocampal Area CA2: An Overlooked but Promising Therapeutic Target. Trends Mol. Med. 2016, 22, 645–655. [Google Scholar] [CrossRef]
- Meira, T.; Leroy, F.; Buss, E.W.; Oliva, A.; Park, J.; Siegelbaum, S.A. A hippocampal circuit linking dorsal CA2 to ventral CA1 critical for social memory dynamics. Nat. Commun. 2018, 9, 4163. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Tischbein, M.; Cox, P.A.; Stommel, E.W. Cyanotoxins and the Nervous System. Toxins 2021, 13, 660. [Google Scholar] [CrossRef]
- Al-Sammak, M.A.; Hoagland, K.D.; Cassada, D.; Snow, D.D. Co-occurrence of the cyanotoxins BMAA, DABA and anatoxin-a in Nebraska reservoirs, fish, and aquatic plants. Toxins 2014, 6, 488–508. [Google Scholar] [CrossRef]
- Main, B.J.; Rodgers, K.J. Assessing the Combined Toxicity of BMAA and Its Isomers 2,4-DAB and AEG In Vitro Using Human Neuroblastoma Cells. Neurotox. Res. 2018, 33, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Violi, J.P.; Pu, L.; Pravadali-Cekic, S.; Bishop, D.P.; Phillips, C.R.; Rodgers, K.J. ffects of the Toxic Non-Protein Amino Acid β-Methylamino-L-Alanine (BMAA) on Intracellular Amino Acid Levels in Neuroblastoma Cells. Toxins 2023, 15, 647. [Google Scholar] [CrossRef] [PubMed]
- Banack, S.A.; Caller, T.; Henegan, P.; Haney, J.; Murby, A.; Metcalf, J.S.; Powell, J.; Cox, P.A.; Stommel, E. Detection of cyanotoxins, beta-N-methylamino-L-alanine and microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins 2015, 7, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.L.; Peccia, J.; Arnold, W. Preliminary Report on Air Sampling of Particle-Associated Microcystins and BMAA Pilot Study in Lee County, Florida: Fall 2018–Winter 2019; Florida Gulf Coast University: Fort Myers, FL, USA, 2019. [Google Scholar]
- Desforges, J.P.; Mikkelsen, B.; Dam, M.; Riget, F.; Sveegaard, S.; Sonne, C.; Dietz, R.; Basu, N. Mercury and neurochemical biomarkers in multiple brain regions of five Arctic marine mammals. Neurotoxicology 2021, 84, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Joiris, C.R.; Holsbeek, L.; Bolbat, D.; Gascard, C.; Stanev, T.; Komakhidze, A.; Baumgartner, W.; Birkun, A. Total and organic mercury in the Black Sea harbour porpoise Phocoena phocoena relicta. Mar. Pollut. Bull. 2001, 42, 905–911. [Google Scholar] [CrossRef]
- Van Elk, C.; van de Bildt, M.; van Run, P.; de Jong, A.; Getu, S.; Verjans, G.; Osterhaus, A.; Kuiken, T. Central nervous system disease and genital disease in harbor porpoises (Phocoena phocoena) are associated with different herpesviruses. Vet. Res. 2016, 47, 28. [Google Scholar] [CrossRef]
- Rubio-Guerri, C.; Melero, M.; Esperon, F.; Belliere, E.N.; Arbelo, M.; Crespo, J.L.; Sierra, E.; Garcia-Parraga, D.; Sanchez-Vizcaino, J.M. Unusual striped dolphin mass mortality episode related to cetacean morbillivirus in the Spanish Mediterranean sea. BMC Vet. Res. 2013, 9, 106. [Google Scholar] [CrossRef]
- Guzman-Verri, C.; Gonzalez-Barrientos, R.; Hernandez-Mora, G.; Morales, J.A.; Baquero-Calvo, E.; Chaves-Olarte, E.; Moreno, E. Brucella ceti and brucellosis in cetaceans. Front. Cell Infect. Microbiol. 2012, 2, 3. [Google Scholar] [CrossRef]
- Leblanc, P.; Vorberg, I.M. Viruses in neurodegenerative diseases: More than just suspects in crimes. PLoS Pathog. 2022, 18, e1010670. [Google Scholar] [CrossRef]
- Geraci, J.R.; Lounsbury, V.L.; Yates, N. Marine Mammals Ashore, A Field Guide for Strandings, 2nd ed.; National Aquarium in Baltimore, Inc.: Baltimore, MD, USA, 2005; pp. 198–199. [Google Scholar]
- Breathnach, A.S.; Goldby, F. The amygdaloid nuclei, hippocampus and other parts of the rhinencephalon in the porpoise (Phocaena phocaena). J. Anat. 1954, 88, 267–291. [Google Scholar]
- Marino, L.; Sudheimer, K.; Sarko, D.; Sirpenski, G.; Johnson, J.I. Neuroanatomy of the harbor porpoise (Phocoena phocoena) from magnetic resonance images. J. Morphol. 2003, 257, 308–347. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, R.G.; Perrin, W.F.; Dizon, A.E. Phylogenetic Relationships Among the Delphinid Cetaceans Based on Full Cytochrome B Sequences. Mar. Mammal Sci. 1999, 15, 619–648. [Google Scholar] [CrossRef]
- Chen, I.H.; Chou, L.S.; Chou, S.J.; Wang, J.H.; Stott, J.; Blanchard, M.; Jen, I.F.; Yang, W.C. Selection of suitable reference genes for normalization of quantitative RT-PCR in peripheral blood samples of bottlenose dolphins (Tursiops truncatus). Sci. Rep. 2015, 5, 15425. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Su, A.I.; Cooke, M.P.; Ching, K.A.; Hakak, Y.; Walker, J.R.; Wiltshire, T.; Orth, A.P.; Vega, R.G.; Sapinoso, L.M.; Moqrich, A.; et al. Large-scale analysis of the human and mouse transcriptomes. Proc. Natl. Acad. Sci. USA 2002, 99, 4465–4470. [Google Scholar] [CrossRef]
- Mirra, S.S.; Hart, M.N.; Terry, R.D. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch. Pathol. Lab. Med. 1993, 117, 132–144. [Google Scholar] [PubMed]
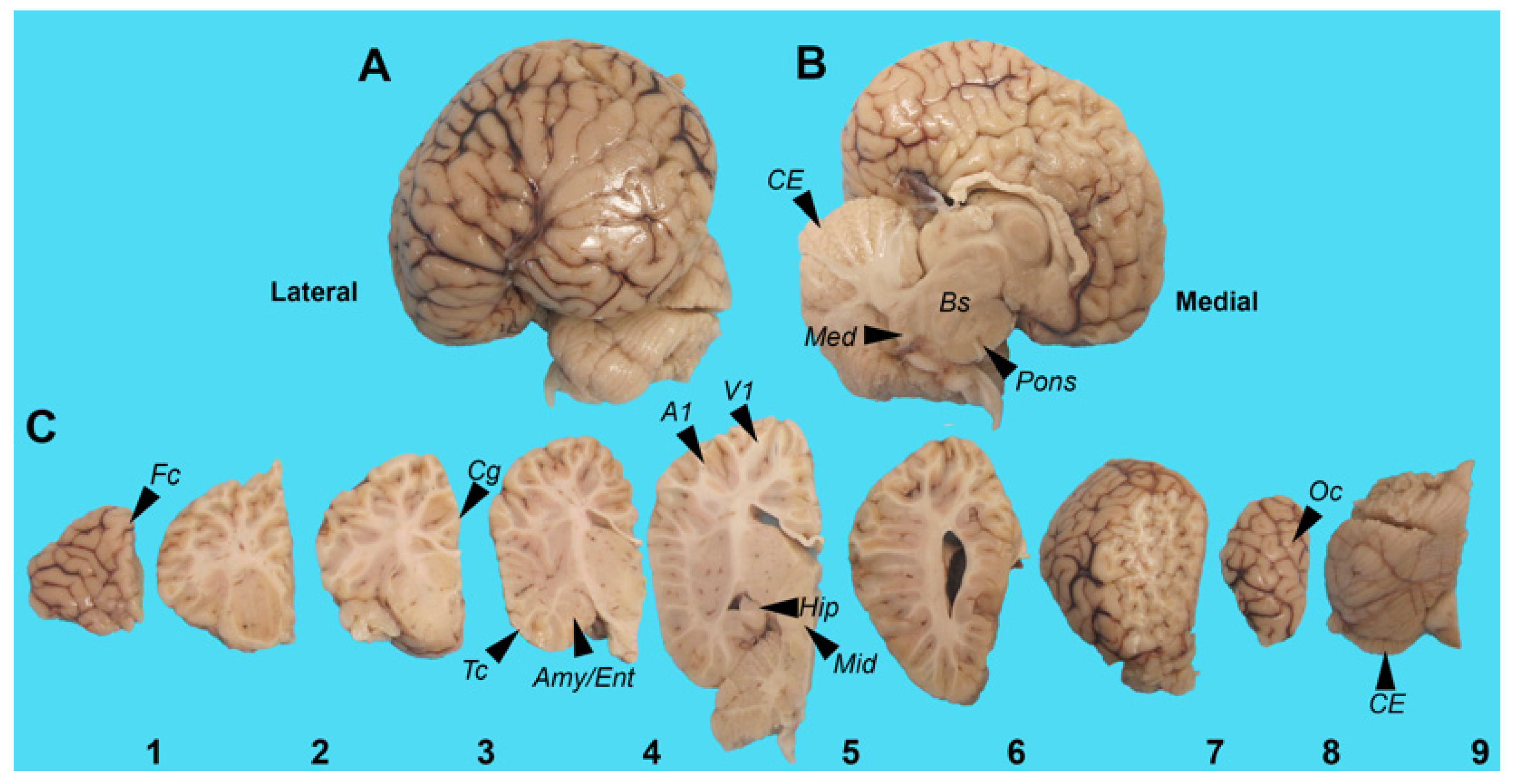
| Brain Regions | BMAA (μg/g) | AEG (μg/g) | 2,4-DAB (μg/g) |
|---|---|---|---|
| PcA1 (n = 4) | 93.6 ± 9.1 ns | 36.5 ± 7.7 p = 0.001 | 282.6 ± 21.2 ns |
| PcV1 (n = 5) | 93.1 ± 7.5 | ND | 245.0 ± 15.4 |
| Mean + S.E. | 93.3 ± 5.4 | 16.2 ± 7.2 | 261.7 ± 13.6 |
| Brain Regions | TDP-43 pSer 409/410 | β-Amyloid 1–16 (clone 6E10) | P62/SQSTM1 | SM: Argyrophilic Neurons and Plaques |
|---|---|---|---|---|
| Amy | + | + | + | + |
| CE | (−)/+ | + | ++ | + |
| Cg | + | + | (−) | + |
| Ent | + | + | + | + |
| Fc | + | + | −/+ | + |
| Hipp | + | + | + | + |
| Med | + | + | (−) | + |
| Mid | + | + | (−) | + |
| Oc | (−) | + | (−) | + |
| PcA1 | + | + | (−) | + |
| Pons | + | + | (−) | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garamszegi, S.P.; Brzostowicki, D.J.; Coyne, T.M.; Vontell, R.T.; Davis, D.A. TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA. Toxins 2024, 16, 42. https://doi.org/10.3390/toxins16010042
Garamszegi SP, Brzostowicki DJ, Coyne TM, Vontell RT, Davis DA. TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA. Toxins. 2024; 16(1):42. https://doi.org/10.3390/toxins16010042
Chicago/Turabian StyleGaramszegi, Susanna P., Daniel J. Brzostowicki, Thomas M. Coyne, Regina T. Vontell, and David A. Davis. 2024. "TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA" Toxins 16, no. 1: 42. https://doi.org/10.3390/toxins16010042
APA StyleGaramszegi, S. P., Brzostowicki, D. J., Coyne, T. M., Vontell, R. T., & Davis, D. A. (2024). TDP-43 and Alzheimer’s Disease Pathology in the Brain of a Harbor Porpoise Exposed to the Cyanobacterial Toxin BMAA. Toxins, 16(1), 42. https://doi.org/10.3390/toxins16010042





