Dynamics and Molecular Interactions of GPI-Anchored CD59
Abstract
1. Introduction
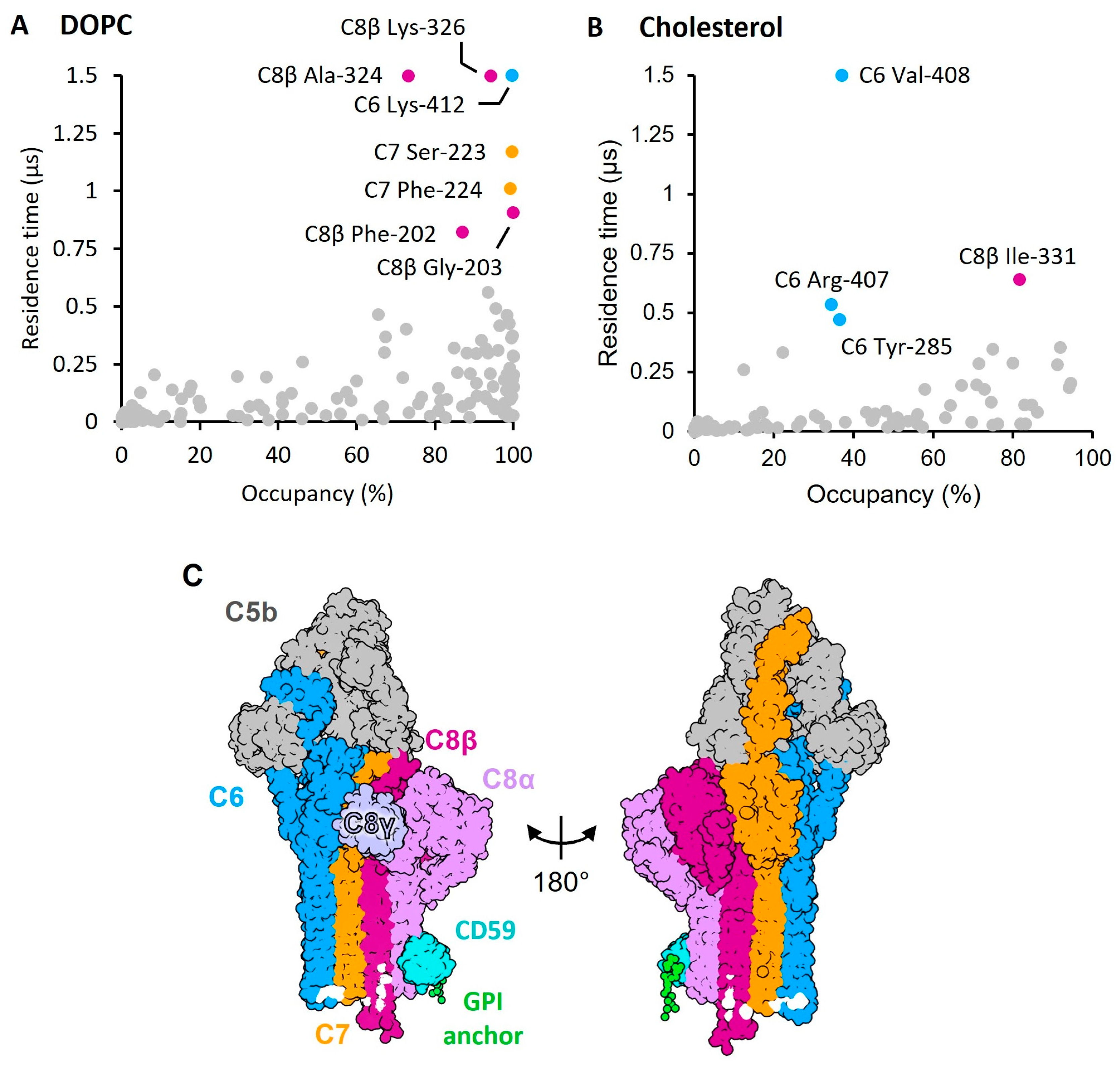
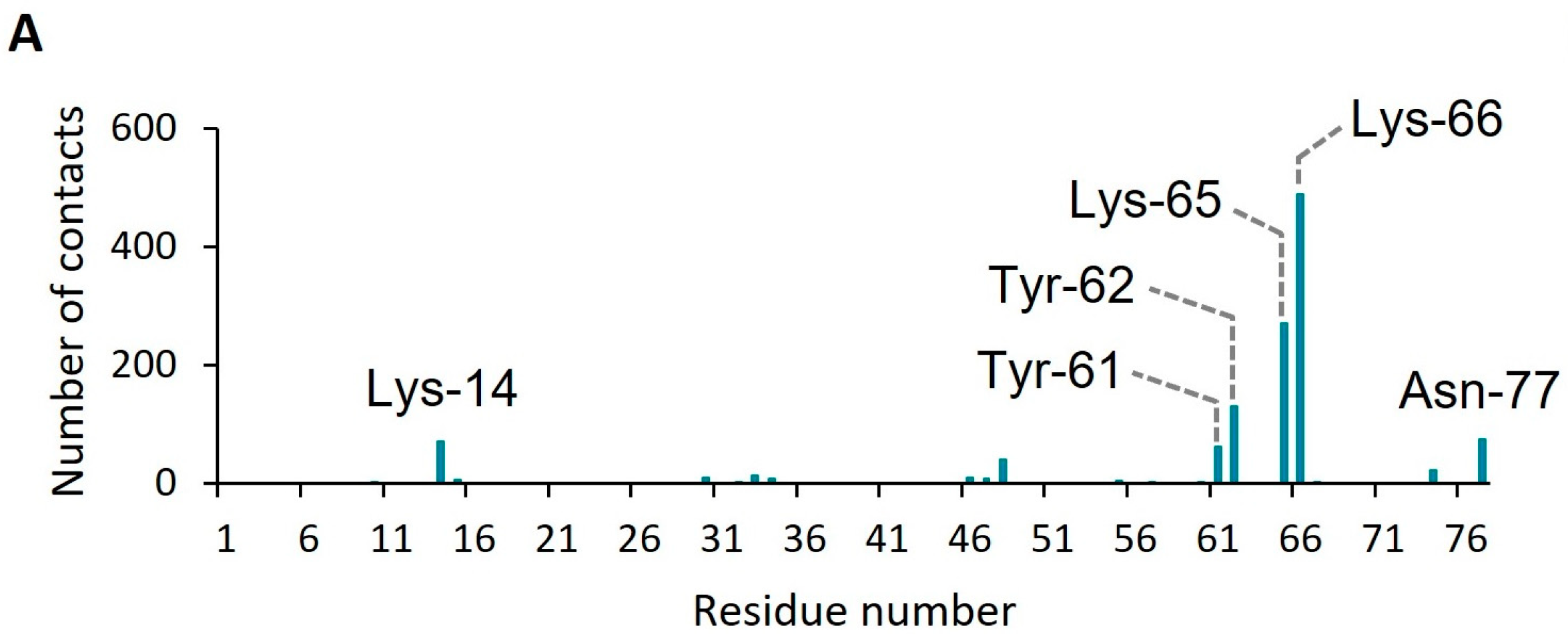
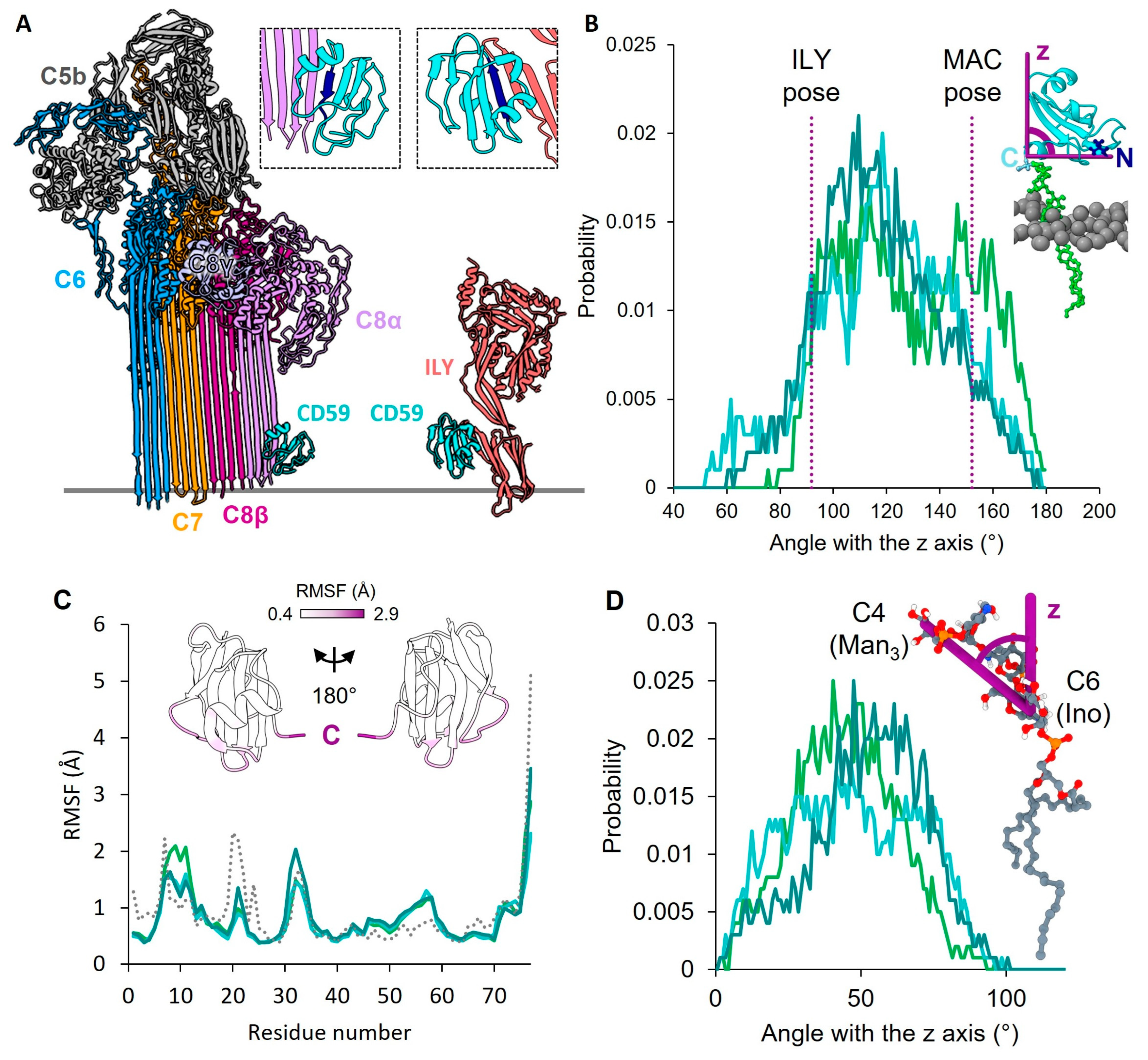
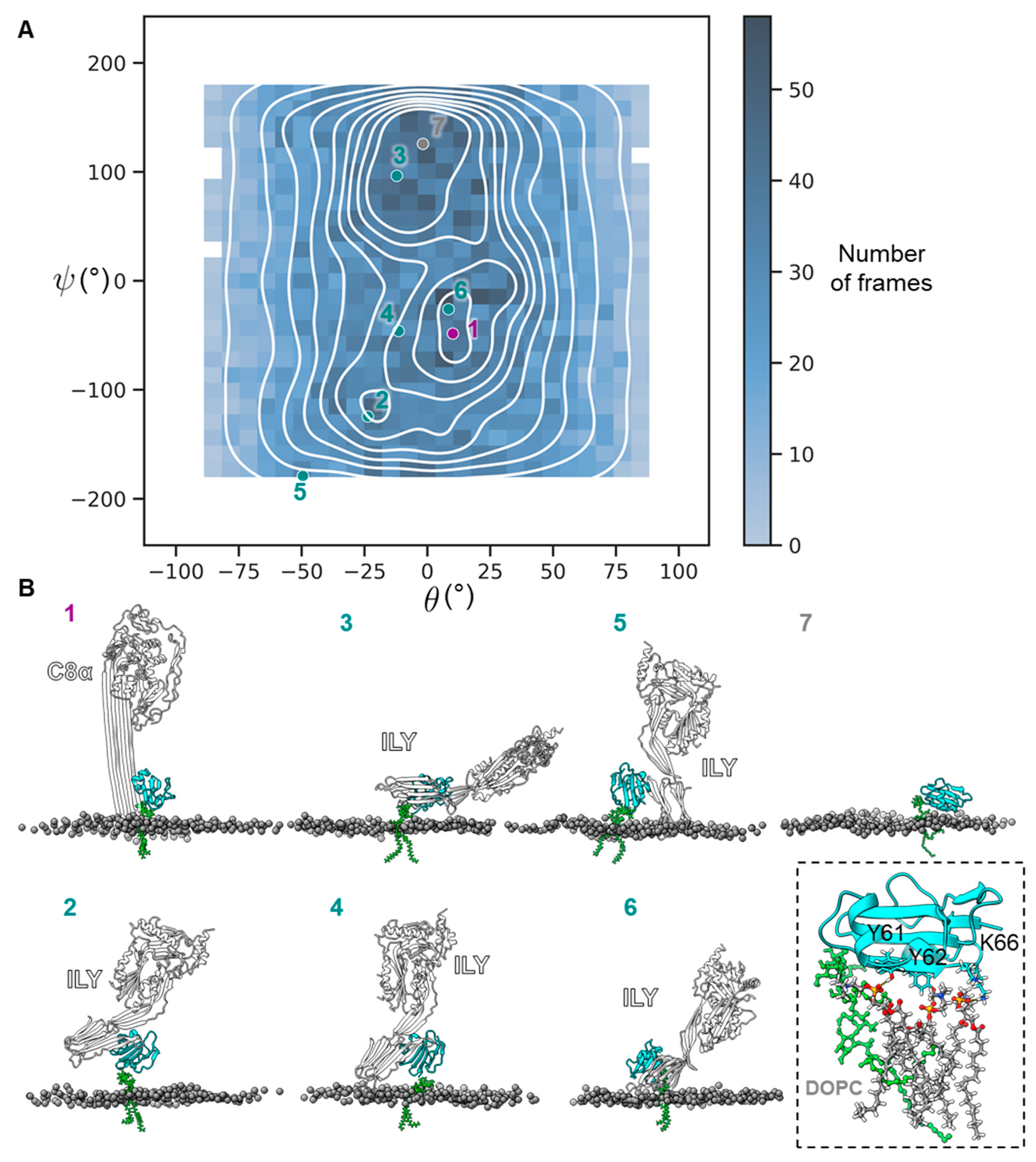
2. Results
3. Discussion
4. Materials and Methods
4.1. Analysis of Coarse-Grain CD59-C5b8 Simulations
4.2. Analysis of Atomistic CD59 Interactions with the Membrane
4.3. Analysis of CD59 Flexibility
4.4. CD59 Orientation Plot
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudd, P.M.; Morgan, B.P.; Wormald, M.R.; Harvey, D.J.; van den Berg, C.W.; Davis, S.J.; Ferguson, M.A.J.; Dwek, R.A. The Glycosylation of the Complement Regulatory Protein, Human Erythrocyte CD59. J. Biol. Chem. 1997, 272, 7229–7244. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Simmons, D.L.; Hale, G.; Harrison, R.A.; Tighe, H.; Lachmann, S.P.J.; Waldmanns, H. CD59, an LY-6-like Protein Expressed in Human Lymphoid Cells, Regulates the Action of the Complement Membrane Attack Complex on Homologous Cells. J. Exp. Med. 1989, 170, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Reis, E.S.; Lambris, J.D. Complement in Disease: A Defence System Turning Offensive. Nat. Rev. Nephrol. 2016, 12, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Farkas, I.; Baranyi, L.; Ishikawa, Y.; Okada, N.; Bohata, C.; Budai, D.; Fukuda, A.; Imai, M.; Okada, H. CD59 Blocks Not Only the Insertion of C9 into MAC but Inhibits Ion Channel Formation by Homologous C5b-8 as Well as C5b-9. J. Physiol. 2002, 539, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Meri, S.; Morgan, B.P.; Davies, A.; Daniels, R.H.; Olavesen, M.G.; Waldmann, H. Human Protectin (CD59), an 18,000-20,000 MW Complement Lysis Restricting Factor, Inhibits C5b-8 Catalysed Insertion of C9 into Lipid Bilayers. Immunology 1990, 71, 1–9. [Google Scholar]
- Nevo, Y.; Ben-Zeev, B.; Tabib, A.; Straussberg, R.; Anikster, Y.; Shorer, Z.; Fattal-Valevski, A.; Ta-Shma, A.; Aharoni, S.; Rabie, M.; et al. CD59 Deficiency Is Associated with Chronic Hemolysis and Childhood Relapsing Immune-Mediated Polyneuropathy. Blood 2013, 121, 129–135. [Google Scholar] [CrossRef]
- Fishelson, Z. Obstacles to Cancer Immunotherapy: Expression of Membrane Complement Regulatory Proteins (MCRPs) in Tumors. Mol. Immunol. 2003, 40, 109–123. [Google Scholar] [CrossRef]
- Shao, F.; Gao, Y.; Wang, W.; He, H.; Xiao, L.; Geng, X.; Xia, Y.; Guo, D.; Fang, J.; He, J.; et al. Silencing EGFR-Upregulated Expression of CD55 and CD59 Activates the Complement System and Sensitizes Lung Cancer to Checkpoint Blockade. Nat. Cancer 2022, 3, 1192–1210. [Google Scholar] [CrossRef]
- Giddings, K.S.; Zhao, J.; Sims, P.J.; Tweten, R.K. Human CD59 Is a Receptor for the Cholesterol-Dependent Cytolysin Intermedilysin. Nat. Struct. Mol. Biol. 2004, 11, 1173–1178. [Google Scholar] [CrossRef]
- Gelber, S.E.; Aguilar, J.L.; Lewis, K.L.T.; Ratner, A.J. Functional and Phylogenetic Characterization of Vaginolysin, the Human-Specific Cytolysin from Gardnerella Vaginalis. J. Bacteriol. 2008, 190, 3896–3903. [Google Scholar] [CrossRef]
- Wickham, S.E.; Hotze, E.M.; Farrand, A.J.; Polekhina, G.; Nero, T.L.; Tomlinson, S.; Parker, M.W.; Tweten, R.K. Mapping the Intermedilysin-Human CD59 Receptor Interface Reveals a Deep Correspondence with the Binding Site on CD59 for Complement Binding Proteins C8α and C9. J. Biol. Chem. 2011, 286, 20952–20962. [Google Scholar] [CrossRef]
- Boyd, C.M.; Parsons, E.S.; Smith, R.A.G.; Seddon, J.M.; Ces, O.; Bubeck, D. Disentangling the Roles of Cholesterol and CD59 in Intermedilysin Pore Formation. Sci. Rep. 2016, 6, 38446. [Google Scholar] [CrossRef]
- Couves, E.C.; Gardner, S.; Voisin, T.B.; Bickel, J.K.; Stansfeld, P.J.; Tate, E.W.; Bubeck, D. Structural Basis for Membrane Attack Complex Inhibition by CD59. Nat. Commun. 2023, 14, 890. [Google Scholar] [CrossRef]
- Johnson, S.; Brooks, N.J.; Smith, R.A.G.; Lea, S.M.; Bubeck, D. Structural Basis for Recognition of the Pore-Forming Toxin Intermedilysin by Human Complement Receptor CD59. Cell Rep. 2013, 3, 1369–1377. [Google Scholar] [CrossRef]
- Lawrence, S.L.; Gorman, M.A.; Feil, S.C.; Mulhern, T.D.; Kuiper, M.J.; Ratner, A.J.; Tweten, R.K.; Morton, C.J.; Parker, M.W. Structural Basis for Receptor Recognition by the Human CD59-Responsive Cholesterol-Dependent Cytolysins. Structure 2016, 24, 1488–1498. [Google Scholar] [CrossRef]
- Ivanova, M.E.; Lukoyanova, N.; Malhotra, S.; Topf, M.; Trapani, J.A.; Voskoboinik, I.; Saibil, H.R. The Pore Conformation of Lymphocyte Perforin. Sci. Adv. 2022, 8, eabk3147. [Google Scholar] [CrossRef]
- Pang, S.S.; Bayly-Jones, C.; Radjainia, M.; Spicer, B.A.; Law, R.H.P.; Hodel, A.W.; Parsons, E.S.; Ekkel, S.M.; Conroy, P.J.; Ramm, G.; et al. The Cryo-EM Structure of the Acid Activatable Pore-Forming Immune Effector Macrophage-Expressed Gene 1. Nat. Commun. 2019, 10, 4288. [Google Scholar] [CrossRef]
- Ni, T.; Jiao, F.; Yu, X.; Aden, S.; Ginger, L.; Williams, S.I.; Bai, F.; Pražák, V.; Karia, D.; Stansfeld, P.; et al. Structure and Mechanism of Bactericidal Mammalian Perforin-2, an Ancient Agent of Innate Immunity. Sci. Adv. 2020, 6, eaax8286. [Google Scholar] [CrossRef]
- Menny, A.; Serna, M.; Boyd, C.M.; Gardner, S.; Joseph, A.P.; Morgan, B.P.; Topf, M.; Brooks, N.J.; Bubeck, D. CryoEM Reveals How the Complement Membrane Attack Complex Ruptures Lipid Bilayers. Nat. Commun. 2018, 9, 5316. [Google Scholar] [CrossRef]
- Rosado, C.J.; Buckle, A.M.; Law, R.H.P.; Butcher, R.E.; Kan, W.-T.; Bird, C.H.; Ung, K.; Browne, K.A.; Baran, K.; Bashtannyk-Puhalovich, T.A.; et al. A Common Fold Mediates Vertebrate Defense and Bacterial Attack. Science 2007, 317, 1548–1551. [Google Scholar] [CrossRef]
- Shatursky, O.; Heuck, A.P.; Shepard, L.A.; Rossjohn, J.; Parker, M.W.; Johnson, A.E.; Tweten, R.K. The Mechanism of Membrane Insertion for a Cholesterol-Dependent Cytolysin: A Novel Paradigm for Pore-Forming Toxins. Cell 1999, 99, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.M.; Bubeck, D. Advances in CryoEM and Its Impact on β-Pore Forming Proteins. Curr. Opin. Struct. Biol. 2018, 52, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Parsons, E.S.; Stanley, G.J.; Pyne, A.L.B.; Hodel, A.W.; Nievergelt, A.P.; Menny, A.; Yon, A.R.; Rowley, A.; Richter, R.P.; Fantner, G.E.; et al. Single-Molecule Kinetics of Pore Assembly by the Membrane Attack Complex. Nat. Commun. 2019, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Serna, M.; Giles, J.L.; Morgan, B.P.; Bubeck, D. Structural Basis of Complement Membrane Attack Complex Formation. Nat. Commun. 2016, 7, 10587. [Google Scholar] [CrossRef] [PubMed]
- Vögele, M.; Bhaskara, R.M.; Mulvihill, E.; van Pee, K.; Yildiz, Ö.; Kühlbrandt, W.; Müller, D.J.; Hummer, G. Membrane Perforation by the Pore-Forming Toxin Pneumolysin. Proc. Natl. Acad. Sci. USA 2019, 116, 13352–13357. [Google Scholar] [CrossRef]
- Ninomiya, H.; Sims, P.J. The Human Complement Regulatory Protein CD59 Binds to the Alpha-Chain of C8 and to the “b”Domain of C9. J. Biol. Chem. 1992, 267, 13675–13680. [Google Scholar] [CrossRef]
- Lockert, D.H.; Kaufman, K.M.; Chang, C.-P.; Hüsler, T.; Sodetz, J.M.; Sims, P.J. Identity of the Segment of Human Complement C8 Recognized by Complement Regulatory Protein CD59. J. Biol. Chem. 1995, 270, 19723–19728. [Google Scholar] [CrossRef]
- Soltani, C.E.; Hotze, E.M.; Johnson, A.E.; Tweten, R.K. Specific Protein-Membrane Contacts Are Required for Prepore and Pore Assembly by a Cholesterol-Dependent Cytolysin. J. Biol. Chem. 2007, 282, 15709–15716. [Google Scholar] [CrossRef]
- Rossjohn, J.; Feil, S.C.; McKinstry, W.J.; Tweten, R.K.; Parker, M.W. Structure of a Cholesterol-Binding, Thiol-Activated Cytolysin and a Model of Its Membrane Form. Cell 1997, 89, 685–692. [Google Scholar] [CrossRef]
- Petranka, J.; Zhao, J.; Norris, J.; Tweedy, N.B.; Ware, R.E.; Sims, P.J.; Rosse, W.F. Structure-Function Relationships of the Complement Regulatory Protein, CD59. Blood Cells Mol. Dis. 1996, 22, 281–296. [Google Scholar] [CrossRef]
- Huang, Y.; Qiao, F.; Abagyan, R.; Hazard, S.; Tomlinson, S. Defining the CD59-C9 Binding Interaction. J. Biol. Chem. 2006, 281, 27398–27404. [Google Scholar] [CrossRef]
- Cragg, M.S.; Morgan, S.M.; Chan, H.T.C.; Morgan, B.P.; Filatov, A.V.; Johnson, P.W.M.; French, R.R.; Glennie, M.J. Complement-Mediated Lysis by Anti-CD20 MAb Correlates with Segregation into Lipid Rafts. Blood 2003, 101, 1045–1052. [Google Scholar] [CrossRef]
- Song, W.; Corey, R.A.; Duncan, A.L.; Ansell, T.B.; Stansfeld, P.J.; Sansom, M.S. PyLipID: A Python Toolkit for Analysis of Lipid-Protein Interactions from MD Simulations. Biophys. J. 2021, 120, 48a. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. JOSS 2021, 6, 3021. [Google Scholar] [CrossRef]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement: A Key System for Immune Surveillance and Homeostasis. Nat. Immunol. 2010, 11, 785–797. [Google Scholar] [CrossRef]
- Zaigraev, M.M.; Lyukmanova, E.N.; Paramonov, A.S.; Shenkarev, Z.O.; Chugunov, A.O. Orientational Preferences of GPI-Anchored Ly6/UPAR Proteins. Int. J. Mol. Sci. 2022, 24, 11. [Google Scholar] [CrossRef]
- Yesylevskyy, S.O.; Schäfer, L.V.; Sengupta, D.; Marrink, S.J. Polarizable Water Model for the Coarse-Grained MARTINI Force Field. PLoS Comput. Biol. 2010, 6, e1000810. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.-J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI Membrane Builder for Complex Biological Membrane Simulations with Glycolipids and Lipoglycans. J. Chem. Theory Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An Improved Force Field for Folded and Intrinsically Disordered Proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A Toolkit for the Analysis of MolecularDynamics Simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Gowers, R.; Linke, M.; Barnoud, J.; Reddy, T.; Melo, M.; Seyler, S.; Domański, J.; Dotson, D.; Buchoux, S.; Kenney, I.; et al. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. In Proceedings of the 15th Python in Science Conference; SciPy: Austin, TX, USA, 2016; pp. 98–105. [Google Scholar]
- Liu, P.; Agrafiotis, D.K.; Theobald, D.L. Fast Determination of the Optimal Rotational Matrix for Macromolecular Superpositions. J. Comput. Chem. 2010, 31, 1561–1563. [Google Scholar] [CrossRef]
- Theobald, D.L. Rapid Calculation of RMSDs Using a Quaternion-Based Characteristic Polynomial. Acta Cryst. A Found. Cryst. 2005, 61, 478–480. [Google Scholar] [CrossRef]
- Fletcher, C.M.; Harrison, R.A.; Lachmann, P.J.; Neuhausl, D. Structure of a Soluble, Glycosylated Form of the Human Complement Regulatory Protein CD59. Structure 1994, 2, 185–199. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voisin, T.B.; Couves, E.C.; Tate, E.W.; Bubeck, D. Dynamics and Molecular Interactions of GPI-Anchored CD59. Toxins 2023, 15, 430. https://doi.org/10.3390/toxins15070430
Voisin TB, Couves EC, Tate EW, Bubeck D. Dynamics and Molecular Interactions of GPI-Anchored CD59. Toxins. 2023; 15(7):430. https://doi.org/10.3390/toxins15070430
Chicago/Turabian StyleVoisin, Tomas B., Emma C. Couves, Edward W. Tate, and Doryen Bubeck. 2023. "Dynamics and Molecular Interactions of GPI-Anchored CD59" Toxins 15, no. 7: 430. https://doi.org/10.3390/toxins15070430
APA StyleVoisin, T. B., Couves, E. C., Tate, E. W., & Bubeck, D. (2023). Dynamics and Molecular Interactions of GPI-Anchored CD59. Toxins, 15(7), 430. https://doi.org/10.3390/toxins15070430



