1. Introduction
Cyanobacteria (aka blue-green algae) are common in aquatic systems worldwide where they exist in many different forms, and some species produce harmful cyanotoxins. Cyanobacteria can be found floating in the water column (planktonic), growing on the bottom of lakes or rivers (benthic), attached to other algae (epiphytic), attached to substrates (periphyton), or in visible composites that aggregate on the water surface to form floating mats (metaphyton). These varied forms exist year-round in low numbers but can reproduce quickly in favorable conditions to form planktonic or benthic blooms [
1].
During bloom events, the rapid proliferation of cyanobacteria can result in elevated levels of cyanotoxins, which can lead to animal intoxication incidents [
1,
2,
3,
4,
5]. While planktonic blooms and their toxins have dominated the harmful algae literature, there is a growing need for research associated with benthic species and their toxins [
6].
Abiotic factors such as climate change, invasive species colonization, and changing nutrient availability can shift energy production in a system to favor benthic growth, in a process called “benthification” [
7]. Benthic cyanobacteria blooms can be cryptic, forming on the bottom of lakes and rivers attached to the rocky substrate [
8]. As cyanobacterial biomass increases, oxygen bubbles produced by photosynthesis can become trapped among the cyanobacteria, causing the mats of filaments to become positively buoyant [
8]. Benthic mats may become detached and float to the surface through actions such as oxygenation, wind, physical disturbance of the sediment, and/or hydrological movement. When they accumulate on the water’s surface, they are known as metaphyton mats or proliferations, and they may be pushed toward the shore, increasing the potential for exposure to humans, dogs, and other animals. Reports of dogs consuming metaphyton materials have increased in recent years [
4,
5,
6,
9]. However, reports of dog illness or death associated with mat consumption are not routinely followed by pathological testing on the animal. Therefore, it has been difficult to conclude if the cause of death was related to cyanotoxins, or more specifically benthic cyanotoxins.
On 20 February 2021, a dog (referred to as C1) swam in Lake Travis, located near Austin, Texas, and died from suspected neurotoxicosis. Symptoms were consistent with ingestion of cyanobacteria, i.e., trembling, inability to stand, panting, vomiting, tremor, and stumbling. While investigating the death of C1, there were five more reports of illness related to cyanotoxin exposure with one additional canine death reported. All dogs had been swimming in the same area where C1 died, and the first death (C2) occurred 20 January 2021.
Manning et al. [
2] reported the first incidence of a dog fatality and suspected algae-related neurotoxicity in central Texas. However, the cause of death was never confirmed to be cyanotoxin-related. This report is the first to directly link the fatal consumption of metaphyton materials with dihydroanatoxin-a (dhATX), a cyanotoxin associated with the benthic species
Phormidium/
Microcoleus, with a dog death in Texas. The World Health Organization (WHO) does not currently have any reference values for dhATX, but a recent study showed that dhATX is roughly 3- to 4-times more toxic when ingested orally than anatoxin-a (ATX) [
10]. The WHO provisional recreational water health-based reference value for ATX is 60 µg/L [
11].
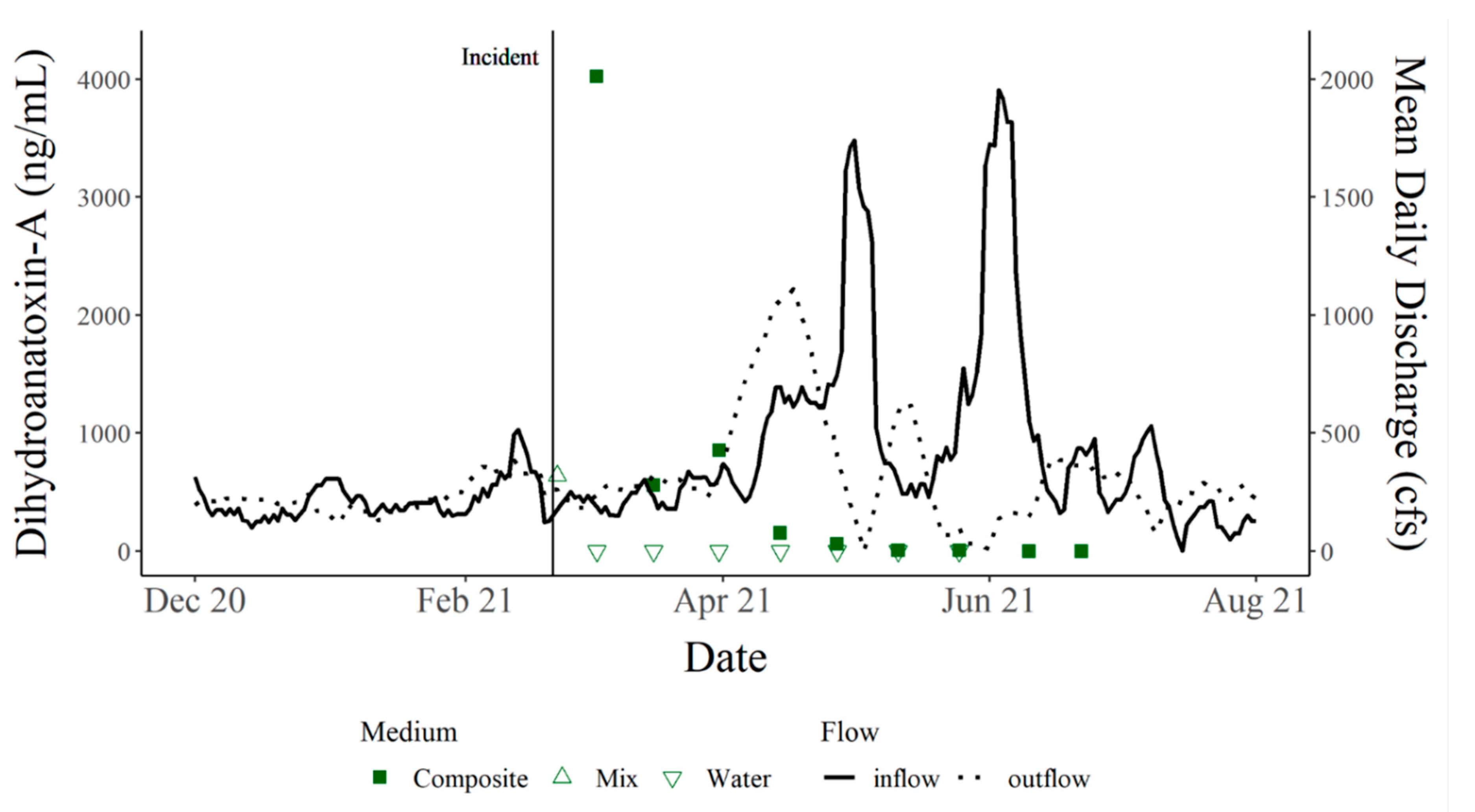
The current study presents data from the necropsy of C1, environmental cyanotoxin data found throughout the site of C1’s death, including the presence of cyanotoxins at the site over time, as well as additional cyanotoxin and cyanobacteria data from nearby lakes. Between the death of C1 and C2, this part of central Texas experienced an unprecedented winter storm. Beginning on 11 February 2021, temperatures ranged from 3 to −17 °C for eight days prior to the death of C1.
3. Discussion
Incidents of dogs becoming ill or dying after exposure to cyanobacteria have increased in recent years; however, very few of them are directly linked to cyanotoxins due to lack of supporting necropsy evidence, or incidents are reported too late to collect relevant samples [
2,
3]. The presence of dhATX in the stomach content and stomach tissues of C
1 as well as the high levels found in both metaphyton/periphyton material and water samples at the site of exposure provide strong evidence that C
1 died from dhATX neurotoxicosis. While ATX was also found at the site, recent studies have demonstrated that dhATX is more toxic than ATX when ingested orally with a lethal dose of 8 mg/kg for dhATX and 25 mg/kg for ATX [
10].
By nature, benthic cyanobacteria can be cryptic, making bloom detection difficult and expensive to monitor. Often, cyanobacteria and cyanotoxin monitoring efforts occur during the high-risk seasons for planktonic blooms of summer and fall to curb costs. Survey results collected from residents of Hudson Bend indicate that dogs were becoming ill prior to the death of C
1 and before the onset of the winter storm that impacted Texas (corresponding data [
12]). This information suggests that cyanotoxins and the cyanobacteria producing them were present before the cold temperatures and persisted afterward. Our results have exposed a need to monitor cyanotoxins year-round to accurately categorize the presence/absence of cyanotoxins each season. The persistence of dhATX is supported by the continued monitoring of Hudson Bend that took place following the incident, ending only when two non-detects of dhATX occurred in a row. Additionally, this study indicates that the site of canine intoxication or death may remain toxic for months after the initial event, which could be a result of low flows in the system. Previous work has demonstrated a correlation of low flow and the colonization of benthic mats [
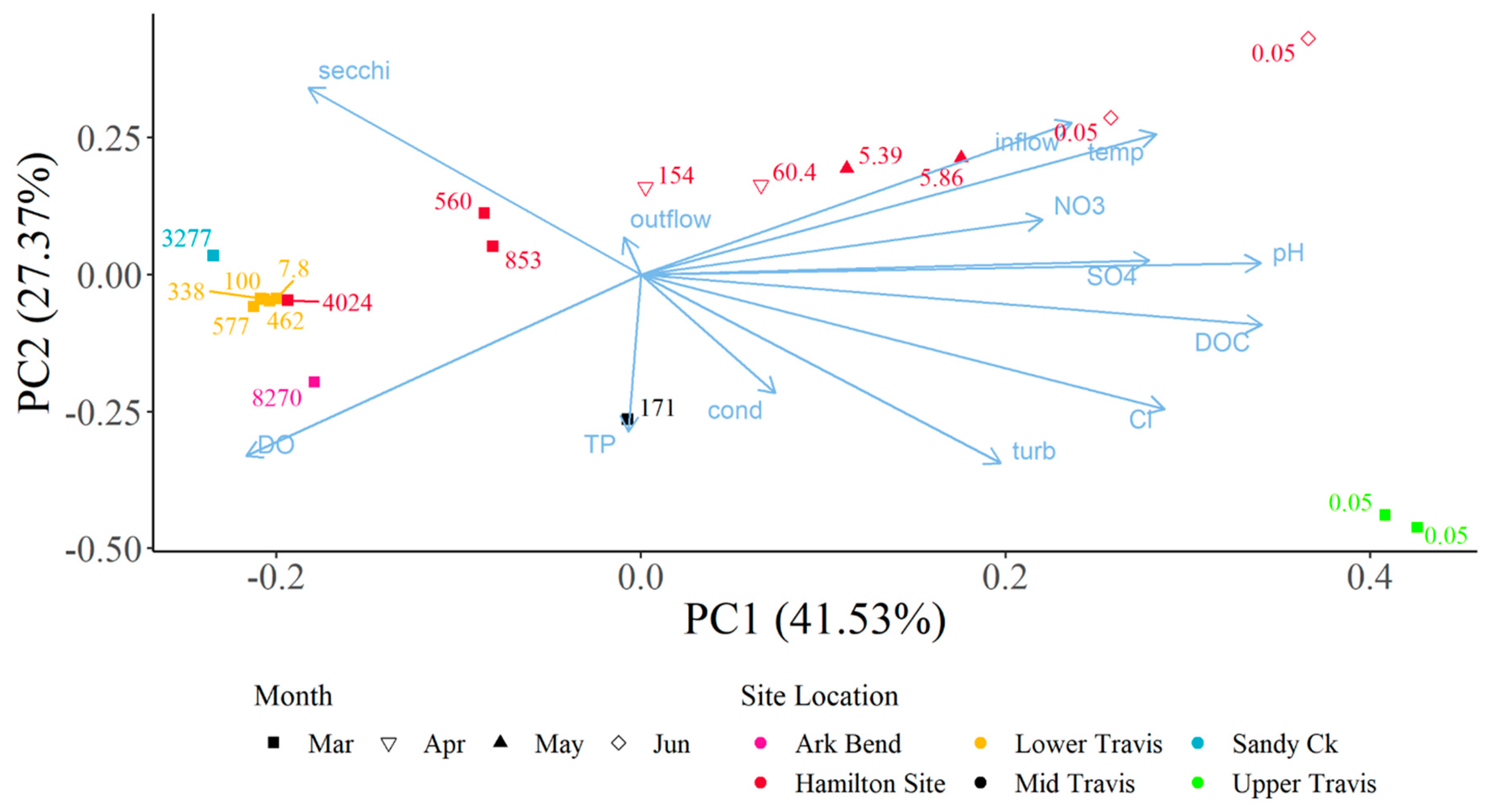
13]. While our study did not quantify mat presence, our PCA shows a correlation between increasing flows and a subsequent decrease in toxin levels. The literature focuses on the presence and abundance of cyanotoxins at the time of the dog’s death, but it remains unclear whether follow-up monitoring was conducted following the incidents [
2,
6,
9,
14].
The survey of Lake Travis showed that high levels of dhATX can be present in mat material, while whole water samples collected directly next to mats can be non-detects for dhATX. This observation supports previous work suggesting that consumption of lake water alone is often not enough to cause intoxication events, as cyanotoxins can have low stability in the water matrix, and cyanotoxin-related deaths are most likely caused by consumption of mat material [
5,
14]. Additionally, the presence of dhATX while ATX is non-detect demonstrates the importance of identifying the genus or species of cyanobacteria present in a sample to identify which cyanotoxin analytical tests should target. Some may consider dhATX a degradation product of ATX, but recent work has suggested that it can be present intracellularly within certain cyanobacteria species [
10]. This suggests that dhATX may occur independently of ATX; therefore, screening methods for cyanotoxins should not rule out the presence of dhATX based on the absence of ATX.
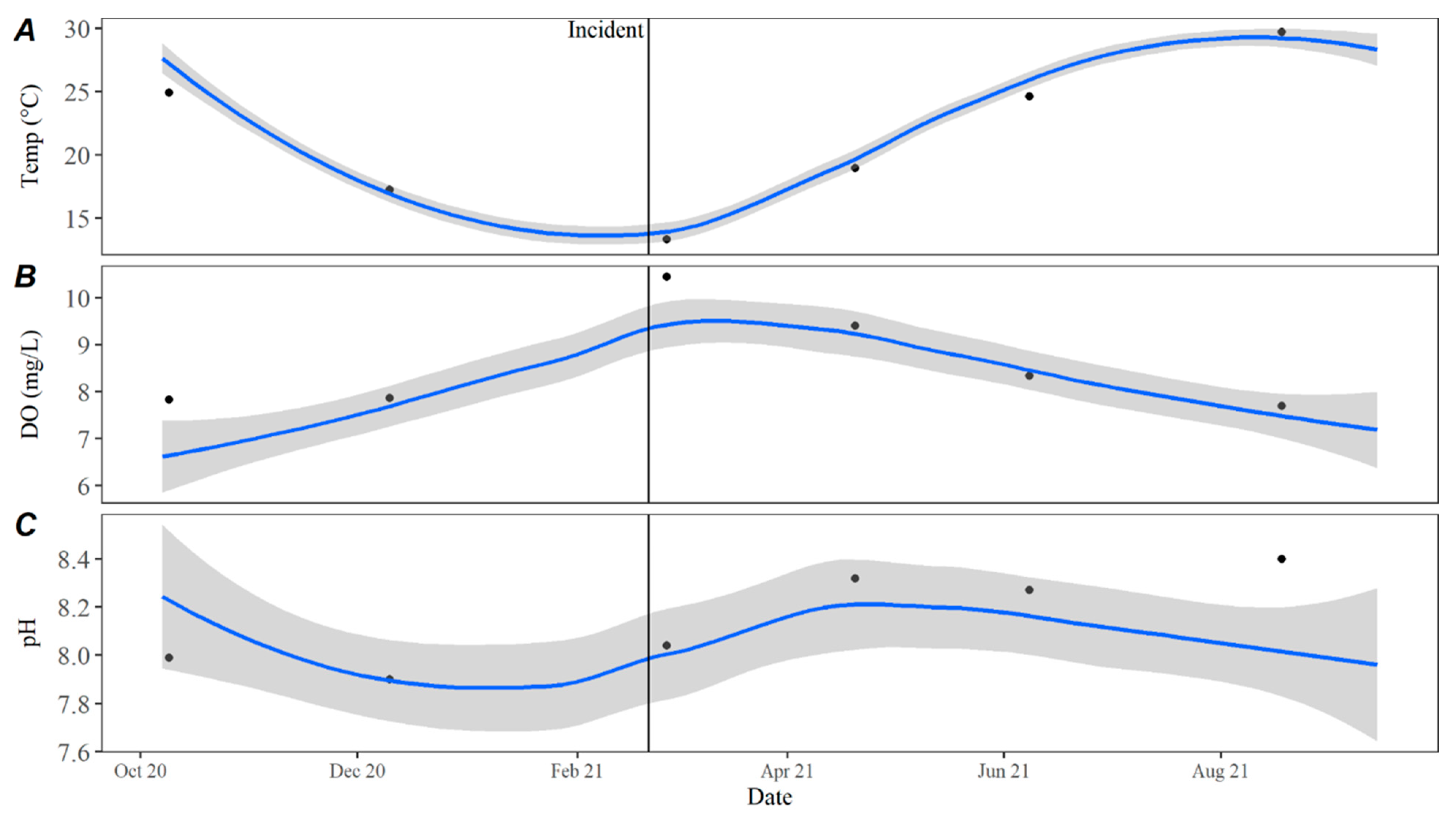
Water quality parameters collected from Lake Travis before, during, and after the dhATX exposure event can shed light on how these events may be impacted by water quality. While water and ambient air temperatures were unusually cold in the week leading up to C
1’s death, historical routine water quality data in Lake Travis showed that water temperatures in the months before, during, and after the death of C
1 were consistent with observed trends from the previous five years. Reinl et al. 2023 defined a cold water cyanobacterial bloom as a cyanobacterial bloom that occurs in freshwater lakes when water temperatures are below 15 °C [
15]. In the week leading up to the death of C
1, ambient air and water temperatures in Texas were historically low (<15 °C), with an extreme temperature difference potentially leading to turnover in Lake Travis. Physical disturbances or extreme storms can disturb the benthos and cause benthic proliferations to float to the surface [
8,
15], which could explain the sudden onset of benthic material along the shorelines of Lake Travis.
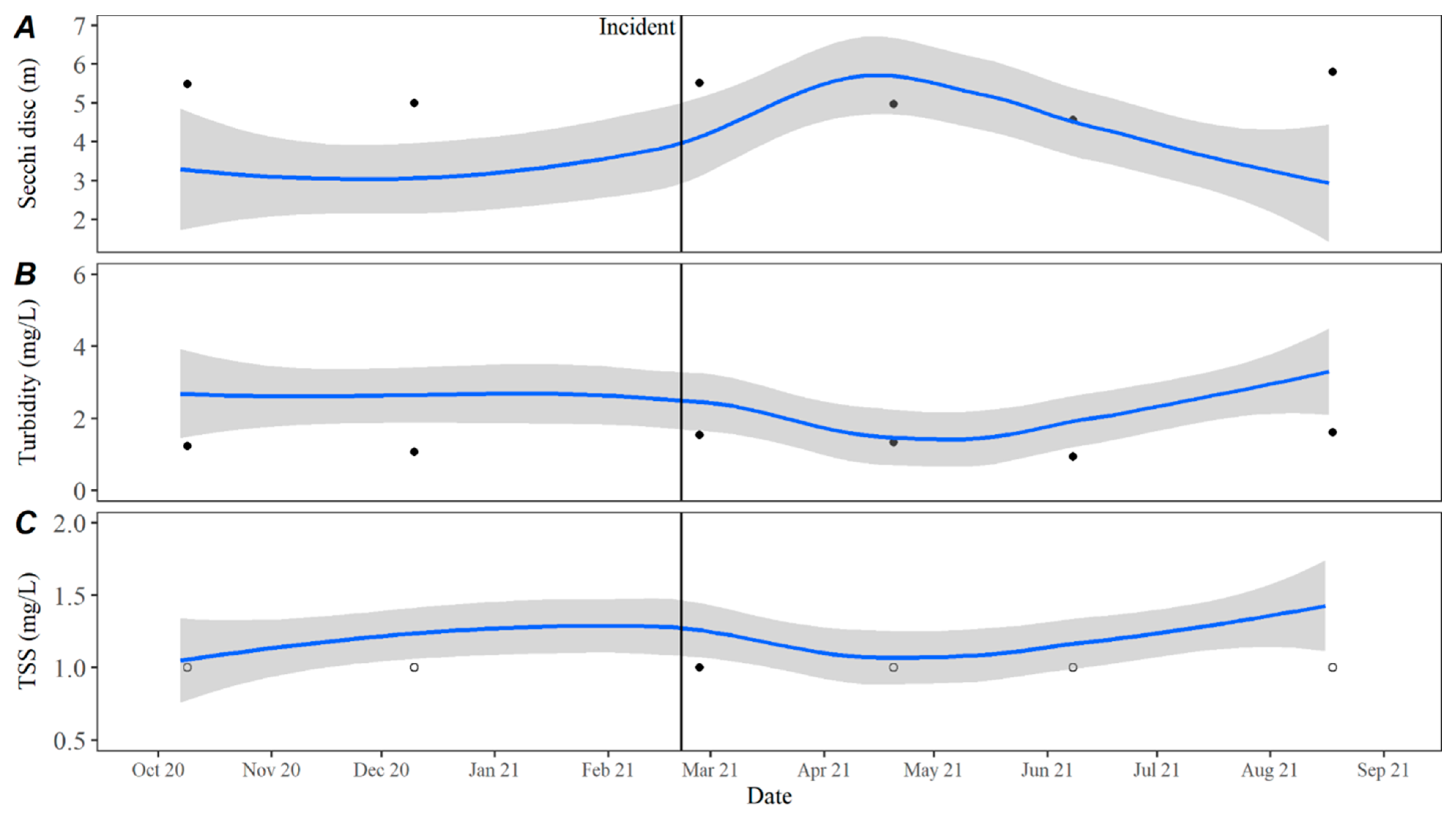
The decrease in turbidity and increase in secchi depth values in Lake Travis over the past five years may be a result of the continuing invasion and colonization by zebra mussels,
Dreissena polymorpha. Zebra mussels were first detected in Lake Travis in 2017 [
16] and have since colonized and maintained a breeding population (Lower Colorado River Authority, unpublished data). Established zebra mussel colonies can lead to benthification within a system through enhanced water clarity that allows greater benthic photosynthesis opportunities and increased deposition of nutrients to the benthos to support more biomass [
7]. A review by Vadeboncoeur et al. [
17] demonstrated that benthic algal proliferations can specifically benefit from an increase in zebra mussel presence due to their ability to capture nutrients from the water column and deposit them into the benthos. Another study found that inland lakes in Michigan with established zebra mussel populations had around 3.3 times higher microcystin levels when compared to uninvaded lakes [
18]. The established presence of a reproducing zebra mussel population in Lake Travis could explain why benthic proliferations of cyanobacteria are becoming more common.
5. Materials and Methods
5.1. Study Site
Lake Travis (30.42° N, 97.91° W) is an impoundment of the Texas Colorado River created after the construction of Mansfield Dam was completed in 1942. It is a water supply reservoir and is the fifth reservoir in the Highland Lakes chain. Hudson Bend is the most downstream major bend in Lake Travis. Residence time and water levels in Lake Travis fluctuate based on inflows and management of the reservoir according to a stakeholder-driven and state-approved water management plan. The Lower Colorado River Authority collects routine water quality data from Lake Travis and the Texas Colorado River basin as a partner in the Texas Clean Rivers Program (CRP) administered by the Texas Commission on Environmental Quality (TCEQ) according to Texas Water Code, Section 26.0135. Historical data used to identify five-year trends in Lake Travis were collected from a CRP site located in front of Mansfield Dam (30° 23′ 31.1994″, −97° 54′ 18″).
5.2. Sample Collection and Analysis
Whole water samples were collected at the surface (approximately 0.3 m depth) and contained no proliferations or algal material. Composite samples consisted of any floating mats, or epiphytic and/or periphytic algal material found at the site. Both whole water and composite samples were collected in amber bottles and frozen until processed. Historic water quality data from Lake Travis were collected following protocols outlined by the TCEQ Surface Water Quality Monitoring Procedures, Volume 1 [
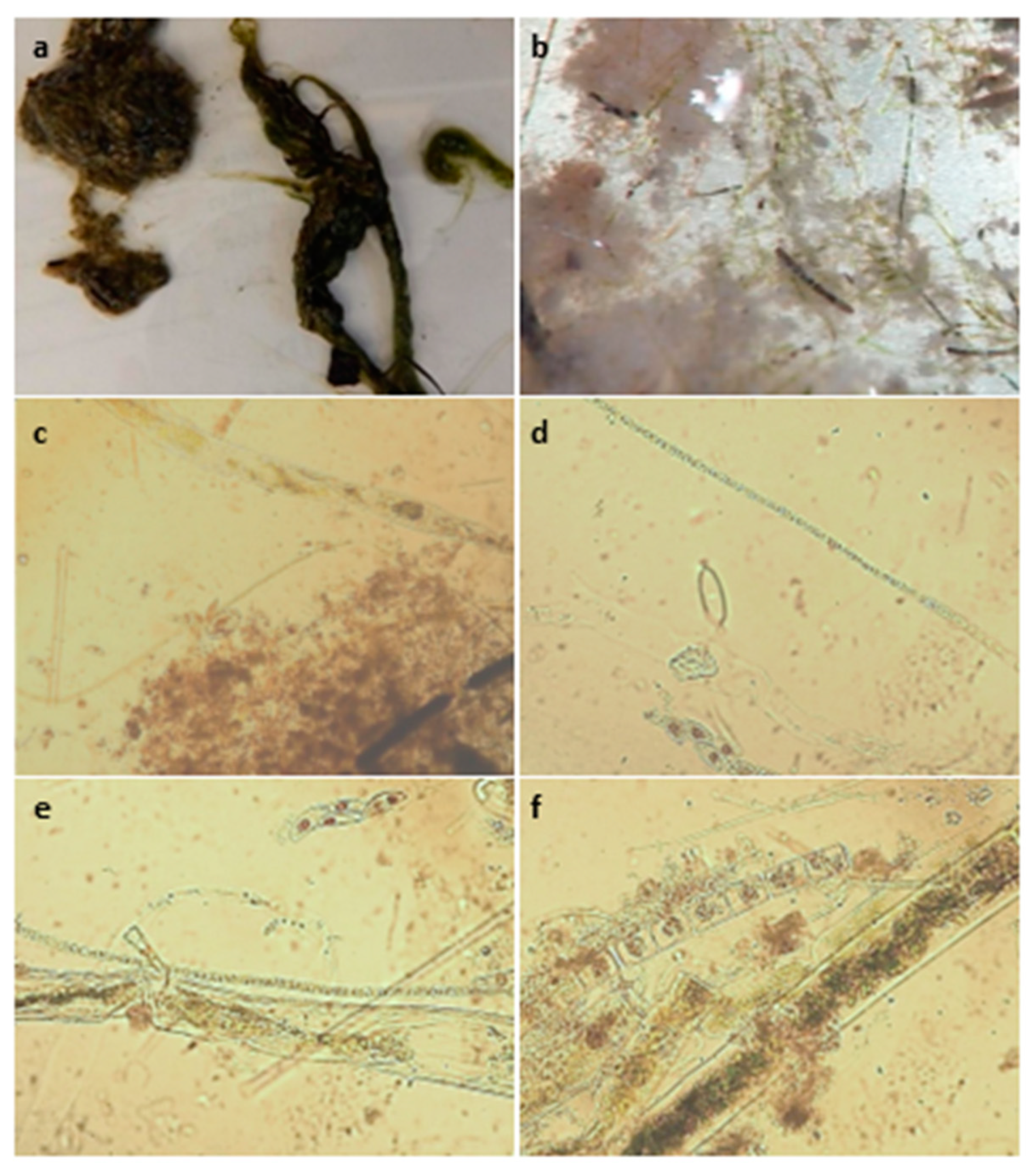
19]. Potentially toxigenic cyanobacteria were identified by GreenWater Laboratories using a Nikon TE200 inverted microscope equipped with phase contrast and epifluorescence (green light excitation, 510–560 nm). Samples were prepared as wet mount slides and scanned at 400× and 100× to narrow down which cyanobacteria were present and determine which cyanotoxins would be tested for. Full microscopy methods and photos of PTOX cyanobacteria from this analysis can be found in the corresponding data set [
12].
5.3. Animal Necropsy and Histopathology
A full necropsy of C
1 was performed by Texas A&M Veterinary Medical Diagnostic Laboratory, which included examining the musculoskeletal, respiratory, circulatory, digestive, hepatobiliary, hemolymphatic, urinary, reproductive, endocrine, and nervous systems. Necropsy included a toxicology screen for a broad range of organophosphates, some carbamates and organochlorine insecticides, as well as permethrin, petroleum products, and some drugs. Necropsy also included a screen for botulism A, B, and C. Twenty-three tissue samples were collected for histopathology. The full necropsy report can be accessed in the corresponding data set [
12]. Additional digestive tissues (jejunum, duodenum, and stomach content fluid) were reserved for high-performance liquid-chromatography–high-resolution mass spectrometry (HPLC-HRMS).Stomach contents, fluids, and tissue samples were analyzed by UC Davis Veterinary Medicine using liquid chromatography (LC) coupled with mass spectrometry (MS) (see report in corresponding data [
12]). Microcystins (LA, LR, RR, YR) and cylindrospermopsin were analyzed using LC-MS/MS and STX and ATX were analyzed using LC-MS/MS/MS. Additional digestive tissues (jejunum, duodenum, and stomach content fluid) were reserved for HPLC-HRMS to analyze for dhATX. The full necropsy report can be accessed in the corresponding data set [
12].
5.4. Toxin Analysis
Water samples and composite metaphyton/periphyton material samples were analyzed by GreenWater Laboratories, sample analysis methods are described in the corresponding data set, and the full reports can be viewed [
12]. Environmental and necropsy samples were extracted, enriched, and prepared for biochemical analysis. Extraction and enrichment steps were performed in dimmed lighting, and sample tubes were wrapped in foil to minimize photolysis. Prior to processing, samples were centrifuged for 5 min at 2100×
g to sediment debris. Water and solid/biomass extracts were processed by C18 solid-phase chromatography to enrich cyanotoxins and analyzed by HPLC-HRMS, according to procedures described in Manning et al., 2020 [
2]. Sediments (15 g) were extracted using double-distilled water (ddH
2O) with sonication. Zebra mussels (six bodies) were shucked and extracted in ddH
2O with 1% HCl (Fisher Chemical, Fair Lawn Industrial Park, Fair Lawn, NJ, USA) assisted by heating (60 °C) and sonication for 6 h; and 12 zebra mussel half shells were extracted in ddH
2O for 2 h with sonication. Necropsy liquids and tissues were extracted in methanol and 1% HCl with sonication for 6 h. Extracts were dried and resuspended in 5% MeOH in preparation for analysis by HPLC-HRMS. All samples were performed in duplicate.
Clarified lake water (50 mL) and extracts (25 mL) were passed over Waters Sep Pak C18 solid-phase extraction (SPE) cartridges (Waters Corporation, Milford, Massachusetts, USA) to enrich targeted compounds. SPE cartridges were activated with 5 mL of 100% MeOH (Fisher Chemical, Fair Lawn Industrial Park, NJ, USA) and conditioned using 5 mL of ddH2O. Then, the water or algal sample supernatant was slowly pushed through the column to adsorb metabolites. The eluate was retained as the sample load, and 5 mL of 100% MeOH was pushed through the cartridge to collect metabolites adsorbed on the column. Methanolic fractions were dried under nitrogen at 40 °C in pre-weighed glass vials. Once dried, extract residues were resuspended in 1 mL of 5% MeOH (aqueous), representing a 25- to 50-times enrichment from the original samples. All samples were prepared for analysis containing 0.1 ng/µL of caffeine internal standard (Fisher Chemical, Fair Lawn Industrial Park, NJ, USA) to monitor injection consistency and detection sensitivity; 100 µL of each sample with standard was transferred to a glass insert in a 2 mL amber glass vial in preparation for HPLC-HRMS.
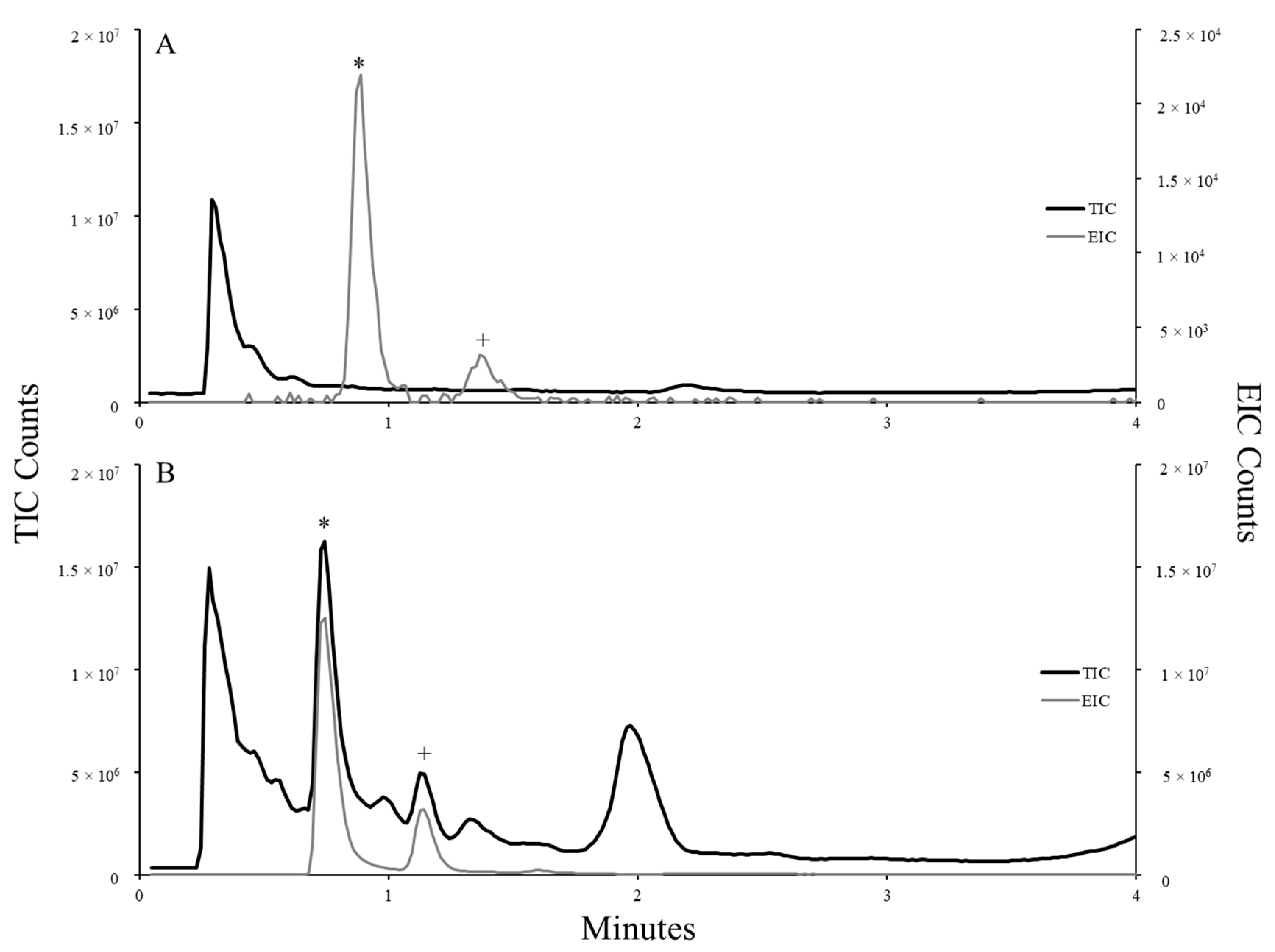
Samples were analyzed on an Agilent 6530 QTOF instrument (Agilent, Santa Clara, CA, USA). Samples (10 µL) were injected and separated on an Agilent Zorbax Eclipse Plus C18 column (50 mm length, 2.1 mm ID, 5 µm particle size, packed; Santa Clara, CA, USA) using a water:methanol gradient with 0.1% formic acid addition (Fisher Chemical, Fair Lawn Industrial Park, Fair Lawn, NJ, USA). The gradient was ramped from 1.0 to 95.0% MeOH over 12 min and held at 100% MeOH for 6 min. Mass spectrometry data were collected following electrospray ionization (ESI) operated in positive mode to produce the total ion chromatogram (TIC) and related extraction ion chromatograms (EIC) for target toxins.
Due to an absence of commercial reference materials, quantitative analysis of dhATX in samples was estimated using the caffeine internal standard as a proxy analyte of similar mass and polarity. The area underneath each peak was combined to represent the quantity of dhATX present in each injection and extrapolated to estimate the amount of dhATX per L or water or per g or biomass sampled (n = 2).
5.5. Statistical Analysis of Water Quality Parameters
Principal Component Analysis (PCA) was used to assess composite sample dhATX concentrations alongside multiple water quality parameters collected in open water locations within Lake Travis using the prcomp function in the R Statistical Software (v4.3.0; R Core Team) [
20]. Toxins from algal samples were collected from 3 March 2021 to 22 June 2021. Water quality parameters were collected as part of the Texas Clean Rivers Program (CRP) in lower Lake Travis, the Sandy Creek arm, the middle of Lake Travis, near Arkansas Bend, and in upper Lake Travis. Parameters collected and used within the PCA include specific conductivity, dissolved oxygen, water temperature, pH, turbidity, inflow from the upstream dam, outflow from the downstream dam, secchi disc depth, nitrate, chloride, sulfate, dissolved organic carbon, and total phosphorus. Composite samples collected at sites 1, 2, 3, 4, 5, and 6 corresponded to water quality data collected in lower Lake Travis (30.3920° N, 97.9050° W; 12,302); those at site 7 corresponded to data in the Sandy Creek arm (30.4786° N, 97.9056° W; 12,307); those at site 8 corresponded to data in the middle of Lake Travis (30.3731° N, 97.9636° W; 15,428); those at site 9 corresponded to data near Arkansas Bend (30.3960° N, 97.9510° W; 12,309); and those at sites 10 and 11 corresponded to data in upper Lake Travis (30.4990° N, 98.1040° W; 12,316).
Composite samples were not collected on the same day as the water quality samples, so water quality parameters were imputed and estimated based on mathematical formulas, using equations for Julian day. The formulas were derived from water quality samples collected every other month at each CRP site from 25 February 2021 to 14 December 2021, for a total of six samples at each site. The frequency of observed values would not allow for the evaluation of acute changes to water quality, but it is appropriate to allow the authors to evaluate general trends of water quality within the site location during the event. Quadratic to quintic equations were chosen as imputation equations for each parameter and site based on the fit of the equation. The R2 values of the imputation equations ranged from 0.1537 to 1.0; however, 54% of the equations had an R2 value above 0.9 and an additional 27% of the equations had an R2 over 0.7. The majority of the imputation equations fit the data well with only a few instances where no equation fit the data well.
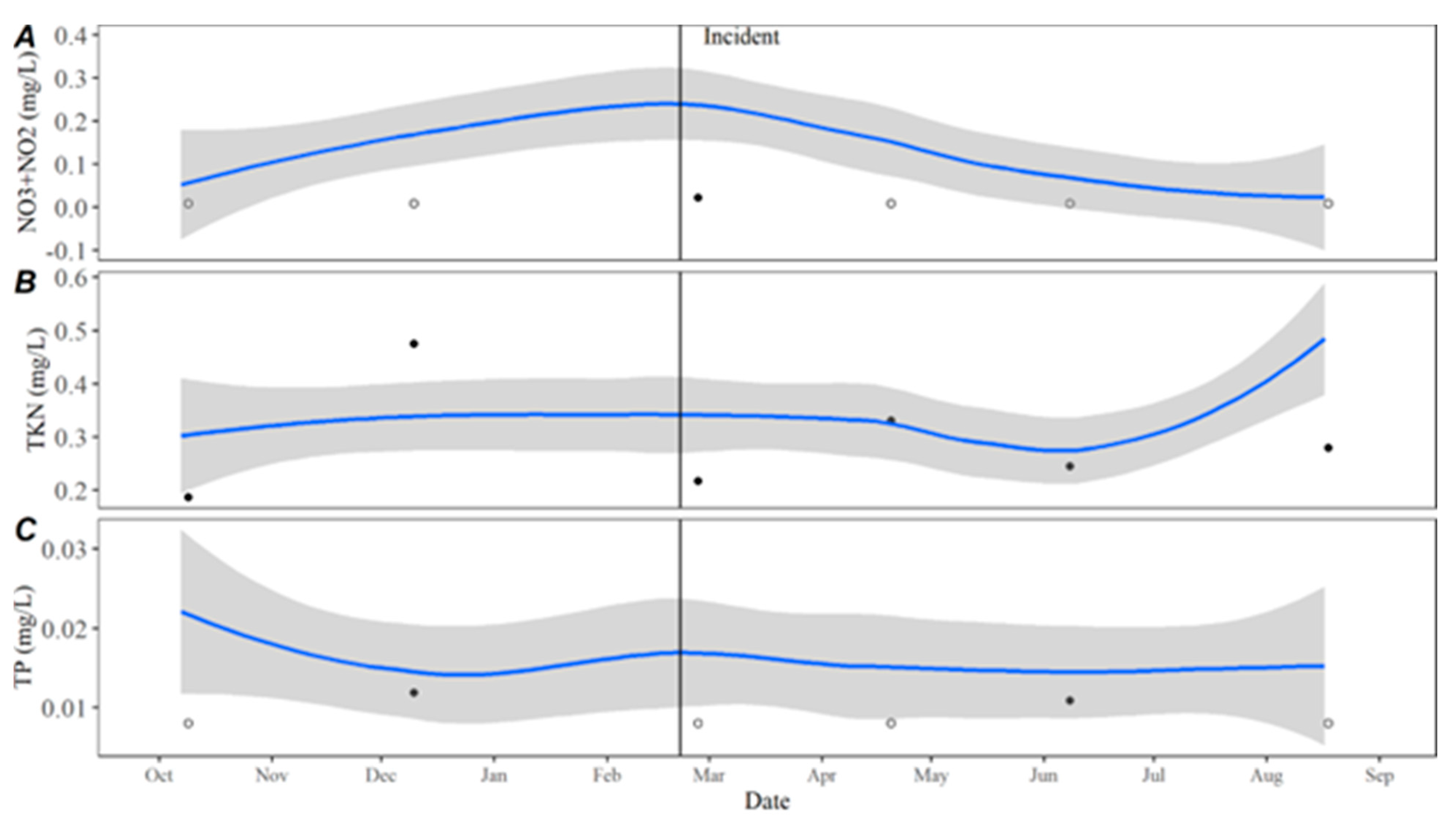
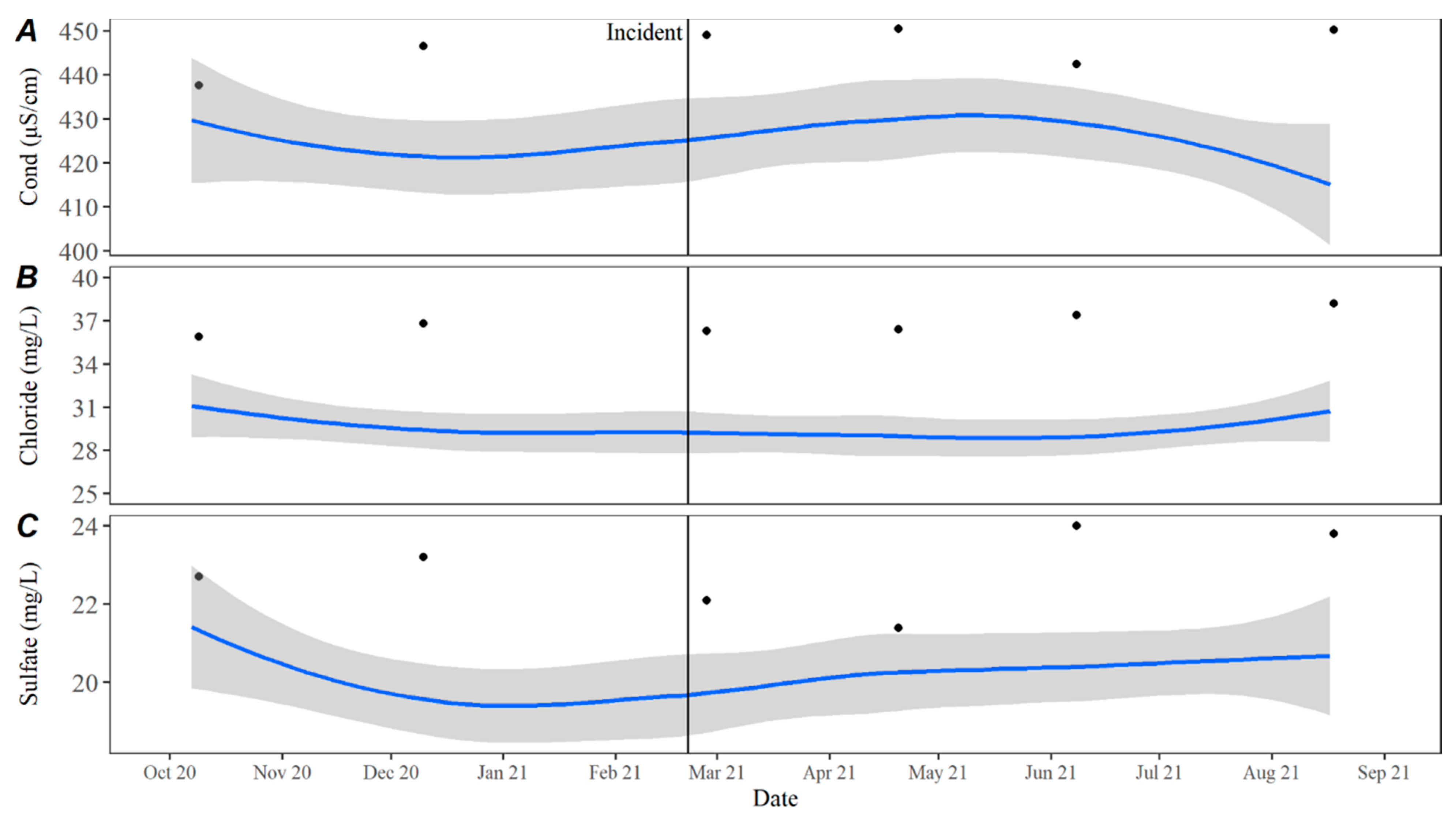
Time series averages and 95% confidence intervals for water quality parameters were constructed in lower Travis based on data collected from October 2015 to October 2020. Samples were typically collected throughout each year in February, April, May, June, July, August, September, October, and December. For all parameters except TKN, the total sample size for computing the time series averages was
n = 46. There were quality control issues with one TKN sample and the sample size for TKN was
n = 45. The average for each parameter by day was calculated by transforming the date to Julian day and applying locally estimated scatterplot smoothing (LOESS [
21]), also known as local polynomial regression, to the collected data using the LOESS function in the R Statistical Software R Statistical Software (v4.3.0; R Core Team 2023) [
20]. Using LOESS allows for flexibility in fitting the model by using localized subsets rather than a global formula, which allows the average to move throughout the year based on seasonal environmental conditions. Water quality data collected from October 2020 to September 2021 were compared to the averages to evaluate how conditions directly prior to, during, and after the incident compared to historically collected data.