Hepatorenal Toxicity after 7-Day Oral Administration of Low-Dose Tetrodotoxin and Its Distribution in the Main Tissues in Mice
Abstract
1. Introduction
2. Results
2.1. Single Oral Administration of TTX in Mice: Temporal Profile of Its Distribution in Blood and Selected Tissues
2.2. Toxicity of TTX after Daily Oral Administration for 7 Days
2.2.1. TTX Lethality and Distribution in Selected Tissues and Feces
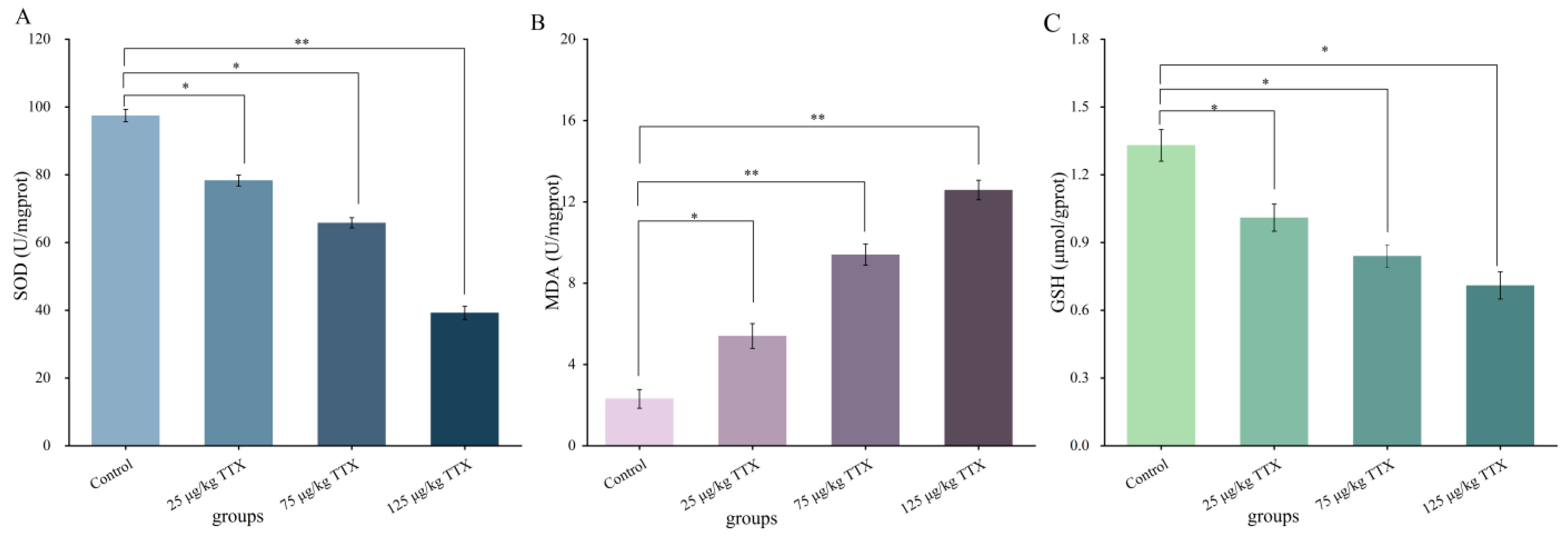
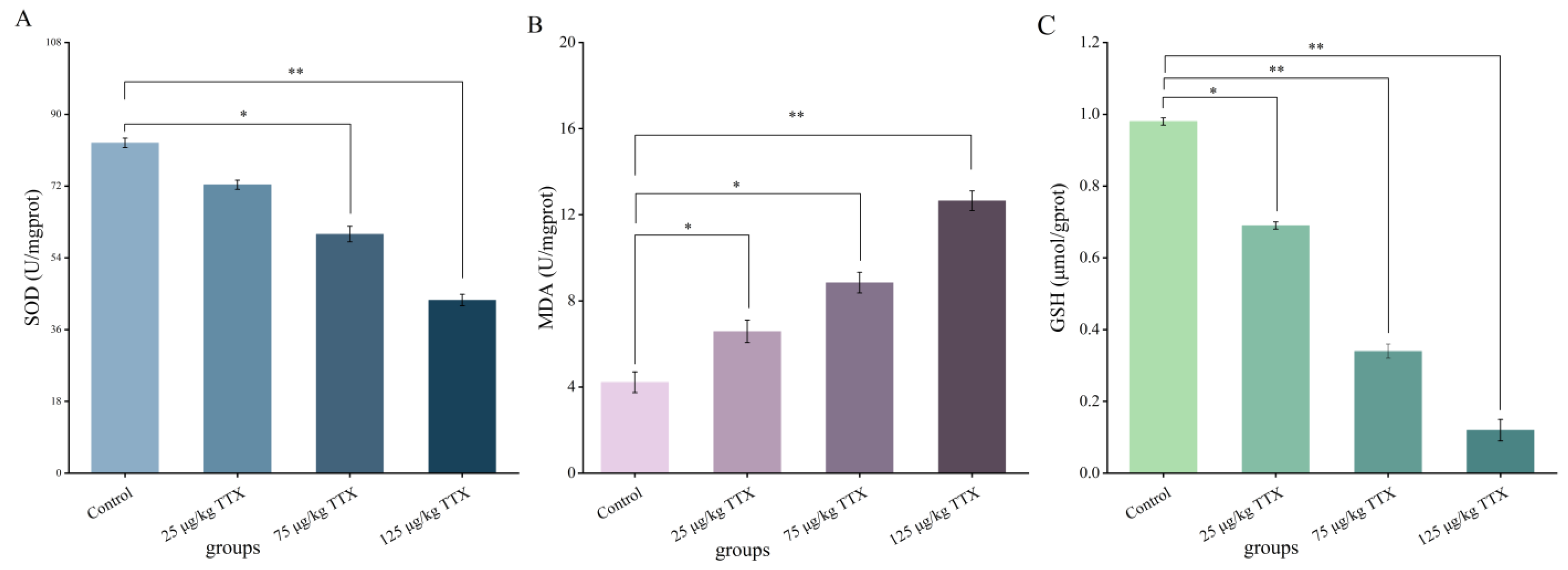
2.2.2. Effects of Different Doses of TTX on Oxidative Stress Markers in Mouse Liver
2.2.3. Effects of Different Doses of TTX on Oxidative Stress Markers in Mouse Kidney
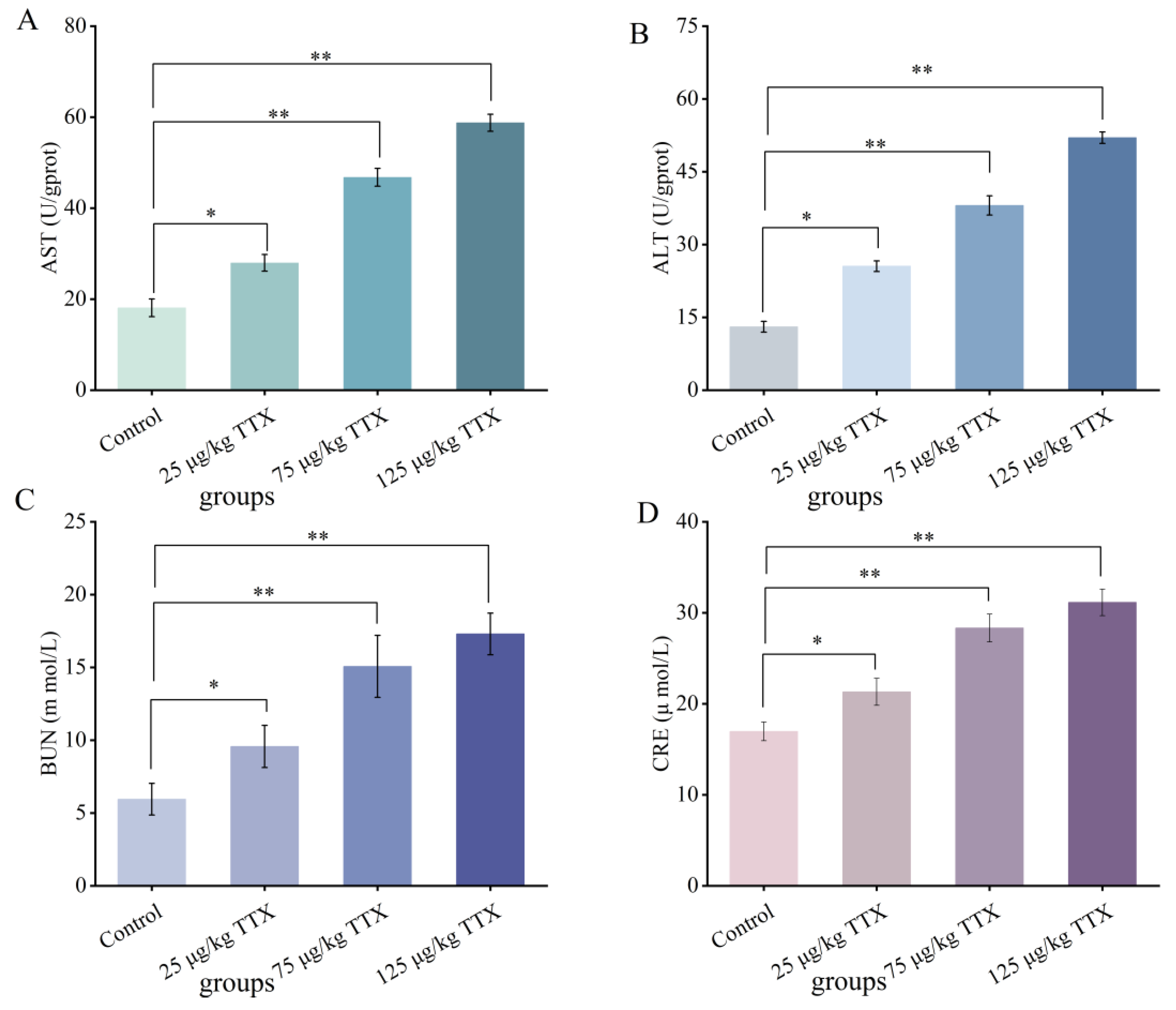
2.2.4. Effects of Different Doses of TTX on the Liver and Kidney Function Markers in Mouse Blood
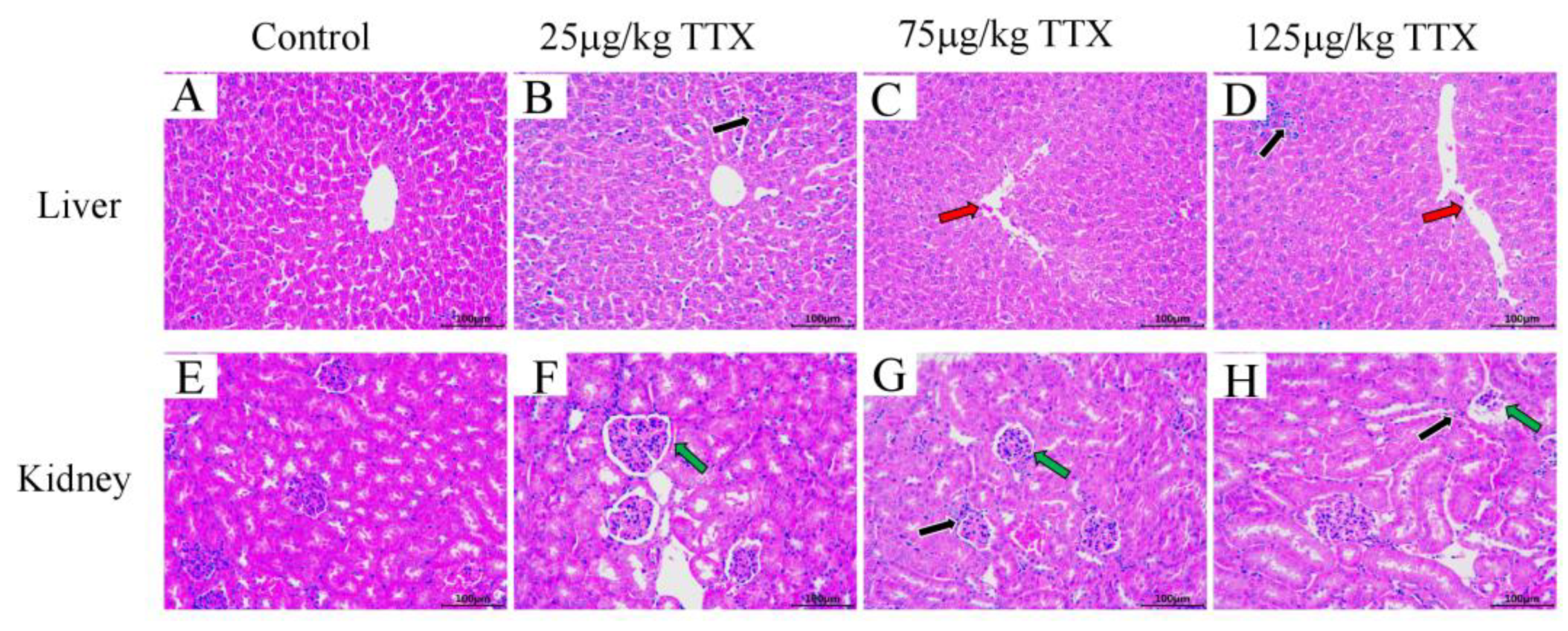
2.2.5. Histopathological Effects of TTX on Mice’s Liver and Kidney
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Animals
5.3. Single-Dose Administration
5.4. Daily Oral Administration for 7 Days
5.5. HPLC-MS/MS Analysis
5.5.1. TTX Extraction
5.5.2. HPLC-MS/MS Analysis of TTX
5.6. Liver and Kidney Function Markers
5.7. Serum Biochemical Indexes
5.8. Histological Analysis
5.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campbell, K.; Barnes, P.; Haughey, S.A.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C.T. Development and single laboratory validation of an optical biosensor assay for tetrodotoxin detection as a tool to combat emerging risks in European seafood. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef]
- Guzman, A.; Fernandez de Henestrosa, A.R.; Marin, A.P.; Ho, A.; Borroto, J.I.; Carasa, I.; Pritchard, L. Evaluation of the genotoxic potential of the natural neurotoxin Tetrodotoxin (TTX) in a battery of in vitro and in vivo genotoxicity assays. Mutat. Res. 2007, 634, 14–24. [Google Scholar] [CrossRef]
- Tukker, A.M.; Vrolijk, M.F.; van Kleef, R.; Sijm, D.; Westerink, R.H.S. Mixture effects of tetrodotoxin (TTX) and drugs targeting voltage-gated sodium channels on spontaneous neuronal activity in vitro. Toxicol. Lett. 2023, 373, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.; Rodriguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef]
- Melnikova, D.I.; Khotimchenko, Y.S.; Magarlamov, T.Y. Addressing the Issue of Tetrodotoxin Targeting. Mar. Drugs 2018, 16, 352. [Google Scholar] [CrossRef]
- Katrine, K.H.; Jan, A.; Lars, B.; Margherita, B.; Beat, B.; Sandra, C.; Bruce, C.; Michael, D.; Lutz, E.; Bettina, G.-K.; et al. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. Eur. Food Saf. Auth. 2017, 15, e04752. [Google Scholar] [CrossRef]
- Leonardo, S.; Kiparissis, S.; Rambla-Alegre, M.; Almarza, S.; Roque, A.; Andree, K.B.; Christidis, A.; Flores, C.; Caixach, J.; Campbell, K.; et al. Detection of tetrodotoxins in juvenile pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the North Aegean Sea (Greece) by an electrochemical magnetic bead-based immunosensing tool. Food Chem. 2019, 290, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.P.; Rodrigues, S.M.; Gouveia, N.; Timóteo, V.; Costa, P.R. Tetrodotoxin and saxitoxin in two native species of puffer fish, Sphoeroides marmoratus and Lagocephalus lagocephalus, from NE Atlantic Ocean (Madeira Island, Portugal). Mar. Environ. Res. 2019, 151, 104780. [Google Scholar] [CrossRef]
- Katikou, P. Public Health Risks Associated with Tetrodotoxin and Its Analogues in European Waters: Recent Advances after The EFSA Scientific Opinion. Toxins 2019, 11, 240. [Google Scholar] [CrossRef]
- Boente-Juncal, A.; Otero, P.; Rodríguez, I.; Camiña, M.; Rodriguez-Vieytes, M.; Vale, C.; Botana, L.M. Oral Chronic Toxicity of the Safe Tetrodotoxin Dose Proposed by the European Food Safety Authority and Its Additive Effect with Saxitoxin. Toxins 2020, 12, 312. [Google Scholar] [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First occurrence of tetrodotoxins in bivalve mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O. Tetrodotoxin—Distribution and accumulation in aquatic organisms, and cases of human intoxication. Mar. Drugs 2008, 6, 220–242. [Google Scholar] [CrossRef] [PubMed]
- Abal, P.; Louzao, M.C.; Antelo, A.; Alvarez, M.; Cagide, E.; Vilarino, N.; Vieytes, M.R.; Botana, L.M. Acute Oral Toxicity of Tetrodotoxin in Mice: Determination of Lethal Dose 50 (LD50) and No Observed Adverse Effect Level (NOAEL). Toxins 2017, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zong, J.; Chen, S.; Li, M.; Lu, Y.; Wang, R.; Xu, H. Accumulation and Elimination of Tetrodotoxin in the Pufferfish Takifugu obscurus by Dietary Administration of the Wild Toxic Gastropod Nassarius semiplicata. Toxins 2020, 12, 278. [Google Scholar] [CrossRef]
- Shiro, I.; Hiroyuki, U.; Riko, Y.; Mitsuki, T.; Tatsunori, S.; Shotaro, O.; Yoshiki, W.; Ryuya, O.; Hikaru, O.; Takahiro, S.; et al. Including planocerid flatworms in the diet effectively toxifies the pufferfish, Takifugu niphobles. Sci. Rep. 2018, 8, 12302. [Google Scholar] [CrossRef]
- Kono, M.; Matsui, T.; Furukawa, K.; Takase, T.; Yamamori, K.; Kaneda, H.; Aoki, D.; Jang, J.-H.; Yotsu-Yamashita, M. Examination of transformation among tetrodotoxin and its analogs in the living cultured juvenile puffer fish, kusafugu, Fugu niphobles by intramuscular administration. Toxicon 2008, 52, 714–720. [Google Scholar] [CrossRef]
- Wang, J.; Araki, T.; Tatsuno, R.; Nina, S.; Ikeda, K.; Takatani, T.; Arakawa, O. Transfer profile of orally and intramuscularly administered tetrodotoxin to artificial hybrid specimens of the pufferfish Takifugu rubripes and Takifugu porphyreus. Food Hyg. Saf. Sci. 2012, 53, 33–38. [Google Scholar] [CrossRef][Green Version]
- Kasteel, E.E.J.; Westerink, R.H.S. Comparison of the acute inhibitory effects of Tetrodotoxin (TTX) in rat and human neuronal networks for risk assessment purposes. Toxicol. Lett. 2017, 270, 12–16. [Google Scholar] [CrossRef]
- Annelies, N.; Mengying, Z.; van Bennard, R.; Rietjens, I.M.C.M. Use of physiologically based kinetic modeling-facilitated reverse dosimetry to predict in vivo acute toxicity of tetrodotoxin in rodents. Toxicol. Sci. 2022, 187, 127–138. [Google Scholar] [CrossRef]
- Finch, S.C.; Boundy, M.J.; Harwood, D.T. The Acute Toxicity of Tetrodotoxin and Tetrodotoxin–Saxitoxin Mixtures to Mice by Various Routes of Administration. Toxins 2018, 10, 423. [Google Scholar] [CrossRef]
- Kim, Y.C.; Yim, H.K.; Jung, Y.S.; Park, J.H.; Kim, S.Y. Hepatic injury induces contrasting response in liver and kidney to chemicals that are metabolically activated: Role of male sex hormone. Toxicol. Appl. Pharmacol. 2007, 223, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Cui, Y.; Lu, H.; Ma, X. Effects of long-term feeding diets supplemented with Lactobacillus reuteri 1 on growth performance, digestive and absorptive function of the small intestine in pigs. J. Funct. Foods 2020, 71, 104010. [Google Scholar] [CrossRef]
- Boente-Juncal, A.; Vale, C.; Cifuentes, M.; Otero, P.; Camina, M.; Rodriguez-Vieytes, M.; Botana, L.M. Chronic In Vivo Effects of Repeated Exposure to Low Oral Doses of Tetrodotoxin: Preliminary Evidence of Nephrotoxicity and Cardiotoxicity. Toxins 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Zhe, W.; Yingjie, W.; Yufei, W.; Kaining, Z.; Yongchao, L. Robust and reliable detection of malondialdehyde in biological samples via microprobe-triggered surface-enhanced Raman spectroscopy. Microchem. J. 2022, 181, 107815. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, K.; Yang, P.; Qiao, L.; Liu, B. Sensitive and fast beverage/fruit antioxidant evaluation by TiO2-Au/graphene nanocomposites coupled with MALDI-MS. Rapid Commun. Mass Spectrom. 2016, 30, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide Dismutase and Stress Tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Marta, K.; Thomas, P.; Michal, P.; Agnieszka, S.; Wojciech, K.; Jolanta, D. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef]
- Masataka, S.; Yusuke, G.; Marina, Y.; Vandebroek, A.A.; Masashi, M.; Kenji, H.; Masato, Y.; Jumpei, S. Serum D-serine accumulation after proximal renal tubular damage involves neutral amino acid transporter Asc-1. Sci. Rep. 2019, 9, 16075. [Google Scholar] [CrossRef]
- Abal, P.; Louzao, M.C.; Vilarino, N.; Vieytes, M.R.; Botana, L.M. Acute Toxicity Assessment: Macroscopic and Ultrastructural Effects in Mice Treated with Oral Tetrodotoxin. Toxins 2019, 11, 305. [Google Scholar] [CrossRef]
- Hong, B.; Chen, H.; Han, J.; Xie, Q.; He, J.; Bai, K.; Dong, Y.; Yi, R. A Study of 11-[(3)H]-Tetrodotoxin Absorption, Distribution, Metabolism and Excretion (ADME) in Adult Sprague-Dawley Rats. Mar. Drugs 2017, 15, 159. [Google Scholar] [CrossRef]
- Tatsuno, R.; Shikina, M.; Shirai, Y.; Wang, J.; Soyano, K.; Nishihara, G.N.; Takatani, T.; Arakawa, O. Change in the transfer profile of orally administered tetrodotoxin to non-toxic cultured pufferfish Takifugu rubripes depending of its development stage. Toxicon 2013, 65, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.I.; Magarlamov, T.Y. An Overview of the Anatomical Distribution of Tetrodotoxin in Animals. Toxins 2022, 14, 576. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Isbister, G.K.; Lin, C.S.-Y.; David, B.; Hugh, B. Acute tetrodotoxin-induced neurotoxicity after ingestion of puffer fish. Ann. Neurol. 2005, 57, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef]
- Rodriguez, I.; Alfonso, A.; Gonzalez-Jartin, J.M.; Vieytes, M.R.; Botana, L.M. A single run UPLC-MS/MS method for detection of all EU-regulated marine toxins. Talanta 2018, 189, 622–628. [Google Scholar] [CrossRef]

| Group | Liver (ng/g) | Kidney (ng/g) | Blood (ng/g) | Small Intestine (ng/g) | Feces (ng/g) |
|---|---|---|---|---|---|
| 25 μg/kg TTX | 34 ± 2 | 14 ± 3 | 27 ± 2 | 34 ± 2 | 50 ± 3 |
| 75 μg/kg TTX | 41 ± 1 | 16 ± 2 | 32 ± 2 | 57 ± 3 | 99 ± 3 |
| 125 μg/kg TTX | 46 ± 2 | 20 ± 2 | 42 ± 2 | 86 ± 2 | 150 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Zhang, X.; Yang, Q.; Wang, Q. Hepatorenal Toxicity after 7-Day Oral Administration of Low-Dose Tetrodotoxin and Its Distribution in the Main Tissues in Mice. Toxins 2023, 15, 564. https://doi.org/10.3390/toxins15090564
Zhong Y, Zhang X, Yang Q, Wang Q. Hepatorenal Toxicity after 7-Day Oral Administration of Low-Dose Tetrodotoxin and Its Distribution in the Main Tissues in Mice. Toxins. 2023; 15(9):564. https://doi.org/10.3390/toxins15090564
Chicago/Turabian StyleZhong, Yaqian, Xiaojun Zhang, Qiyu Yang, and Qianfeng Wang. 2023. "Hepatorenal Toxicity after 7-Day Oral Administration of Low-Dose Tetrodotoxin and Its Distribution in the Main Tissues in Mice" Toxins 15, no. 9: 564. https://doi.org/10.3390/toxins15090564
APA StyleZhong, Y., Zhang, X., Yang, Q., & Wang, Q. (2023). Hepatorenal Toxicity after 7-Day Oral Administration of Low-Dose Tetrodotoxin and Its Distribution in the Main Tissues in Mice. Toxins, 15(9), 564. https://doi.org/10.3390/toxins15090564





