Identification of the Enterotoxigenic Potential of Staphylococcus spp. from Raw Milk and Raw Milk Cheeses
Abstract
:1. Introduction
2. Results
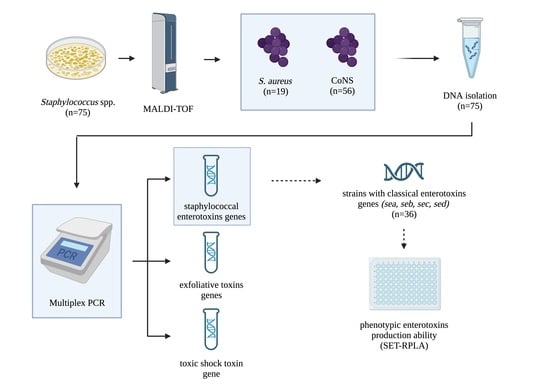
2.1. Isolates Identification
2.2. Genotypic Characterization of Enterotoxigenic Potential of Staphylococcal Isolates
2.3. Phenotypic Ability to Produce Enterotoxins—SET-RPLA Method
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Isolates Identification
5.2. Genotypic Characterization of Enterotoxigenic Potential of Staphylococcal Isolates
5.2.1. DNA Extraction
5.2.2. Detection of Staphylococcal Superantigens by Multiplex PCR
5.3. Phenotypic Ability to Produce (RPLA Method)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/reg/2005/2073/oj (accessed on 12 July 2023).
- Chajęcka-Wierzchowska, W.; Gajewska, J.; Wiśniewski, P.; Zadernowska, A. Enterotoxigenic potential of coagulase-negative staphylococci from ready-to-eat food. Pathogens 2020, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 20, e07666. [Google Scholar] [CrossRef]
- Balaban, N.; Rasooly, A. Staphylococcal enterotoxins. Int. J. Food Microbiol. 2000, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; De Lourdes, M.; Souza, R. De Staphylococcal enterotoxins: Molecular aspects and detection methods. J. Public Health Epidemiol. 2010, 2, 29–42. [Google Scholar]
- Fijałkowski, K.; Struk, M.; Karakulska, J.; Paszkowska, A.; Giedrys-Kalemba, S.; Masiuk, H.; Czernomysy-Furowicz, D.; Nawrotek, P. Comparative analysis of superantigen genes in Staphylococcus xylosus and Staphylococcus aureus isolates collected from a single mammary quarter of cows with mastitis. J. Microbiol. 2014, 52, 366–372. [Google Scholar] [CrossRef]
- Ono, H.K.; Hirose, S.; Narita, K.; Sugiyama, M.; Asano, K.; Hu, D.-L.; Nakane, A. Histamine release from intestinal mast cells induced by staphylococcal enterotoxin A (SEA) evokes vomiting reflex in common marmoset. PLoS Pathogens 2019, 15, e1007803. [Google Scholar] [CrossRef]
- Ono, H.K.; Hirose, S.; Naito, I.; Sato’o, Y.; Asano, K.; Hu, D.-L.; Omoe, K.; Nakane, A. The emetic activity of staphylococcal enterotoxins, SEK, SEL, SEM, SEN and SEO in a small emetic animal model, the house musk shrew. Microbiol. Immunol. 2017, 61, 12–16. [Google Scholar] [CrossRef]
- Aung, M.S.; Urushibara, N.; Kawaguchiya, M.; Ito, M.; Habadera, S.; Kobayashi, N. Prevalence and Genetic Diversity of Staphylococcal Enterotoxin (-Like) Genes sey, selw, selx, selz, sel26 and sel27 in Community-Acquired Methicillin-Resistant Staphylococcus aureus. Toxins 2020, 12, 347. [Google Scholar] [CrossRef]
- Omoe, K.; Hu, D.-L.; Ono, H.K.; Shimizu, S.; Takahashi-Omoe, H.; Nakane, A.; Uchiyama, T.; Shinagawa, K.; Imanishi, K. Emetic potentials of newly identified staphylococcal enterotoxin-like toxins. Infect. Immun. 2013, 81, 3627–3631. [Google Scholar] [CrossRef]
- Ono, H.K.; Sato’o, Y.; Narita, K.; Naito, I.; Hirose, S.; Hisatsune, J.; Asano, K.; Hu, D.L.; Omoe, K.; Sugai, M.; et al. Identification and characterization of a novel staphylococcal emetic toxin. Appl. Environ. Microbiol. 2015, 81, 7034–7040. [Google Scholar] [CrossRef]
- Le Loir, Y.; Baron, F.; Gautier, M. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2003, 2, 63–76. [Google Scholar] [PubMed]
- Park, J.Y.; Fox, L.K.; Seo, K.S.; McGuire, M.A.; Park, Y.H.; Rurangirwa, F.R.; Sischo, W.M.; Bohach, G.A. Detection of classical and newly described staphylococcal superantigen genes in coagulase-negative staphylococci isolated from bovine intramammary infections. Vet. Microbiol. 2011, 147, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Padovani, C.R.; Miya, N.T.; Sant’ana, A.S.; Pereira, J.L. High incidence of enterotoxin D producing Staphylococcus spp. in Brazilian cow’s raw milk and its relation with coagulase and thermonuclease enzymes. Foodborne Pathog Dis. 2011, 8, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Nasaj, M.; Saeidi, Z.; Tahmasebi, H.; Dehbashi, S.; Arabestani, M.R. Prevalence and distribution of resistance and enterotoxins/enterotoxin-like genes in different clinical isolates of coagulase-negative Staphylococcus. Eur. J. Med. Res. 2020, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Krakowiak, S.; Bania, J. Wytwarzanie enterotoksyn gronkowcowych w zywności. Med. Weter. 2018, 74, 16–22. [Google Scholar] [CrossRef]
- Hennekinne, J.-A.; De Buyser, M.-L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.C.; Garland-Lewis, G.; Trufan, S.; Meschke, S.J.; Fowler, H.; Shean, R.C.; Greninger, A.L.; Rabinowitz, P.M. Distribution of Staphylococcus species in dairy cows, workers and shared farm environments. FEMS Microbiol. Lett. 2018, 365, fny146. [Google Scholar] [CrossRef]
- Yildirim, T.; SAdati, F.; Kocaman, B.; Siriken, B. Staphylococcus aureus and Staphylococcal enterotoxin detection in raw milk and cheese origin coagulase positive isolates. Int. J. Sci. Lett. 2019, 1, 30–41. [Google Scholar] [CrossRef]
- Cardozo, M.V.; Nespolo, N.; Delfino, T.C.; de Almeida, C.C.; Pizauro, L.J.L.; Valmorbida, M.K.; Pereira, N.; de Ávila, F.A. Raw milk cheese as a potential infection source of pathogenic and toxigenic food born pathogens. Food Sci. Technol. 2021, 41, 355–358. [Google Scholar] [CrossRef]
- Johler, S.; Giannini, P.; Jermini, M.; Hummerjohann, J.; Baumgartner, A.; Stephan, R. Further evidence for staphylococcal food poisoning outbreaks caused by egc-Encoded enterotoxins. Toxins 2015, 7, 997–1004. [Google Scholar] [CrossRef]
- Aydin, A.; Sudagidan, M.; Muratoglu, K. Prevalence of staphylococcal enterotoxins, toxin genes and genetic-relatedness of foodborne Staphylococcus aureus strains isolated in the Marmara Region of Turkey. Int. J. Food Microbiol. 2011, 148, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.Á.; Mendoza, M.C.; Rodicio, M.R. Food Poisoning and Staphylococcus aureus Enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Rall, V.L.M.; Miranda, E.S.; Castilho, I.G.; Camargo, C.H.; Langoni, H.; Guimarães, F.F.; Araújo Júnior, J.P.; Fernandes Júnior, A. Diversity of Staphylococcus species and prevalence of enterotoxin genes isolated from milk of healthy cows and cows with subclinical mastitis. J. Dairy Sci. 2014, 97, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Podkowik, M.; Park, J.Y.; Seo, K.S.; Bystroń, J.; Bania, J. Enterotoxigenic potential of coagulase-negative staphylococci. Int. J. Food Microbiol. 2013, 163, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Helak, I.; Daczkowska-Kozon, E.G.; Dłubała, A.A. Short communication: Enterotoxigenic potential of coagulase-negative staphylococci isolated from bovine milk in Poland. J. Dairy Sci. 2020, 103, 3076–3081. [Google Scholar] [CrossRef] [PubMed]
- França, A.; Gaio, V.; Lopes, N.; Melo, L.D.R. Virulence Factors in Coagulase-Negative Staphylococci. Pathogens 2021, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Banaszkiewicz, S.; Schubert, J.; Tabiś, A.; Król, J.; Stefaniak, T.; Węsierska, E.; Bania, J. Staphylococcal Enterotoxin Genes in Coagulase-Negative Staphylococci—Stability, Expression, and Genomic Context. Int. J. Mol. Sci. 2022, 23, 2560. [Google Scholar] [CrossRef]
- Salamandane, A.; Oliveira, J.; Coelho, M.; Ramos, B.; Cunha, M.V.; Brito, L. Enterotoxin- and Antibiotic-Resistance-Encoding Genes Are Present in Both Coagulase-Positive and Coagulase-Negative Foodborne Staphylococcus Strains. Appl. Microbiol. 2022, 2, 367–380. [Google Scholar] [CrossRef]
- Elal Muş, T.; Çetinkaya, F.; Soyutemiz, G.E.; Erten, B. Toxigenic Genes of Coagulase-Negative Staphylococci and Staphylococcus aureus from Milk and Dairy. J. Agric. Sci. 2023, 29, 924–932. [Google Scholar] [CrossRef]
- Smyth, D.S.; Hartigan, P.J.; Meaney, W.J.; Fitzgerald, J.R.; Deobald, C.F.; Bohach, G.A.; Smyth, C.J. Superantigen genes encoded by the egc cluster and SaPlbov are predominant among Staphylococcus aureus isolates from cows, goats, sheep, rabbits and poultry. J. Med. Microbiol. 2005, 54, 401–411. [Google Scholar] [CrossRef]
- Poli, A.; Guglielmini, E.; Sembeni, S.; Spiazzi, M.; Dellaglio, F.; Rossi, F.; Torriani, S. Detection of Staphylococcus aureus and enterotoxin genotype diversity in Monte Veronese, a Protected Designation of Origin Italian cheese. Lett. Appl. Microbiol. 2007, 45, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, D.M.; Gallina, S.; Bellio, A.; Chiesa, F.; Civera, T.; Decastelli, L. Enterotoxin gene profiles of Staphylococcus aureus isolated from milk and dairy products in Italy. Lett. Appl. Microbiol. 2014, 58, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Macori, G.; Bellio, A.; Acutis, P.L.; Gallina, S.; Decastelli, L. Short communication: Characterization of Staphylococcus aureus isolated along the raw milk cheese production process in artisan dairies in Italy. J. Dairy Sci. 2018, 101, 2915–2920. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, S.A.; Lam, T.J.G.M.; Hoekstra, J.; Rutten, V.P.M.G.; Tessema, T.S.; Broens, E.M.; Riesebos, A.E.; Spaninks, M.P.; Koop, G. Characterization of Staphylococcus aureus isolated from milk samples of dairy cows in small holder farms of North-Western Ethiopia. BMC Vet. Res. 2018, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Kalorey, D.R.; Shanmugam, Y.; Kurkure, N.V.; Chousalkar, K.K.; Barbuddhe, S.B. PCR-based detection of genes encoding virulence determinants in Staphylococcus aureus from bovine subclinical mastitis cases. J. Vet. Sci. 2007, 8, 151–154. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, B.R.; Singh, R.S. Genetic determinants of antibiotic resistance in Staphylococcus aureus isolates from milk of mastitic crossbred cattle. Curr. Microbiol. 2010, 60, 379–386. [Google Scholar] [CrossRef]
- Carfora, V.; Caprioli, A.; Marri, N.; Sagrafoli, D.; Boselli, C.; Giacinti, G.; Giangolini, G.; Sorbara, L.; Dottarelli, S.; Battisti, A.; et al. Enterotoxin genes, enterotoxin production, and methicillin resistance in Staphylococcus aureus isolated from milk and dairy products in Central Italy. Int. Dairy J. 2015, 42, 12–15. [Google Scholar] [CrossRef]
- Riva, A.; Borghi, E.; Cirasola, D.; Colmegna, S.; Borgo, F.; Amato, E.; Pontello, M.M.; Morace, G. Methicillin-resistant Staphylococcus aureus in raw milk: Prevalence, SCCmec typing, enterotoxin characterization, and antimicrobial resistance patterns. J. Food Prot. 2015, 78, 1142–1146. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, S.; Dahiya, D.K.; Khan, A.; Mathur, M.; Sharma, A. Coagulase gene polymorphism, enterotoxigenecity, biofilm production, and antibiotic resistance in Staphylococcus aureus isolated from bovine raw milk in North West India. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 65. [Google Scholar] [CrossRef]
- Zschöck, M.; Kloppert, B.; Wolter, W.; Hamann, H.P.; Lämmler, C. Pattern of enterotoxin genes seg, seh, sei and sej positive Staphylococcus aureus isolated from bovine mastitis. Vet. Microbiol. 2005, 108, 243–249. [Google Scholar] [CrossRef]
- Haveri, M.; Hovinen, M.; Roslöf, A.; Pyörälä, S. Molecular types and genetic profiles of Staphylococcus aureus strains isolated from bovine intramammary infections and extramammary sites. J. Clin. Microbiol. 2008, 46, 3728–3735. [Google Scholar] [CrossRef] [PubMed]
- Nawrotek, P.; Czernomysy-Furowicz, D.; Borkowski, J.; Fijałkowski, K.; Pobucewicz, A. The effect of auto-vaccination therapy on the phenotypic variation of one clonal type of Staphylococcus aureus isolated from cows with mastitis. Vet. Microbiol. 2012, 155, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.J.; Mathisen, T.; Løvseth, A.; Omoe, K.; Qvale, K.S.; Loncarevic, S. An outbreak of staphylococcal food poisoning caused by enterotoxin H in mashed potato made with raw milk. FEMS Microbiol. Lett. 2005, 252, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.J.; Mork, T.; Høgåsen, H.R.; Rørvik, L.M. Enterotoxigenic Staphylococcus aureus in bulk milk in Norway. J. Appl. Microbiol. 2005, 99, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Johler, S.; Weder, D.; Bridy, C.; Huguenin, M.C.; Robert, L.; Hummerjohann, J.; Stephan, R. Outbreak of staphylococcal food poisoning among children and staff at a Swiss boarding school due to soft cheese made from raw milk. J. Dairy Sci. 2015, 98, 2944–2948. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.X.; Silva, N.C.C.; Trevilin, J.H.; Cruzado, M.M.B.; Mui, T.S.; Duarte, F.R.S.; Castillo, C.J.C.; Canniatti-Brazaca, S.G.; Porto, E. Molecular characterization and antibiotic resistance of Staphylococcus spp. isolated from cheese processing plants. J. Dairy Sci. 2017, 100, 5167–5175. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. Staphylococcal Superantigens: Pyrogenic Toxins Induce Toxic Shock. Toxins 2019, 11, 178. [Google Scholar] [CrossRef]
- Schlievert, P.M.; Cahill, M.P.; Hostager, B.S.; Brosnahan, A.J.; Klingelhutz, A.J.; Gourronc, F.A.; Bishop, G.A.; Leung, D.Y.M. Staphylococcal Superantigens Stimulate Epithelial Cells through CD40 To Produce Chemokines. mBio 2019, 10, e00214-19. [Google Scholar] [CrossRef]
- Omoe, K.; Hu, D.; Takahashi-Omoe, H.; Nakane, A.; Shinagawa, K. Identification and characterization of a new staphylococcal enterotoxin-related putative toxin encoded by two kinds of plasmids. Infect. Immun. 2003, 71, 6088–6094. [Google Scholar] [CrossRef]
- Van Leeuwen, W.B.; Melles, D.C.; Alaidan, A.; Al-Ahdal, M.; Boelens, H.A.M.; Snijders, S.V.; Wertheim, H.; Van Duijkeren, E.; Peeters, J.K.; Van Der Spek, P.J.; et al. Host- and tissue-specific pathogenic traits of Staphylococcus aureus. J. Bacteriol. 2005, 187, 4584–4591. [Google Scholar] [CrossRef]
- Sospedra, I.; Soriano, J.M.; Mañes, J. Enterotoxinomics: The omic sciences in the study of staphylococcal toxins analyzed in food matrices. Food Res. Int. 2013, 54, 1052–1060. [Google Scholar] [CrossRef]
- Morandi, S.; Brasca, M.; Lodi, R.; Cremonesi, P.; Castiglioni, B. Detection of classical enterotoxins and identification of enterotoxin genes in Staphylococcus aureus from milk and dairy products. Vet. Microbiol. 2007, 124, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zouharova, M.; Rysanek, D. Multiplex PCR, and RPLA identification of Staphylococcus aureus enterotoxigenic strains from bulk tank milk. Zoonoses Public Health 2008, 55, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Mclauchlin, J.; Narayanan, G.L.; Mithani, V.; O’Neill, G. The detection of enterotoxins and toxic shock syndrome toxin genes in Staphylococcus aureus by polymerase chain reaction. J. Food Prot. 2000, 63, 479–488. [Google Scholar] [CrossRef]
- Da Cunha, M.D.L.R.D.S.; Peresi, E.; Oliveira Calsolari, R.A.; Araújo, J.P. Detection of enterotoxins genes in coagulase-negative staphylococci isolated from foods. Brazilian J. Microbiol. 2006, 37, 70–74. [Google Scholar] [CrossRef]
- Fujikawa, H.; Igarashi, H. Rapid latex agglutination test for detection of Staphylococcal enterotoxins A to E that uses high-density latex particles. Appl. Environ. Microbiol. 1988, 54, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, N.; Gu, H.; Hao, L.; Ye, H.; Gong, W.; Wang, Z. A Review of the methods for detection of Staphylococcus aureus enterotoxins. Toxins 2016, 8, 176. [Google Scholar] [CrossRef]
- Rajkovic, A.; Jovanovic, J.; Monteiro, S.; Decleer, M.; Andjelkovic, M.; Foubert, A.; Beloglazova, N.; Tsilla, V.; Sas, B.; Madder, A.; et al. Detection of toxins involved in foodborne diseases caused by Gram-positive bacteria. Compr Rev Food Sci Food Saf. 2020, 19, 1605–1657. [Google Scholar] [CrossRef]
- Rajkovic, A.; El-Moualij, B.; Uyttendaele, M.; Brolet, P.; Zorzi, W.; Heinen, E.; Foubert, E.; Debevere, J. Immunoquantitative real-time PCR for detection and quantification of Staphylococcus aureus enterotoxin B in foods. Appl Environ Microbiol 2006, 72, 6593–6599. [Google Scholar] [CrossRef]
- Kientz, C.E.; Hulst, A.G.; Wils, E.R. Determination of Staphylococcal enterotoxin B by on-line (micro) liquid chromatography-electrospray mass spectrometry. J. Chromatogr. A 1997, 757, 51–64. [Google Scholar] [CrossRef]
- Poli, M.A.; Rivera, V.R.; Neal, D. Sensitive and specific colorimetric ELISAS for Staphylococcus aureus enterotoxins A and B in urine and buffer. Toxicon 2002, 40, 1723–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.M.; Liu, Z.J.; Li, Y.M.; Li, Q.; Song, C.J.; Xu, Z.W.; Zhang, Y.; Zhang, Y.S.; Ma, Y.; Sun, Y.J. High sensitivity chemiluminescence enzyme immunoassay for detecting Staphylococcal enterotoxin A in multi-matrices. Anal. Chim. Acta 2013, 796, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Kostov, Y.; Bruck, H.A.; Rasooly, A. Gold nanoparticle-based enhanced chemiluminescence immunosensor for detection of Staphylococcal enterotoxin B (SEB) in food. Int. J. Food Microbiol. 2009, 133, 265–271. [Google Scholar] [CrossRef]
- Soelberg, S.D.; Stevens, R.C.; Limaye, A.P.; Furlong, C.E. Surface plasmon resonance detection using antibody-linked magnetic nanoparticles for analyte capture, purification, concentration, and signal amplification. Anal. Chem. 2009, 81, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Duan, N.; Ma, X.Y.; Xia, Y.; Wang, H.X.; Wang, Z.P. A highly sensitive fluorescence resonance energy transfer aptasensor for Staphylococcal enterotoxin B detection based on exonuclease-catalyzed target recycling strategy. Anal. Chim. Acta 2013, 782, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Chajęcka-Wierzchowska, W. Biofilm formation ability and presence of adhesion genes among coagulase-negative and coagulase-positive staphylococci isolates from raw cow’s milk. Pathogens 2020, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, A.J.; Zarzecka, U.; Chajęcka-Wierzchowska, W.; Zadernowska, A. A Comparison of Methods for Identifying Enterobacterales Isolates from Fish and Prawns. Pathogens 2022, 11, 410. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial Resistance and Virulence Characterization of Listeria monocytogenes Strains Isolated from Food and Food Processing Environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Chajęcka-Wierzchowska, W.; Zadernowska, A. High-Pressure Processing—Impacts on the Virulence and Antibiotic Resistance of Listeria monocytogenes Isolated from Food and Food Processing Environments. Foods 2023, 12, 3899. [Google Scholar] [CrossRef]
- Zhang, S.; Iandolo, J.J.; Stewart, G.C. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej). FEMS Microbiol. Lett. 1998, 168, 227–233. [Google Scholar] [CrossRef]
- Jarraud, S.; Mougel, C.; Thioulouse, J.; Lina, G.; Meugnier, H.; Forey, F.; Nesme, X.; Etienne, J.; Vandenesch, F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002, 70, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, S.; Grumann, D.; Schmudde, M.; Nguyen, H.T.T.; Eichler, P.; Strommenger, B.; Kopron, K.; Kolata, J.; Giedrys-Kalemba, S.; Steinmetz, I.; et al. Clonal distribution of superantigen genes in clinical Staphylococcus aureus isolates. J. Clin. Microbiol. 2007, 45, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Fijałkowski, K.; Peitler, D.; Karakulska, J. Staphylococci isolated from ready-to-eat meat—Identification, antibiotic resistance and toxin gene profile. Int. J. Food Microbiol. 2016, 238, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, X.; Yang, Y.; Zheng, Y.; Wang, C.; Deng, L.; Liu, L.; Li, C.; Shang, Y.; Zhao, C.; et al. Superantigen gene profiles and presence of exfoliative toxin genes in community-acquired meticillin-resistant Staphylococcus aureus isolated from Chinese children. J. Med. Microbiol. 2011, 60, 35–45. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Kobayashi, N.; Cui, L.; Oguchi, A.; Aoki, K.I.; et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]

| No. | Toxin Genes Occurrence | Source of Isolate (Number of Isolates) | Species (Number of Isolates) (%) | Total (Number of Isolates) (%) | Number of Genes |
|---|---|---|---|---|---|
| 1 | tst-1 | Raw milk (5), raw milk cheeses (1) | 6 (8.0) | 6 (8.0) | 1 |
| 2 | sed, tst-1 | Raw milk (1) | 1 (1.3) | 23 (30.7) | 2 |
| 3 | see, tst-1 | Raw milk (1) | 1 (1.3) | ||
| 4 | sek, eta | Raw milk (14) | 14 (18.7) | ||
| 5 | sel, ser | Raw milk (1) | 1 (1.3) | ||
| 6 | sem, tst-1 | Raw milk (1) | 1 (1.3) | ||
| 7 | seq, tst-1 | Raw milk (2), raw milk cheese (3) | 5 (6.7) | ||
| 8 | seb, seq, tst-1 | Raw milk (1) | 1 (1.3) | 17 (22.7) | 3 |
| 9 | sec, seh, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 10 | sed, etd, tst-1 | Raw milk (1) | 1 (1.3) | ||
| 11 | sed, see, seu | Raw milk (1) | 1 (1.3) | ||
| 12 | sed, seq, tst-1 | Raw milk (3) | 3 (4.0) | ||
| 13 | sei, ser, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 14 | sek, ser, eta | Raw milk (6) | 6 (8.0) | ||
| 15 | sel, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 16 | sen, seq, tst-1 | Raw milk (1) | 1 (1.3) | ||
| 17 | seq, ser, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 18 | sec, sed, seh, tst-1 | Raw milk cheese (1) | 1 (1.3) | 11 (14.7) | 4 |
| 19 | sec, seh, seq, tst-1 | Raw milk cheese (4) | 4 (5.4) | ||
| 20 | sed, seg, seq, tst-1 | Raw milk (1) | 1 (1.3) | ||
| 21 | sed, seq, etd, tst-1 | Raw milk (5) | 5 (6.7) | ||
| 22 | sea, sec, seh, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | 8 (10.7) | 5 |
| 23 | sec, sed, seh, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 24 | sec, seh, sel, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 25 | sec, seh, sel, ser, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 26 | sec, seh, seo, seq tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 27 | sec, seh, seq, ser, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 28 | sed, seh, sek, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 29 | sed, seo, seg, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 30 | sec, seh, sel, seo, seq, tst-1 | Raw milk cheese (2) | 2 (2.6) | 4 (5.3) | 6 |
| 31 | sed, see, seq, etd, eta, tst-1 | Raw milk (1) | 1 (1.3) | ||
| 32 | sed, seg, seq, etd, eta, tst-1 | Raw milk (1) | 1 (1.3) | ||
| 33 | sea, sed, seh, sei, sej, selo, tst-1 | Raw milk cheese (1) | 1 (1.3) | 2 (2.7) | 7 |
| 34 | sec, seg, seh, sell, seo, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| 35 | sec, sed, seg, seh, selk, seo, seq, tst-1 | Raw milk cheese (1) | 1 (1.3) | 2 (2.7) | 8 |
| 36 | sec, seg, seh, sej, sek, seq, sei, tst-1 | Raw milk cheese (1) | 1 (1.3) | ||
| Negative for toxin genes | Raw milk (2) | 2 (2.6) | 2 (2.7) | 0 | |
| Total | 75 (100.0) | 75 (100.0) |
| Strains | Superantigen Gene (SAg) | References |
|---|---|---|
| FRI913 | sea, sec, see, sek, sel, seq, tst-1 | [75] |
| FRI137 | sec, seh, sel, seu | |
| TY114 | etd | |
| A920210 | eta | |
| Col | seb, sek, seq | |
| FRI1151m | sed, sej, ser | [73] |
| N315 | sec, seg, sei, sel, sem, sen, seo, sep, tst1 | [76] |
| 8325-4 | No genes of SAg | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewski, P.; Gajewska, J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Identification of the Enterotoxigenic Potential of Staphylococcus spp. from Raw Milk and Raw Milk Cheeses. Toxins 2024, 16, 17. https://doi.org/10.3390/toxins16010017
Wiśniewski P, Gajewska J, Zadernowska A, Chajęcka-Wierzchowska W. Identification of the Enterotoxigenic Potential of Staphylococcus spp. from Raw Milk and Raw Milk Cheeses. Toxins. 2024; 16(1):17. https://doi.org/10.3390/toxins16010017
Chicago/Turabian StyleWiśniewski, Patryk, Joanna Gajewska, Anna Zadernowska, and Wioleta Chajęcka-Wierzchowska. 2024. "Identification of the Enterotoxigenic Potential of Staphylococcus spp. from Raw Milk and Raw Milk Cheeses" Toxins 16, no. 1: 17. https://doi.org/10.3390/toxins16010017
APA StyleWiśniewski, P., Gajewska, J., Zadernowska, A., & Chajęcka-Wierzchowska, W. (2024). Identification of the Enterotoxigenic Potential of Staphylococcus spp. from Raw Milk and Raw Milk Cheeses. Toxins, 16(1), 17. https://doi.org/10.3390/toxins16010017