Intersexual Differences in the Gene Expression of Phoneutria depilata (Araneae, Ctenidae) Toxins Revealed by Venom Gland Transcriptome Analyses
Abstract
1. Introduction
2. Results
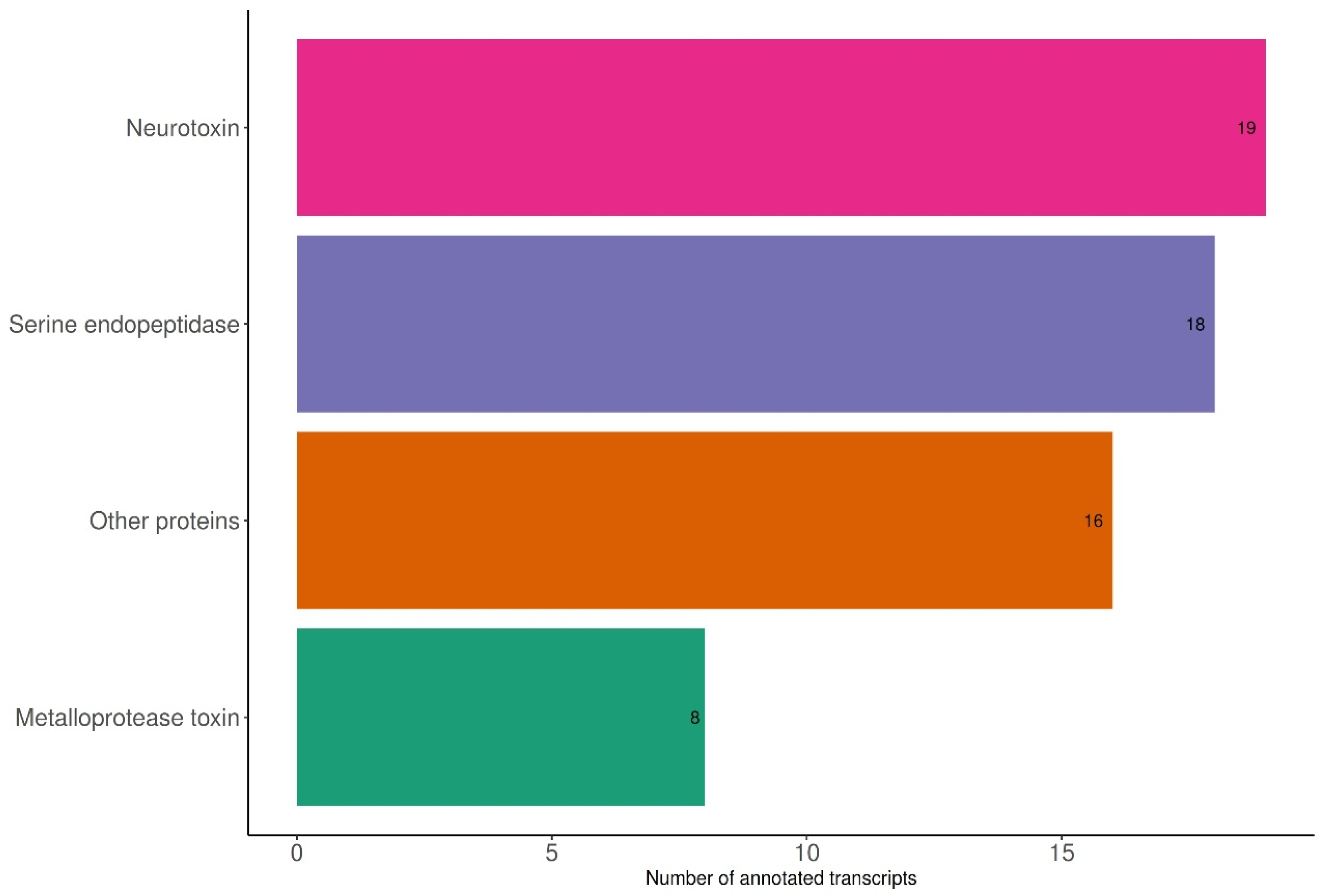
2.1. Transcriptome Sequencing, De Novo Assembly, and Transcript Analysis
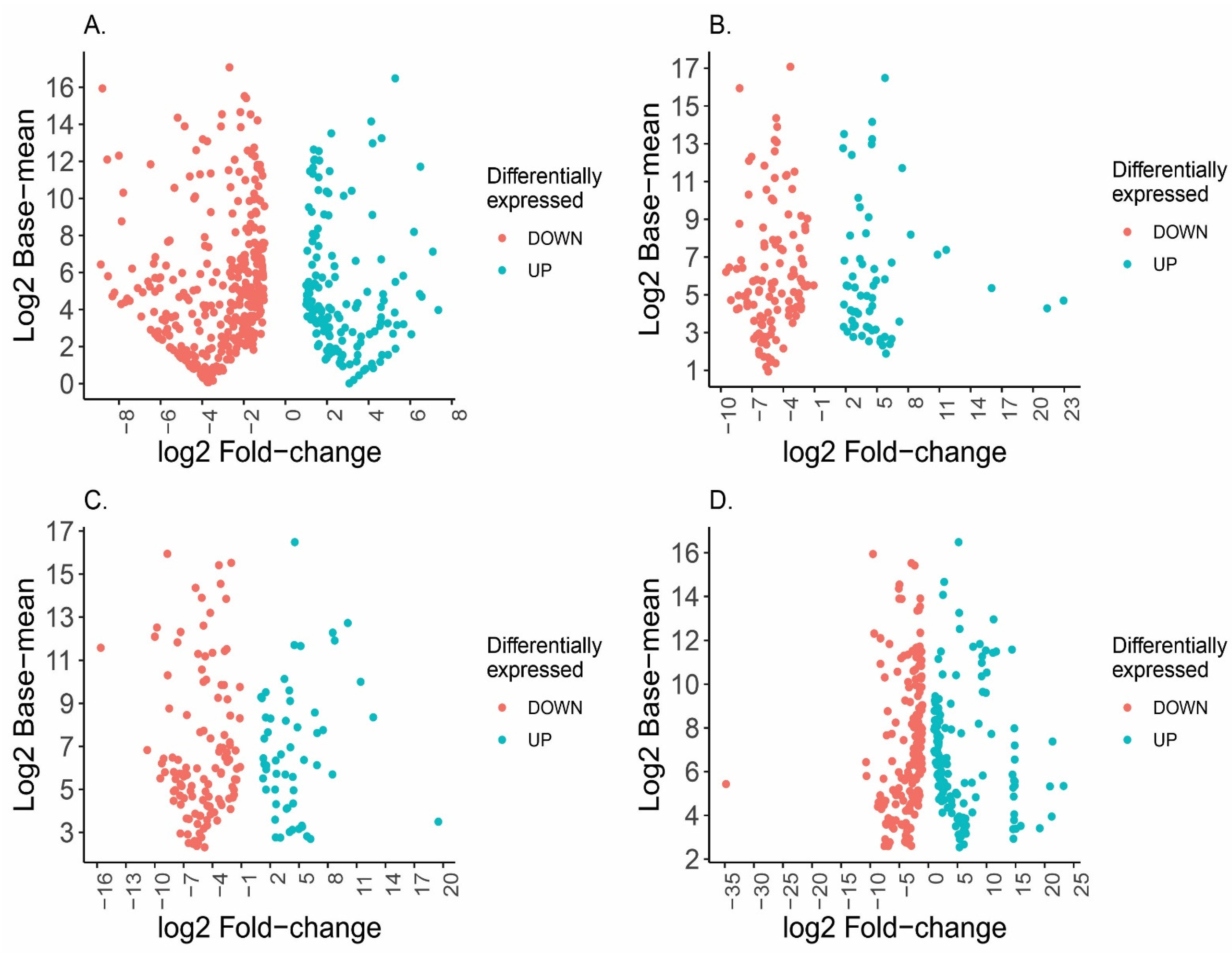
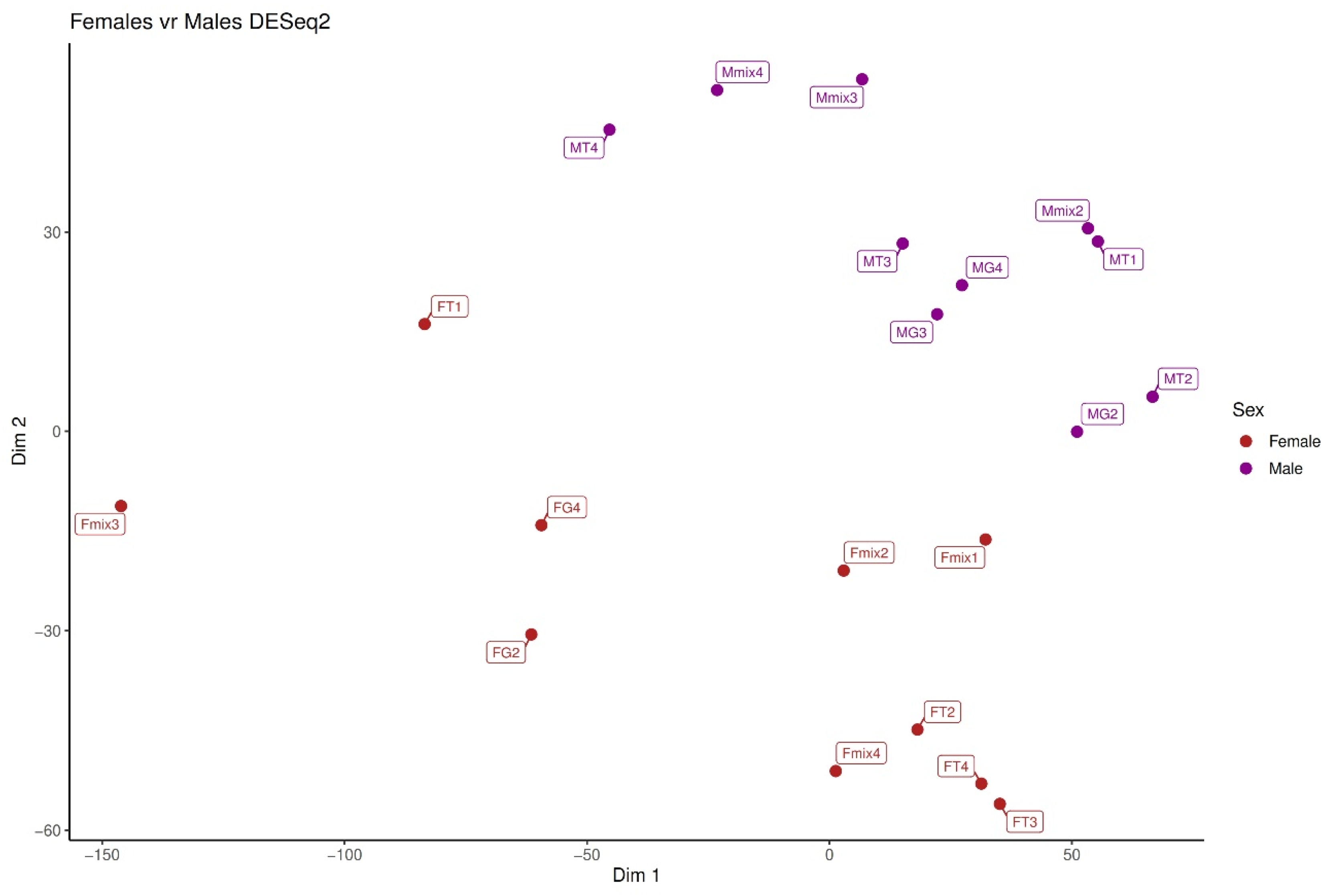
2.2. Differential Expression
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Collection and Differential Diet Experiment
5.2. RNA Extraction, Library Construction, and Sequencing
5.3. De Novo Transcriptome Assembly
5.4. Clustering and Functional Annotation of Transcripts
5.5. Differential Expression Analysis
5.6. Categorization of Transcripts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DESeq | Differential gene expression analysis |
| DETs | Differentially expressed transcripts |
| DIET | Design including only diet |
| NCBI | National Center for Biotechnology Information |
References
- Arbuckle, K. Special Issue: Evolutionary Ecology of Venom. Toxins 2021, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.; Belov, K. Venom evolution through gene duplications. Gene 2012, 496, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Niermann, C.N.; Tate, T.G.; Suto, A.L.; Barajas, R.; White, H.A.; Guswiler, O.D.; Secor, S.M.; Rowe, A.H.; Rowe, M.P. Defensive Venoms: Is Pain Sufficient for Predator Deterrence? Toxins 2020, 12, 260. [Google Scholar] [CrossRef]
- Sunagar, K.; Moran, Y. The Rise and Fall of an Evolutionary Innovation: Contrasting Strategies of Venom Evolution in Ancient and Young Animals. PLoS Genet. 2015, 11, e1005596. [Google Scholar] [CrossRef]
- Zancolli, G.; Reijnders, M.; Waterhouse, R.M.; Robinson-Rechavi, M. Convergent evolution of venom gland transcriptomes across Metazoa. Proc. Natl. Acad. Sci. USA 2022, 119, e2111392119. [Google Scholar] [CrossRef]
- Catalog, W.S. World Spider Catalog, Version 23.5; Natural History Museum Bern: Bern, Switzerland, 2022. [Google Scholar]
- Lüddecke, T.; Herzig, V.; von Reumont, B.M.; Vilcinskas, A. The biology and evolution of spider venoms. Biol. Rev. Camb. Philos. Soc. 2022, 97, 163–178. [Google Scholar] [CrossRef]
- Betz, L.; Tscharntke, T. Enhancing spider families and spider webs in Indian rice fields for conservation biological control, considering local and landscape management. J. Insect Conserv. 2017, 21, 495–508. [Google Scholar] [CrossRef]
- Michalko, R.; Pekár, S.; Entling, M.H. An updated perspective on spiders as generalist predators in biological control. Oecologia 2019, 189, 21–36. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Pekár, S.; Toft, S. Trophic specialisation in a predatory group: The case of prey-specialised spiders (Araneae). Biol. Rev. Camb. Philos. Soc. 2015, 90, 744–761. [Google Scholar] [CrossRef]
- Nyffeler, M.; Gibbons, J.W. Spiders (Arachnida: Araneae) feeding on snakes (Reptilia: Squamata). J. Arachnol. 2021, 49, 1–27. [Google Scholar] [CrossRef]
- Nyffeler, M.; Gibbons, J.W. Spiders feeding on vertebrates is more common and widespread than previously thought, geographically and taxonomically. J. Arachnol. 2022, 50, 121–134. [Google Scholar] [CrossRef]
- Nyffeler, M.; Olson, E.J.; Symondson, W.O. Plant-eating by spiders. J. Arachnol. 2016, 44, 15–27. [Google Scholar] [CrossRef]
- Valdez, J.W. Arthropods as vertebrate predators: A review of global patterns. Glob. Ecol. Biogeogr. 2020, 29, 1691–1703. [Google Scholar] [CrossRef]
- Cooper, A.M.; Nelsen, D.R.; Hayes, W.K.J.E.V.A.T.T. The strategic use of venom by spiders. In Evolution of Venomous Animals and Their Toxins; Springer: Dordrecht, The Netherlands, 2015; pp. 1–18. [Google Scholar]
- Pineda, S.S.; Chin, Y.K.; Undheim, E.A.B.; Senff, S.; Mobli, M.; Dauly, C.; Escoubas, P.; Nicholson, G.M.; Kaas, Q.; Guo, S.; et al. Structural venomics reveals evolution of a complex venom by duplication and diversification of an ancient peptide-encoding gene. Proc. Natl. Acad. Sci. USA 2020, 117, 11399–11408. [Google Scholar] [CrossRef] [PubMed]
- Davies, E.L.; Arbuckle, K. Coevolution of Snake Venom Toxic Activities and Diet: Evidence that Ecological Generalism Favours Toxicological Diversity. Toxins 2019, 11, 711. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.; Dugon, M.M.; Healy, K. Diet Breadth Mediates the Prey Specificity of Venom Potency in Snakes. Toxins 2020, 12, 74. [Google Scholar] [CrossRef]
- Saez, N.J.; Herzig, V. Versatile spider venom peptides and their medical and agricultural applications. Toxicon Off. J. Int. Soc. Toxinology 2019, 158, 109–126. [Google Scholar] [CrossRef]
- Wu, T.; Wang, M.; Wu, W.; Luo, Q.; Jiang, L.; Tao, H.; Deng, M. Spider venom peptides as potential drug candidates due to their anticancer and antinociceptive activities. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e146318. [Google Scholar] [CrossRef] [PubMed]
- King, G.F.; Gentz, M.C.; Escoubas, P.; Nicholson, G.M. A rational nomenclature for naming peptide toxins from spiders and other venomous animals. Toxicon Off. J. Int. Soc. Toxinology 2008, 52, 264–276. [Google Scholar] [CrossRef] [PubMed]
- De Roodt, A.R.; Lanari, L.C.; Laskowicz, R.D.; Costa de Oliveira, V.; Irazu, L.E.; González, A.; Giambelluca, L.; Nicolai, N.; Barragán, J.H.; Ramallo, L.; et al. Toxicity of the venom of Latrodectus (Araneae: Theridiidae) spiders from different regions of Argentina and neutralization by therapeutic antivenoms. Toxicon Off. J. Int. Soc. Toxinology 2017, 130, 63–72. [Google Scholar] [CrossRef]
- Zobel-Thropp, P.A.; Bodner, M.R.; Binford, G.J. Comparative analyses of venoms from American and African Sicarius spiders that differ in sphingomyelinase D activity. Toxicon Off. J. Int. Soc. Toxinology 2010, 55, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Duran, L.H.; Rymer, T.L.; Wilson, D.T. Variation in venom composition in the Australian funnel-web spiders Hadronyche valida. Toxicon X 2020, 8, 100063. [Google Scholar] [CrossRef]
- Gonçalves de Andrade, R.M.; De Oliveira, K.C.; Giusti, A.L.; Dias da Silva, W.; Tambourgi, D.V. Ontogenetic development of Loxosceles intermedia spider venom. Toxicon Off. J. Int. Soc. Toxinology 1999, 37, 627–632. [Google Scholar] [CrossRef]
- Herzig, V.; Ward, R.J.; Dos Santos, W.F. Ontogenetic changes in Phoneutria nigriventer (Araneae, Ctenidae) spider venom. Toxicon Off. J. Int. Soc. Toxinology 2004, 44, 635–640. [Google Scholar] [CrossRef]
- Santana, R.C.; Perez, D.; Dobson, J.; Panagides, N.; Raven, R.J.; Nouwens, A.; Jones, A.; King, G.F.; Fry, B.G. Venom Profiling of a Population of the Theraphosid Spider Phlogius crassipes Reveals Continuous Ontogenetic Changes from Juveniles through Adulthood. Toxins 2017, 9, 116. [Google Scholar] [CrossRef]
- Binford, G.J. An analysis of geographic and intersexual chemical variation in venoms of the spider Tegenaria agrestis (Agelenidae). Toxicon Off. J. Int. Soc. Toxinology 2001, 39, 955–968. [Google Scholar] [CrossRef]
- Binford, G.J.; Gillespie, R.G.; Maddison, W.P. Sexual dimorphism in venom chemistry in Tetragnatha spiders is not easily explained by adult niche differences. Toxicon Off. J. Int. Soc. Toxinology 2016, 114, 45–52. [Google Scholar] [CrossRef]
- Herzig, V.; John Ward, R.; Ferreira dos Santos, W. Intersexual variations in the venom of the Brazilian ‘armed’ spider Phoneutria nigriventer (Keyserling, 1891). Toxicon Off. J. Int. Soc. Toxinology 2002, 40, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Michálek, O.; Kuhn-Nentwig, L.; Pekár, S. High Specific Efficiency of Venom of Two Prey-Specialized Spiders. Toxins 2019, 11, 687. [Google Scholar] [CrossRef] [PubMed]
- Pekár, S.; Toft, S.; Hrusková, M.; Mayntz, D. Dietary and prey-capture adaptations by which Zodarion germanicum, an ant-eating spider (Araneae: Zodariidae), specialises on the Formicinae. Die Nat. 2008, 95, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Garb, J.E.; Hayashi, C.Y. Molecular evolution of α-latrotoxin, the exceptionally potent vertebrate neurotoxin in black widow spider venom. Mol. Biol. Evol. 2013, 30, 999–1014. [Google Scholar] [CrossRef]
- Thill, V.L.; Moniz, H.A.; Teglas, M.B.; Wasley, M.J.; Feldman, C.R. Preying dangerously: Black widow spider venom resistance in sympatric lizards. R. Soc. Open Sci. 2022, 9, 221012. [Google Scholar] [CrossRef] [PubMed]
- Pekár, S.; Michalko, R.; Korenko, S.; Sedo, O.; Líznarová, E.; Sentenská, L.; Zdráhal, Z. Phenotypic integration in a series of trophic traits: Tracing the evolution of myrmecophagy in spiders (Araneae). Zoology 2013, 116, 27–35. [Google Scholar] [CrossRef]
- Hazzi, N.A.; Hormiga, G. Morphological and molecular evidence support the taxonomic separation of the medically important Neotropical spiders Phoneutria depilata (Strand, 1909) and P. boliviensis (F.O. Pickard-Cambridge, 1897) (Araneae, Ctenidae). ZooKeys 2021, 1022, 13–50. [Google Scholar] [CrossRef]
- Lucas, S.M.; Meier, J. Biology and distribution of spiders of medical importance. In Handbook of: Clinical Toxicology of Animal Venoms and Poisons; CRC Press: Boca Raton, FL, USA, 2017; pp. 239–258. [Google Scholar]
- Hazzi, N.A. Natural history of Phoneutria boliviensis (Araneae: Ctenidae): Habitats, reproductive behavior, postembryonic development and prey-wrapping. J. Arachnol. 2014, 42, 303–310. [Google Scholar] [CrossRef]
- Martins, R.; Bertani, R. The non-Amazonian species of the Brazilian wandering spiders of the genus Phoneutria Perty, 1833 (Araneae: Ctenidae), with the description of a new species. J. Zootaxa 2007, 1526, 1–36. [Google Scholar] [CrossRef]
- Valenzuela-Rojas, J.C.; González-Gómez, J.C.; van der Meijden, A.; Cortés, J.N.; Guevara, G.; Franco, L.M.; Pekár, S.; García, L.F. Prey and Venom Efficacy of Male and Female Wandering Spider, Phoneutria boliviensis (Araneae: Ctenidae). Toxins 2019, 11, 622. [Google Scholar] [CrossRef]
- Sierra Ramírez, D.; Guevara, G.; Franco Pérez, L.M.; van der Meijden, A.; González-Gómez, J.C.; Carlos Valenzuela-Rojas, J.; Prada Quiroga, C.F. Deciphering the diet of a wandering spider (Phoneutria boliviensis; Araneae: Ctenidae) by DNA metabarcoding of gut contents. Ecol. Evol. 2021, 11, 5950–5965. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, B.; Xiao, Z.; Zhou, X.; Liu, Z. Transcriptomic Analysis of the Spider Venom Gland Reveals Venom Diversity and Species Consanguinity. Toxins 2019, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.L.B.; Mudadu, M.A.; Pereira, E.H.T.; Marri, C.A.; Guerra-Duarte, C.; Diniz, M.R.V. Transcriptome analysis of the spider Phoneutria pertyi venom glands reveals novel venom components for the genus Phoneutria. Toxicon Off. J. Int. Soc. Toxinology 2019, 163, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Vásquez-Escobar, J.; Romero-Gutiérrez, T.; Morales, J.A.; Clement, H.C.; Corzo, G.A.; Benjumea, D.M.; Corrales-García, L.L. Transcriptomic Analysis of the Venom Gland and Enzymatic Characterization of the Venom of Phoneutria depilata (Ctenidae) from Colombia. Toxins 2022, 14, 295. [Google Scholar] [CrossRef]
- Gangur, A.N.; Smout, M.; Liddell, M.J.; Seymour, J.E.; Wilson, D.; Northfield, T.D. Changes in predator exposure, but not in diet, induce phenotypic plasticity in scorpion venom. Proc. Biol. Sci. 2017, 284, 20171364. [Google Scholar] [CrossRef]
- Pucca, M.B.; Amorim, F.G.; Cerni, F.A.; Bordon Kde, C.; Cardoso, I.A.; Anjolette, F.A.; Arantes, E.C. Influence of post-starvation extraction time and prey-specific diet in Tityus serrulatus scorpion venom composition and hyaluronidase activity. Toxicon Off. J. Int. Soc. Toxinology 2014, 90, 326–336. [Google Scholar] [CrossRef]
- McElroy, T.; McReynolds, C.N.; Gulledge, A.; Knight, K.R.; Smith, W.E.; Albrecht, E.A. Differential toxicity and venom gland gene expression in Centruroides vittatus. PLoS ONE 2017, 12, e0184695. [Google Scholar] [CrossRef]
- Herzig, V.; Hodgson, W.C. Intersexual variations in the pharmacological properties of Coremiocnemis tropix (Araneae, Theraphosidae) spider venom. Toxicon Off. J. Int. Soc. Toxinology 2009, 53, 196–205. [Google Scholar] [CrossRef]
- Wilder, S.M.; Simpson, S.J.J.F.W. A vertebrate, the fence skink, is a common but relatively low-quality prey for an invertebrate predator, the redback spider. Food Webs 2022, 32, e00236. [Google Scholar] [CrossRef]
- Cordeiro Mdo, N.; de Figueiredo, S.G.; Valentim Ado, C.; Diniz, C.R.; von Eickstedt, V.R.; Gilroy, J.; Richardson, M. Purification and amino acid sequences of six Tx3 type neurotoxins from the venom of the Brazilian ‘armed’ spider Phoneutria nigriventer (Keys). Toxicon Off. J. Int. Soc. Toxinology 1993, 31, 35–42. [Google Scholar] [CrossRef]
- Diniz, M.R.V.; Paiva, A.L.B.; Guerra-Duarte, C.; Nishiyama, M.Y., Jr.; Mudadu, M.A.; Oliveira, U.; Borges, M.H.; Yates, J.R.; Junqueira-de-Azevedo, I.L. An overview of Phoneutria nigriventer spider venom using combined transcriptomic and proteomic approaches. PLoS ONE 2018, 13, e0200628. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Costa-Gonçalves, A.; Lanza, L.F.; Cortes, S.F.; Cordeiro, M.N.; Richardson, M.; Pimenta, A.M.; Webb, R.C.; Leite, R.; De Lima, M.E. Tx2-6 toxin of the Phoneutria nigriventer spider potentiates rat erectile function. Toxicon Off. J. Int. Soc. Toxinology 2008, 51, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, S.G.; de Lima, M.E.; Nascimento Cordeiro, M.; Diniz, C.R.; Patten, D.; Halliwell, R.F.; Gilroy, J.; Richardson, M. Purification and amino acid sequence of a highly insecticidal toxin from the venom of the brazilian spider Phoneutria nigriventer which inhibits NMDA-evoked currents in rat hippocampal neurones. Toxicon Off. J. Int. Soc. Toxinology 2001, 39, 309–317. [Google Scholar] [CrossRef]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN analysis of metagenomic data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef]
- Winter, D.J. rentrez: An R package for the NCBI eUtils API. R J. 2017, 9, 520–526. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Boutet, E.; Lieberherr, D.; Tognolli, M.; Schneider, M.; Bairoch, A. UniProtKB/Swiss-Prot. Methods Mol. Biol. 2007, 406, 89–112. [Google Scholar]
- Karuppasamy, M.P.; Venkateswaran, S.; Subbiah, P. PDB-2-PBv3.0: An updated protein block database. J. Bioinform. Comput. Biol. 2020, 18, 2050009. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, D.S.; Alzate, J.F.; Simone, Y.; van der Meijden, A.; Guevara, G.; Franco Pérez, L.M.; González-Gómez, J.C.; Prada Quiroga, C.F. Intersexual Differences in the Gene Expression of Phoneutria depilata (Araneae, Ctenidae) Toxins Revealed by Venom Gland Transcriptome Analyses. Toxins 2023, 15, 429. https://doi.org/10.3390/toxins15070429
Ramírez DS, Alzate JF, Simone Y, van der Meijden A, Guevara G, Franco Pérez LM, González-Gómez JC, Prada Quiroga CF. Intersexual Differences in the Gene Expression of Phoneutria depilata (Araneae, Ctenidae) Toxins Revealed by Venom Gland Transcriptome Analyses. Toxins. 2023; 15(7):429. https://doi.org/10.3390/toxins15070429
Chicago/Turabian StyleRamírez, Diego Sierra, Juan F. Alzate, Yuri Simone, Arie van der Meijden, Giovany Guevara, Lida Marcela Franco Pérez, Julio César González-Gómez, and Carlos F. Prada Quiroga. 2023. "Intersexual Differences in the Gene Expression of Phoneutria depilata (Araneae, Ctenidae) Toxins Revealed by Venom Gland Transcriptome Analyses" Toxins 15, no. 7: 429. https://doi.org/10.3390/toxins15070429
APA StyleRamírez, D. S., Alzate, J. F., Simone, Y., van der Meijden, A., Guevara, G., Franco Pérez, L. M., González-Gómez, J. C., & Prada Quiroga, C. F. (2023). Intersexual Differences in the Gene Expression of Phoneutria depilata (Araneae, Ctenidae) Toxins Revealed by Venom Gland Transcriptome Analyses. Toxins, 15(7), 429. https://doi.org/10.3390/toxins15070429




