The AwHog1 Transcription Factor Influences the Osmotic Stress Response, Mycelium Growth, OTA Production, and Pathogenicity in Aspergillus westerdijkiae fc-1
Abstract
1. Introduction
2. Results
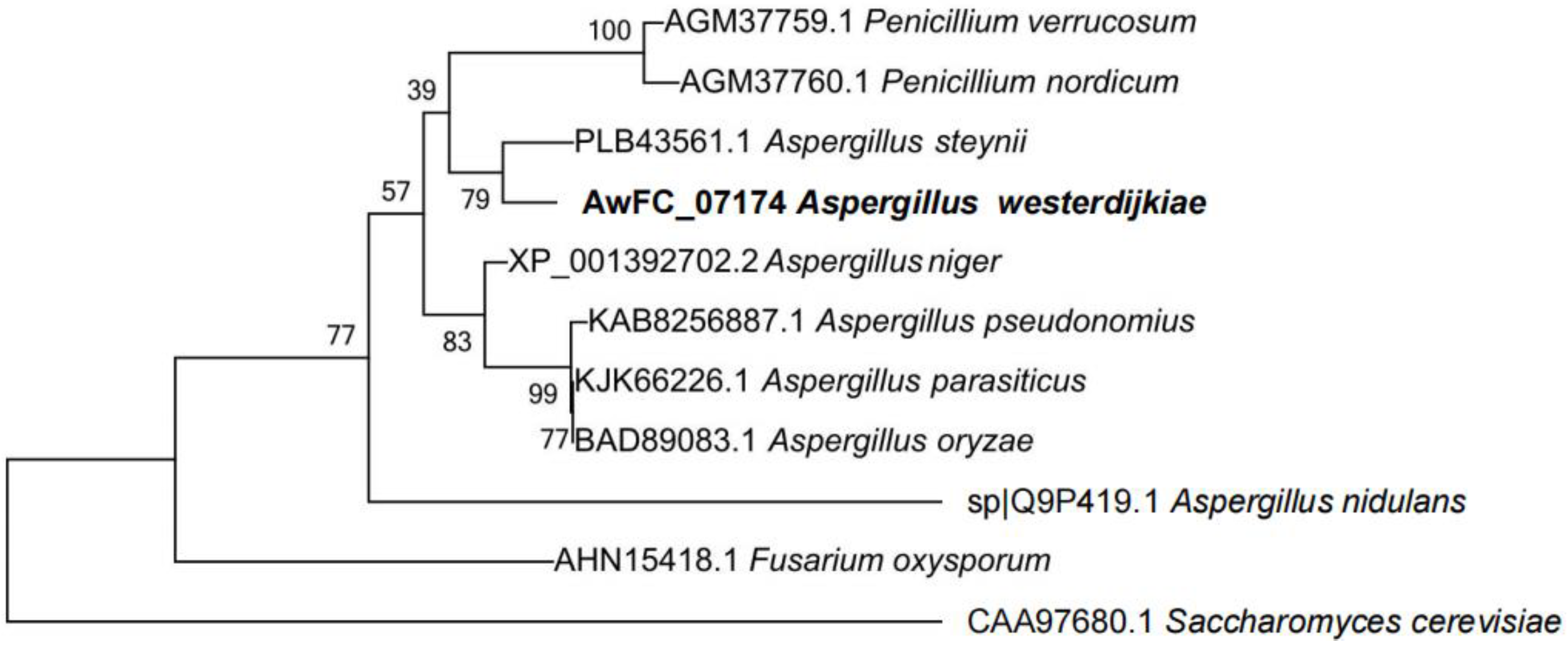
2.1. Characterization of a Hog1 Homology
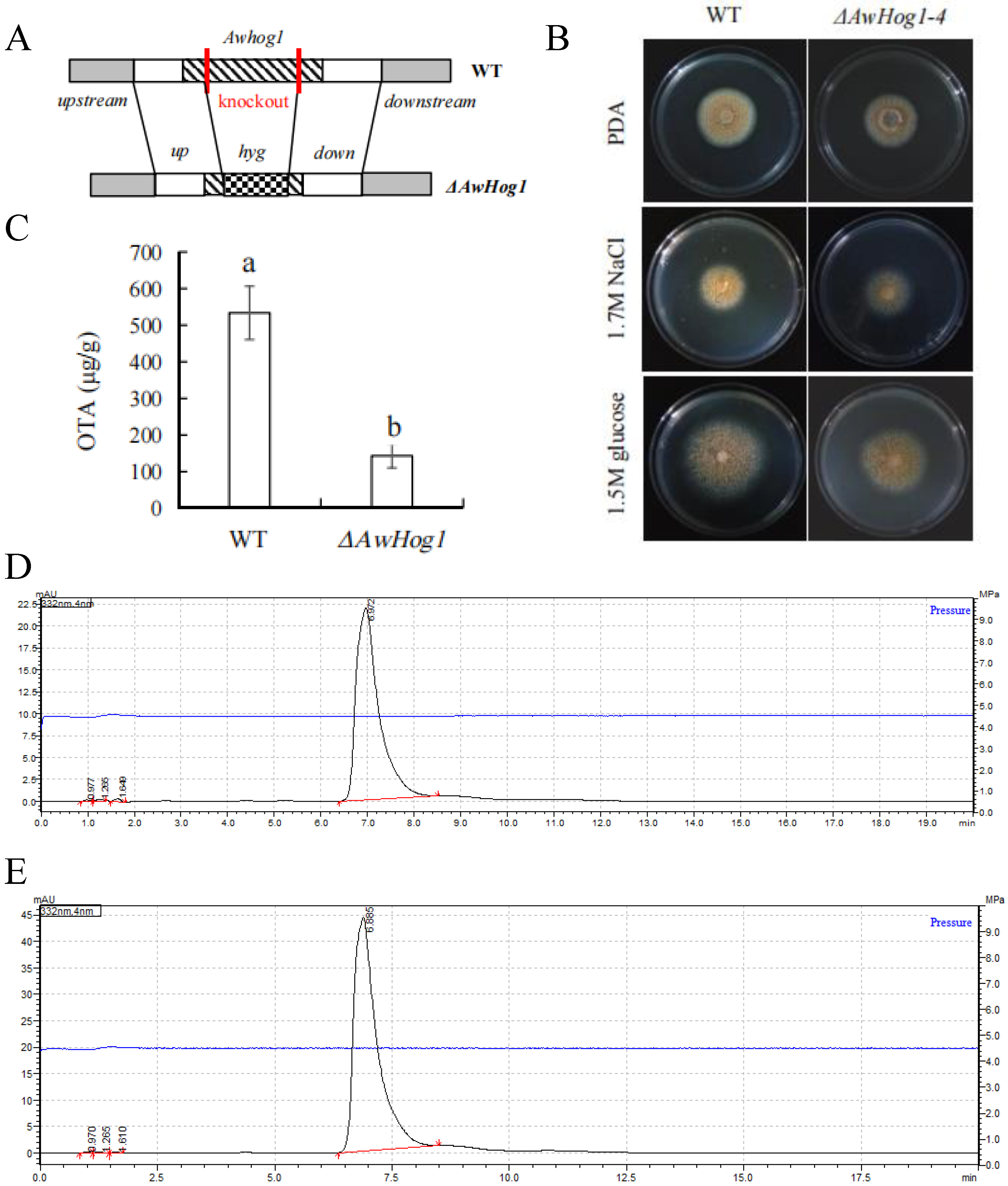
2.2. Construction of ΔAwHog1 Deletion Mutant
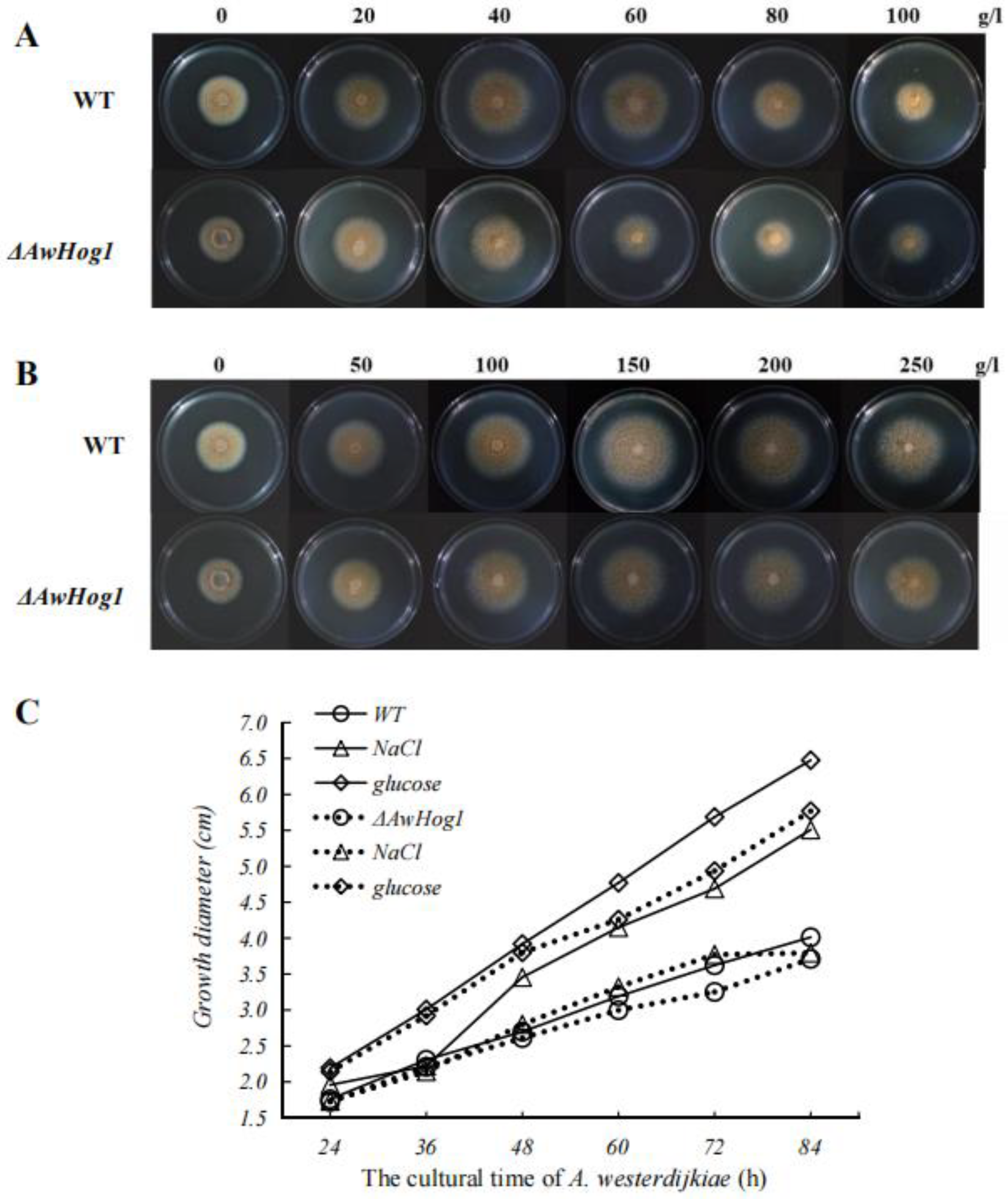
2.3. High Concentrations of NaCl or Glucose Affect Colony Morphology
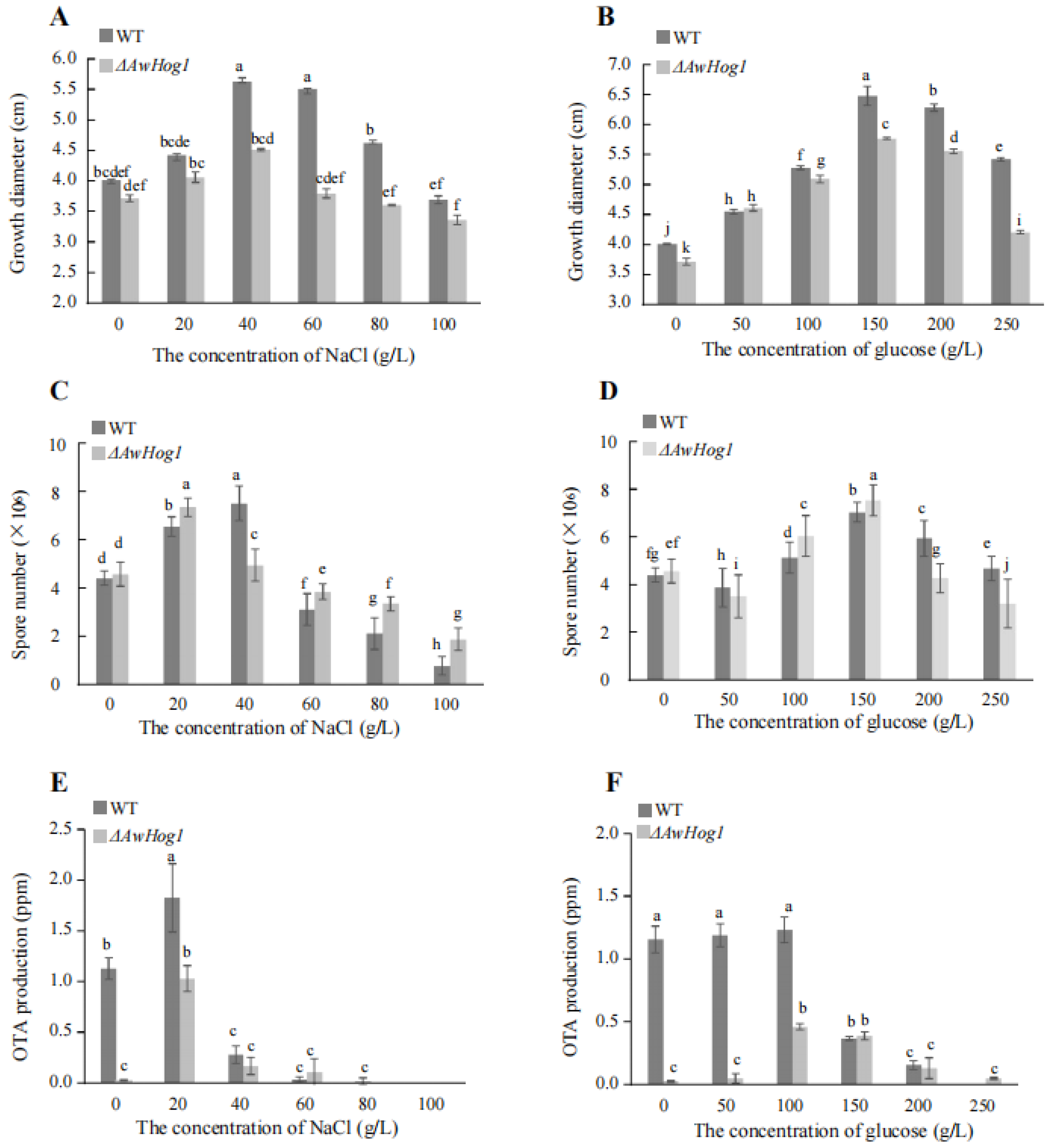
2.4. Growth Diameter, Spores Number, and OTA Production Are Affected and Modulated by AwHog1
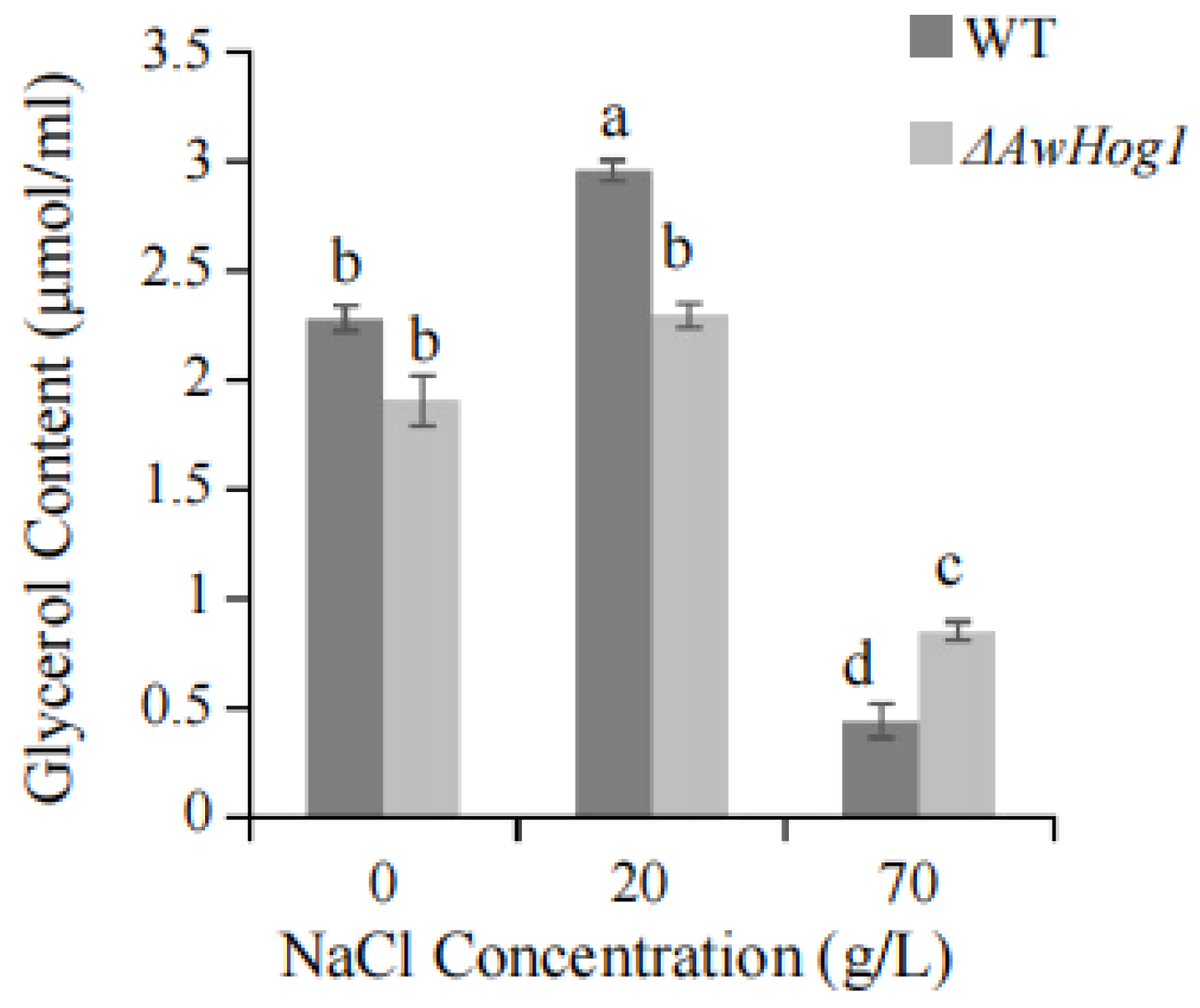
2.5. Glycerol Content Is Affected by AwHog1
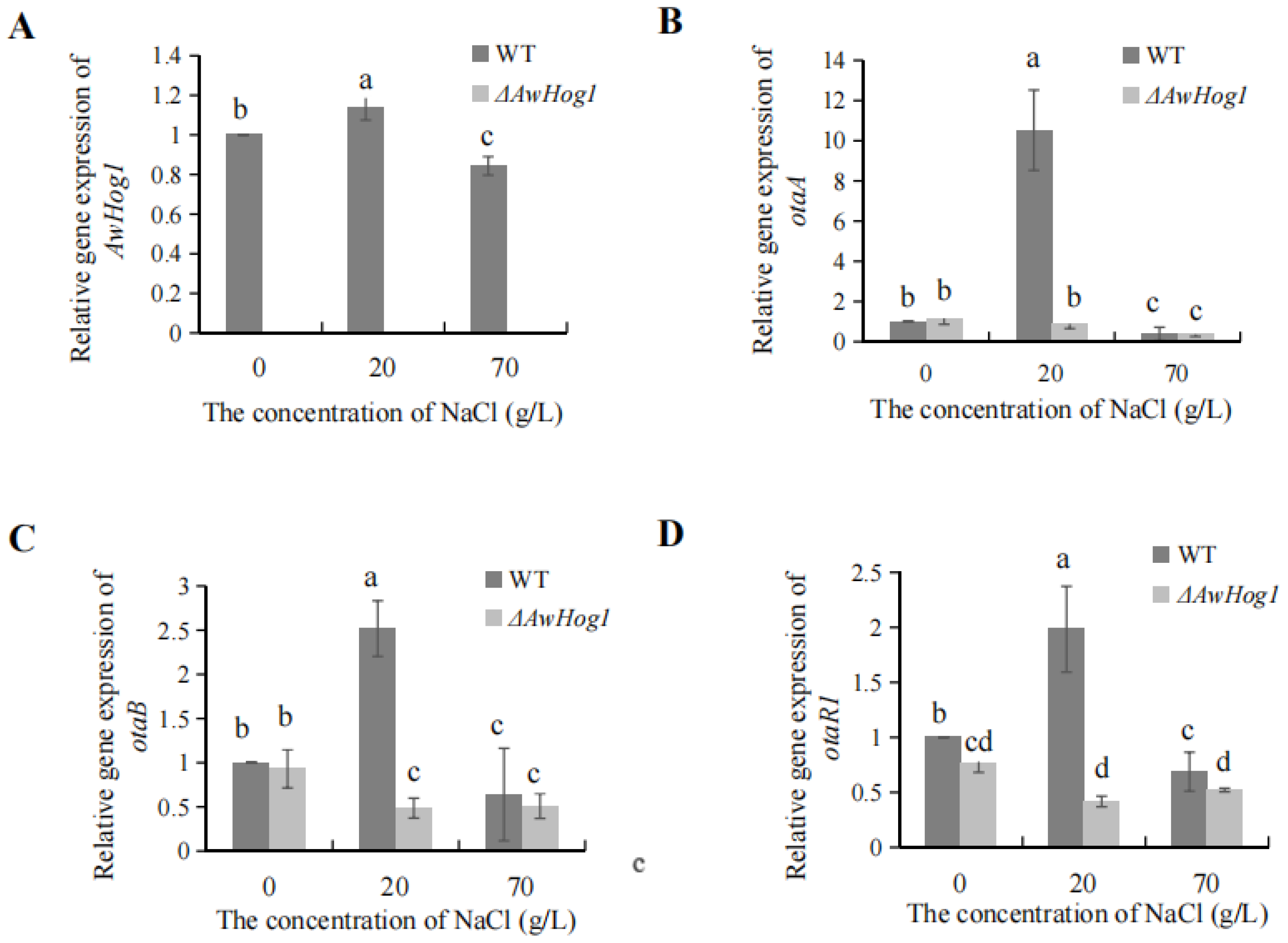
2.6. Relative Expression of AwHog1 and OTA Biosynthetic Genes
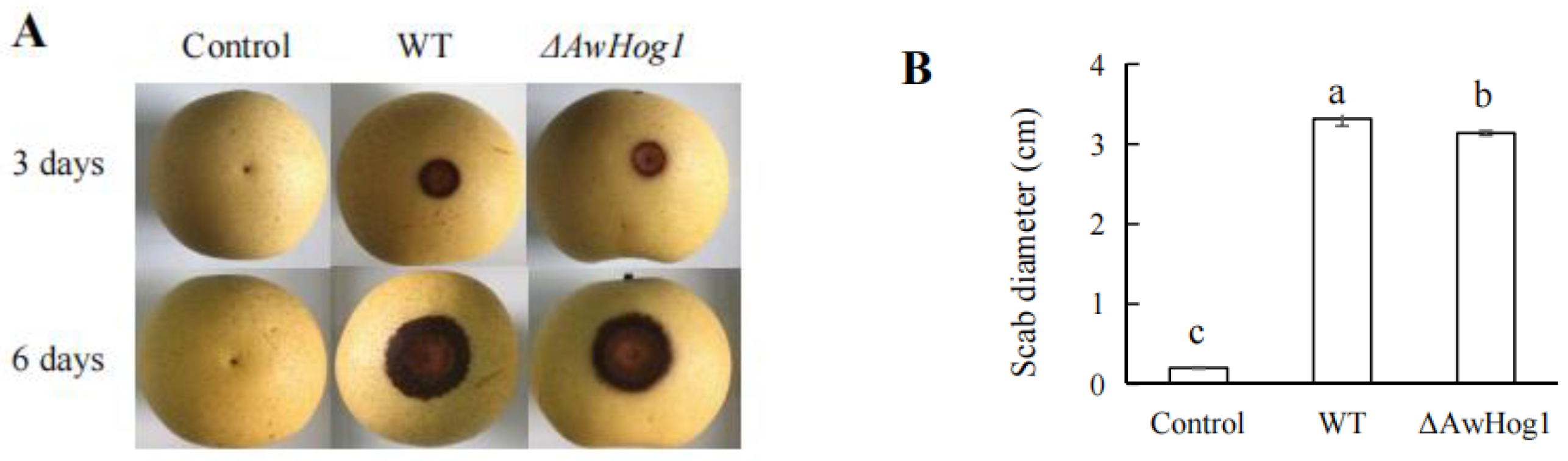
2.7. Transcription Factor AwHog1 in A. westerdijkiae fc-1 Is Essential for Fungi Infection
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains and Media
5.2. Culture Conditions
5.3. Construction of ΔAwHog1 Deletion Strains
5.4. OTA Production Analysis in the ΔAwHog1 Mutants and the Wild Type
5.5. Mycelial Growth, Conidia Count, and OTA Production
5.6. Phenotypic Comparison of Wild Type and ΔAwHog1 Mutants in Different High Permeability Conditions
5.7. Glycerol Content of Wild Type and ΔAwHog1 Mutant on PDA Medium with Increased NaCl Concentration
5.8. Gene Expression under Various NaCl Environments
5.9. Infection Ability in Pears
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Al Ayoubi, M.; Solfrizzo, M.; Gambacorta, L.; Watson, I.; El Darra, N. Risk of exposure to aflatoxin B1, ochratoxin A, and fumonisin B1 from spices used routinely in Lebanese cooking. Food Chem. Toxicol. 2021, 147, 111895. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, J.; Cai, M.; Liu, Y.; Yang, K. Detoxification of mycotoxins in agricultural products by non-thermal physical technologies: A review of the past five years. Crit. Rev. Food Sci. Nutr. 2022; ahead-of-print. [Google Scholar] [CrossRef]
- Wilson, K.A.; Kung, R.W.; Wetmore, S.D. Chapter seven—Toxicology of DNA adducts formed upon human exposure to carcinogens: Insights gained from molecular modeling. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2016; Volume 10, pp. 293–360. [Google Scholar]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.B.; et al. A consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Andretta, E.; Prisco, F.; Russo, V.; Pagnini, F.; Florio, S.; Ciarcia, R. Curcumin modulates nitrosative stress, inflammation and DNA damage and protects against ochratoxin A-induced hepatotoxicity and nephrotoxicity in rats. Antioxidants 2021, 10, 1239. [Google Scholar] [CrossRef]
- Stoll, D.; Schmidt-Heydt, M.; Geisen, R. Differences in the regulation of ochratoxin A by the HOG pathway in Penicillium and Aspergillus in response to high osmolar environments. Toxins 2013, 5, 1282–1298. [Google Scholar] [CrossRef]
- Han, X.; Chakrabortti, A.; Zhu, J.; Liang, Z.-X.; Li, J. Sequencing and functional annotation of the whole genome of the filamentous fungus Aspergillus westerdijkiae. BMC Genom. 2016, 17, 633. [Google Scholar] [CrossRef]
- Rodríguez, A.; Medina, Á.; Córdoba, J.J.; Magan, N. Development of a HOG-based real-time PCR method to detect stress response changes in mycotoxigenic moulds. Food Microbiol. 2016, 57, 109–115. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Graf, E.; Stoll, D.; Geisen, R. The biosynthesis of ochratoxin A by Penicillium as one mechanism for adaptation to NaCl rich foods. Food Microbiol. 2012, 29, 233–241. [Google Scholar] [CrossRef]
- Delgado, J.; da Cruz Cabral, L.; Rodríguez, M.; Rodríguez, A. Influence of ochratoxin A on adaptation of Penicillium nordicum on a NaCl-rich dry-cured ham-based medium. Int. J. Food Microbiol. 2018, 272, 22–28. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Neng, J.; Gao, J.; Yang, B.; Liu, Y. The influence of NaCl and glucose content on growth and ochratoxin A production by Aspergillus ochraceus. Aspergillus carbonarius and Penicillium nordicum. Toxins 2020, 12, 515. [Google Scholar] [CrossRef]
- De Nadal, E.; Posas, F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022, 22, foac013. [Google Scholar] [CrossRef]
- Zhu, J.; Ying, S.; Feng, M. The Pal pathway required for ambient pH adaptation regulates growth, conidiation, and osmotolerance of Beauveria bassiana in a pH-dependent manner. Appl. Microbiol. Biot. 2016, 100, 4423–4433. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Sun, J.; Cui, X. Comparative transcriptomic and proteomic analyses to determine the lignin synthesis pathway involved in the fungal stress response in Panax notoginseng. Physiol. Mol. Plant Pathol. 2022, 11, 101814. [Google Scholar] [CrossRef]
- Dihazi, H.; Kessler, R.; Eschrich, K. High Osmolarity Glycerol (HOG) pathway-induced phosphorylation and activation of 6-phosphofructo-2-kinase are essential for glycerol accumulation and yeast cell proliferation under hyperosmotic stress. J. Biol. Chem. 2004, 279, 23961–23968. [Google Scholar] [CrossRef]
- Álvarez, M.; Rodríguez, A.; Núñez, F.; Silva, A.; Andrade, M.J. In vitro antifungal effects of spices on ochratoxin A production and related gene expression in Penicillium nordicum on a dry-cured fermented sausage medium. Food Control 2020, 114, 107222. [Google Scholar] [CrossRef]
- Graf, E.; Schmidt-Heydt, M.; Geisen, R. HOG MAP kinase regulation of alternariol biosynthesis in Alternaria alternata is important for substrate colonization. Int. J. Food Microbiol. 2012, 157, 353–359. [Google Scholar] [CrossRef]
- Liang, Q.; Li, B.; Wang, J.; Ren, P.; Yao, L.; Meng, Y.; Si, E.; Shang, X.; Wang, H. PGPBS, a mitogen-activated protein kinase kinase, is required for vegetative differentiation, cell wall integrity, and pathogenicity of the barley leaf stripe fungus Pyrenophora graminea. Gene 2019, 696, 95–104. [Google Scholar] [CrossRef]
- Lemos, P.; Ruiz-Roldán, C.; Hera, C. Role of the phosphatase Ptc1 in stress responses mediated by CWI and HOG pathways in Fusarium oxysporum. Fungal Genet. Biol. 2018, 118, 10–20. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Katsuki, Y.; Tanaka, S.; Kawaguchi, R.; Denda, H.; Ikeda, T.; Funato, K.; Tani, M. Protective role of the HOG pathway against the growth defect caused by impaired biosynthesis of complex sphingolipids in yeast Saccharomyces cerevisiae. Mol. Microbiol. 2018, 107, 363–386. [Google Scholar] [CrossRef]
- Zuchman, R.; Koren, R.; Horwitz, B.A. Developmental roles of the hog1 protein phosphatases of the maize pathogen Cochliobolus heterostrophus. J. Fungi 2021, 7, 83. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Z.-C.; Chi, Z.; Liu, G.-L.; Hu, Z.; Chi, Z.-M. cAMP-PKA and HOG1 signaling pathways regulate liamocin production by different ways via the transcriptional activator Msn2 in Aureobasidium melanogenum. Enzym. Microb. Technol. 2021, 143, 109705. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Stoll, D.A.; Mrohs, J.; Geisen, R. Intraspecific variability of HOG1 phosphorylation in Penicillium verrucosum reflects different adaptation levels to salt rich habitats. Int. J. Food Microbiol. 2013, 165, 246–250. [Google Scholar] [CrossRef]
- Kohut, G.; Ádám, A.L.; Fazekas, B.; Hornok, L. N-starvation stress induced FUM gene expression and fumonisin production is mediated via the HOG-type MAPK pathway in Fusarium proliferatum. Int. J. Food Microbiol. 2009, 130, 65–69. [Google Scholar] [CrossRef]
- Gu, Q.; Zhang, C.; Yu, F.; Yin, Y.; Shim, W.-B.; Ma, Z. Protein kinase FgSch9 serves as a mediator of the target of rapamycin and high osmolarity glycerol pathways and regulates multiple stress responses and secondary metabolism in Fusarium graminearum. Environ. Microbiol. 2015, 17, 2661–2676. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, Z.; Guo, Z.; Li, D.; Zhang, F.; Shen, J.; Zhang, Y.; Wang, S.; Zhuang, Z. PbsB regulates morphogenesis, aflatoxin B1 biosynthesis, and pathogenicity of Aspergillus flavus. Front. Cell. Infect. Microbiol. 2018, 8, 162. [Google Scholar] [CrossRef]
- Hayes, B.M.E.; Anderson, M.A.; Traven, A.; van der Weerden, N.L.; Bleackley, M.R. Activation of stress signalling pathways enhances tolerance of fungi to chemical fungicides and antifungal proteins. Cell. Mol. Life Sci. 2014, 71, 2651–2666. [Google Scholar] [CrossRef]
- Lee, D.W.; Hong, C.P.; Thak, E.J.; Park, S.G.; Lee, C.H.; Lim, J.Y.; Seo, J.A.; Kang, H.A. Integrated genomic and transcriptomic analysis reveals unique mechanisms for high osmotolerance and halotolerance in Hyphopichia yeast. Environ. Microbiol. 2021, 23, 3499–3522. [Google Scholar] [CrossRef]
- Li, T.; Xiu, Q.; Wang, J.; Duan, Y.; Zhou, M. A putative MAPK kinase kinase gene Ssos4 is involved in mycelial growth, virulence, osmotic adaptation, and sensitivity to fludioxonil and is essential for SsHog1 phosphorylation in Sclerotinia sclerotiorum. Phytopathology 2021, 111, 521–530. [Google Scholar] [CrossRef]
- Motoyama, T.; Ochiai, N.; Morita, M.; Iida, Y.; Usami, R.; Kudo, T. Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr. Genet. 2008, 54, 185–195. [Google Scholar] [CrossRef]
- Yan, L.; Yang, Q.; Sundin, G.W.; Li, H.; Ma, Z. The mitogen-activated protein kinase kinase BOS5 is involved in regulating vegetative differentiation and virulence in Botrytis cinerea. Fungal Gent. Biol. 2010, 47, 753–760. [Google Scholar] [CrossRef]
- Gong, X.-D.; Feng, S.-Z.; Zhao, J.; Tang, C.; Tian, L.; Fan, Y.-S.; Cao, Z.-Y.; Hao, Z.-M.; Jia, H.; Zang, J.-P.; et al. StPBS2, a MAPK kinase gene, is involved in determining hyphal morphology, cell wall development, hypertonic stress reaction as well as the production of secondary metabolites in Northern Corn Leaf Blight pathogen Setosphaeria turcica. Microbiol. Res. 2017, 201, 30–38. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Graf, E.; Batzler, J.; Geisen, R. The application of transcriptomics to understand the ecological reasons of ochratoxin a biosynthesis by Penicillium nordicum on sodium chloride rich dry cured foods. Trends Food Sci. Technol. 2011, 22, S39–S48. [Google Scholar] [CrossRef]
- Rodríguez, A.; Medina, Á.; Córdoba, J.J.; Magan, N. The influence of salt (NaCl) on ochratoxin A biosynthetic genes, growth and ochratoxin A production by three strains of Penicillium nordicum on a dry-cured ham-based medium. Int. J. Food Microbiol. 2014, 178, 113–119. [Google Scholar] [CrossRef]
- Xu, L.; Wang, H.; Zhang, C.; Wang, J.; Chen, A.; Chen, Y.; Ma, Z. System-wide characterization of subtilases reveals that subtilisin-like protease FgPrb1 of Fusarium graminearum regulates fungal development and virulence. Fungal Genet. Biol. 2020, 144, 103449. [Google Scholar] [CrossRef]
- Román, E.; Correia, I.; Prieto, D.; Alonso, R.; Pla, J. The HOG MAPK pathway in Candida albicans: More than an osmosensing pathway. Int. Microbiol. 2020, 23, 23–29. [Google Scholar] [CrossRef]
- Li, P.; Gong, X.-D.; Jia, H.; Fan, Y.-S.; Zhang, Y.-F.; Cao, Z.-Y.; Hao, Z.-M.; Han, J.-M.; Gu, S.-Q.; Dong, J.-G. MAP kinase gene STK1 is required for hyphal, conidial, and appressorial development, toxin biosynthesis, pathogenicity, and hypertonic stress response in the plant pathogenic fungus Setosphaeria turcica. J. Integr. Agric. 2016, 15, 2786–2794. [Google Scholar] [CrossRef]
- Tian, L.; Yu, J.; Wang, Y.; Tian, C. The C2H2 transcription factor VdMsn2 controls hyphal growth, microsclerotia formation, and virulence of Verticillium dahliae. Fungal Biol. 2017, 121, 1001–1010. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Wang, L.; Wang, Q.; Selvaraj, J.N.; Zhao, Y.; Wang, Y.; Xing, F.; Liu, Y. pH-signaling transcription factor AopacC regulates ochratoxin A biosynthesis in Aspergillus ochraceus. J. Agric. Food Chem. 2018, 66, 4394–4401. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Wang, L.; Wang, Q.; Wang, Y.; Liu, Y. Effect of different media on the growth and toxicity production capacity of Aspergillus ochraceus. J. Nucl. Agric. Sci. 2017, 31, 702–710. [Google Scholar]
- Wang, Y.; Zheng, Y.; Zhou, A.; Neng, J.; Wu, D.; Shen, L.X.; Lou, X. Transcriptomic analysis reveals the inhibition mechanism of pulsed light on fungal growth and ochratoxin A biosynthesis in Aspergillus carbonarius. Food Res. Int. 2023, 165, 112501. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′ to 3′) | Base (bp) | Product Size (bp) |
|---|---|---|---|
| AwHog1-Up | Forward—AGGAACATCTTGCATGGGCA | 20 | 1425 |
| Reverse—CAAAATAGGCATTGATGTGTT GACCTCCCAGCCATACCAGACCAGTCC | 48 | ||
| AwHog1-Down | Forward—CTCGTCCGAGGGCAAAGGAAT AGAGTAGACGATGCTGAGCTACCAGTG | 48 | 1548 |
| Reverse—TCCTGGTGATACGGAGGGAG | 20 | ||
| AwHog1-Knock | Forward—TTGGGGTTCTAGTCCGTGTC | 20 | 3698 |
| Reverse—GGCAGTCACTTACCTCGCAT | 20 | ||
| AwHog1-Out-F | Forward—TTGTGGAGACAAGGAAGCCG | 20 | 860 |
| Reverse—TCGAAGCTGAAAGCACGAGA | 20 | ||
| AwHog1-In-F | Forward—GCGTTTGGACTGGTCTGGTA | 20 | 1795 |
| Reverse—TTGACATGGTCCTTTCCGGG | 20 |
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| GADPH-F | CGGCAAGAAGGTTCAGTT |
| GADPH-R | CTCGTTGGTGGTGAAGAC |
| otaA-F | GGATCTTTATGACCGAATCAG |
| otaA-R | CCTTGACCTGAAGAATGCT |
| otaB-F | ATACCACCAGAGCTCCAAA |
| otaB-R | GAGATGTTCGGTCTGTTCA |
| otaR1-F | GCTTTCAAATCGAATGATTCC |
| otaR1-R | GATCGGTTGGAAGTGTAGAA |
| AwHog1-F | GGTCGCCGTCAAAAAGATTA |
| AwHog1-R | CAGCTCGGTCACGAAGTAGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, F.; Pei, J.; Yan, H.; Wang, Y. The AwHog1 Transcription Factor Influences the Osmotic Stress Response, Mycelium Growth, OTA Production, and Pathogenicity in Aspergillus westerdijkiae fc-1. Toxins 2023, 15, 432. https://doi.org/10.3390/toxins15070432
Wang Y, Liu F, Pei J, Yan H, Wang Y. The AwHog1 Transcription Factor Influences the Osmotic Stress Response, Mycelium Growth, OTA Production, and Pathogenicity in Aspergillus westerdijkiae fc-1. Toxins. 2023; 15(7):432. https://doi.org/10.3390/toxins15070432
Chicago/Turabian StyleWang, Yufei, Fei Liu, Jingying Pei, Hao Yan, and Yan Wang. 2023. "The AwHog1 Transcription Factor Influences the Osmotic Stress Response, Mycelium Growth, OTA Production, and Pathogenicity in Aspergillus westerdijkiae fc-1" Toxins 15, no. 7: 432. https://doi.org/10.3390/toxins15070432
APA StyleWang, Y., Liu, F., Pei, J., Yan, H., & Wang, Y. (2023). The AwHog1 Transcription Factor Influences the Osmotic Stress Response, Mycelium Growth, OTA Production, and Pathogenicity in Aspergillus westerdijkiae fc-1. Toxins, 15(7), 432. https://doi.org/10.3390/toxins15070432





