Abstract
Using alternative feed ingredients in pig diets can lead to deoxynivalenol (DON) contamination. DON has been shown to induce anorexia, inflammation, and—more recently—alterations in the vitamin D, calcium, and phosphorus metabolisms. Adding vitamin D supplementation in the form of vitamin D3 and 25-OH-D3 to the feed could modify the effects of DON in piglets. In this study, vitamin D3 or 25-OH-D3 supplementation was used in a control or DON-contaminated treatment. A repetitive exposure over 21 days to DON in the piglets led to disruptions in the vitamin D, calcium, and phosphorus metabolisms, resulting in a decreased growth performance, increased bone mineralization, and the downregulation of genes related to calcium and to phosphorus intestinal and renal absorption. The DON challenge also decreased blood concentrations of 25-OH-D3, 1,25-(OH)2-D3, and phosphate. The DON contamination likely decreased the piglets’ vitamin D status indirectly by modifying the calcium metabolism response. Vitamin D supplementations did not restore vitamin D status or bone mineralization. After a lipopolysaccharide-induced inflammatory stimulation, feeding a 25-OH-D3 supplementation increased 25-OH-D3 concentration and 1,25-(OH)2-D3 regulations during the DON challenge. DON contamination likely induced a Ca afflux by altering the intestinal barrier, which resulted in hypercalcemia and hypovitaminosis D. The vitamin D supplementation could increase the calcitriol production to face the combined LPS and DON challenge.
Keywords:
piglets; deoxynivalenol; vitamin D; mineralization; calcium; phosphorus; lipopolysaccharides Key Contribution:
Deoxynivalenol (DON) contamination in pig feed ingredients can disrupt vitamin D, calcium, and phosphorus metabolism, thus leading to a decreased growth performance, increased bone mineralization, and a downregulation in the expression of genes related to Ca and P intestinal absorption and renal reabsorption. However, adding vitamin D supplementation during DON challenge, particularly 25-OH-D3, did not mitigate these effects, but it did increase the immune response in piglets facing a lipopolysaccharide challenge.
1. Introduction
In the swine industry, feeding accounts for more than 60% of the production cost. Moreover, it has significantly fluctuated over the last few years, making the production hardly profitable [1]. In this context, reducing feeding costs remains a challenge. One option is to diversify raw materials by adding by-products and low graded grain [2]. However, this type of diversification often leads to the use of ingredients contaminated with mycotoxins. The most common mycotoxin detected in swine diets is deoxynivalenol (DON), which is produced by the Fusarium fungus [3,4]. This secondary metabolite, part of the trichothecene group, is found in wheat, barley, oats, millet, and corn [5]. It can withstand high temperatures and processing, resulting in its widespread occurrence in animal feed with an occurrence ranging from 96% to 100% [6,7]. The effect of DON on growth performance is well known in pigs [3]. DON contamination is reported to decrease feed intake and leads to reduced growth; in addition, it sometimes leads to complete feed refusal at higher doses (3.0–8.0 mg/kg) [8,9,10], even though the concentration of DON found in feed rarely exceeds the 1000 μg/kg limit [7]. At low doses, DON contamination does not always impact growth performance [11,12]. Pigs are very sensitive to DON as it alters immune function [9], as well as induces oxidative stress [13] and intestinal damage [14]. In addition to these known effects, Le Thanh et al. [8] also observed that DON improved calcium (Ca) retention, as well as reduced Ca and phosphorus (P) excretion in pigs, suggesting a possible DON action on the phosphocalcic metabolism.
Vitamin D is produced by UV light photolytic action on the skin, but it can also enter the body through dietary intake [15]. The resulting vitamin D3 is then hydroxylated at the C-25 position to form 25-hydroxyvitamin D3 (25-OH-D3) in the liver by the action of the enzyme 25-hydroxylase, which is regulated by the CYP2R1 gene. The 25-OH-D3 is then hydroxylated at the C-1 position in the kidney to form 1,25-OH2-D3, which is the active and hormonal form of vitamin D and is also called calcitriol. The latter step is controlled by the enzyme 1α-hydroxylase, which is regulated by the CYP27B1 gene. This active form is responsible for most of the biological actions of vitamin D [16,17]. Calcitriol acts on the intestine to increase Ca and P absorption, and on the bone to further increase bone resorption [18,19]. It also decreases parathyroid hormone (PTH) synthesis by negative feedback control (Figure 1) [20]. Increased 1,25-OH2-D3 and PTH also stimulate fibroblast growth factor 23 (FGF23) production, which inhibit the type 2a and 2c sodium-phosphate cotransporters (SLC34 and SLC20) that are responsible, depending on its coreceptor Klotho, for kidney P reabsorption [19,21]. The TRPV5 and TRPV6 channels—found in the kidney and intestine, respectively—are responsible for the apical influx of the active Ca transport, and their regulation follows the serum 1,25-OH2-D3 concentration [22]. Additionally, PTH, FGF23, Klotho, Ca, and P are regulators of the synthesis and activity of 1,25-OH2-D3 [15,23]. It was observed that DON contamination occurred only at a daily dose of 10 mg/kg administered (body weight) perorally for 7 days, and that this decreased 25-OH-D3 concentration in the blood and 25-hydroxylase activity in the liver of rats [24]. Vitamin D also shows immunoregulatory functions that are capable of reducing the inflammatory response [16,25]. Since DON can also induce inflammation and oxidative stress [3,5], adding vitamin D to piglets’ diet may be protective. Moreover, vitamin D supplementation could prevent growth depression [26] and vitamin D deficiency in piglets [27,28].
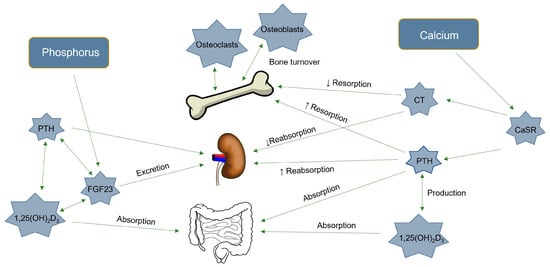
Figure 1.
The calcium and phosphorus metabolism, and the main hormones implicated in the phosphocalcic regulations. CaSR: calcium sensing receptor, PTH: parathormone, CT: calcitonin, 1,25-OH2D3: calcitriol, and FGF23: Fibroblast growth factor 23.
DON contamination is known to impact the integrity of the intestinal barrier. It increases the paracellular permeability in intestinal porcine cells IPEC-1 at a 30 μmol/L dose [29], and damages the apical structure of tight junction zona occludens-1 in the mid-jejunum of pigs that are fed a 3.1 mg/kg DON-contaminated feed [30]. DON contamination also alters the immune system’s response, leading to reduced resistance to pathogen-associated molecular patterns such as lipopolysaccharides (LPS) [31], which also act on the intestinal barrier. The LPS produced by Gram-negative bacteria are commonly found in livestock environments or directly in pigs’ intestinal microbiota [32], and they induce a systemic inflammatory response at low doses [33]. Thus, LPS and DON could interact together to further increase intestinal permeability and bacterial translocation [34], leading to an amplified immune response [35].
The aim of this experiment was to investigate the effect of dietary DON and vitamin D3 supplementation on growth performance, bone mineral content (BMC), blood parameters (including calcium, phosphate, magnesium, 25-OH-D3, and 1,25-OH2-D3), the gene expressions related to the intestinal absorption and renal reabsorption of Ca and P, as well as vitamin D metabolism. Additionally, the study aimed to evaluate the effect of vitamin D supplementation on the same parameters in piglets that are challenged with LPS and fed with a DON-contaminated diet.
2. Results
2.1. Growth Performance, Bone Mineralization
It is worth mentioning that the total Ca was higher than expected in the experimental diet after analysis of the feed composition (Table A1), especially in the DON diets. It is possible that a higher dietary supply of Ca influenced the response of DON on the Ca and vitamin D metabolisms. No interaction between the DON contamination and vitamin D supplementation was observed for growth performance or bone mineralization. The ADG, the ADFI, and the final body weight (BW) were lower in the piglets fed DON-contaminated feed (p < 0.001; Table 1), while the G:F was not affected. The BMC was reduced for the piglets fed DON-contaminated feed compared to the CON (p < 0.001), while the percentage for the BMC/kg of BW was higher for piglets fed the DON-contaminated feed compared to the CON (p < 0.001). Vitamin D supplementation did not impact the growth performance or bone mineralization.

Table 1.
Impact of DON contamination supplementation on growth performances and bone mineralization after a repetitive exposure over 21 days 1.
2.2. Blood Parameters
Piglets unchallenged with LPS and fed DON-contaminated diets had higher serum DON and DOM-1 concentrations than the CON piglets (p < 0.001; Table 2), and High25-OH-D3 supplementation tended to reduce DOM-1 concentration (DON × VitD; p = 0.09). Serum Ca and Mg were not modified by the DON contamination or vitamin D supplementation in piglets that were or were not injected with LPS. DON contamination decreased the plasma concentration of 25-OH-D3 (p < 0.05; Figure 2b), phosphate (p < 0.01; Figure 2a), and the serum concentration of 1,25-(OH)2-D3 (p < 0.01; Figure 2c). The same results were observed in piglets challenged with the LPS for 25-OH-D3 (p < 0.05), phosphate (p < 0.001), and 1,25-(OH)2-D3 (p < 0.05). The High25-OH-D3 treatment tended to prevent a decrease of 25-OH-D3 (DON × VitD; p = 0.09) in the piglets that were fed the DON diet and injected with LPS. There was no interaction between DON contamination and vitamin D supplementation for the serum/plasma concentrations of DON, 1,25-(OH)2-D3, P, Ca, and Mg in piglets that were or were not injected with LPS (Table 2 and Figure 2).

Table 2.
Effect of DON contamination, vitamin D supplementation, and LPS stimulation on blood parameters after a repetitive exposure over 21 days 1.
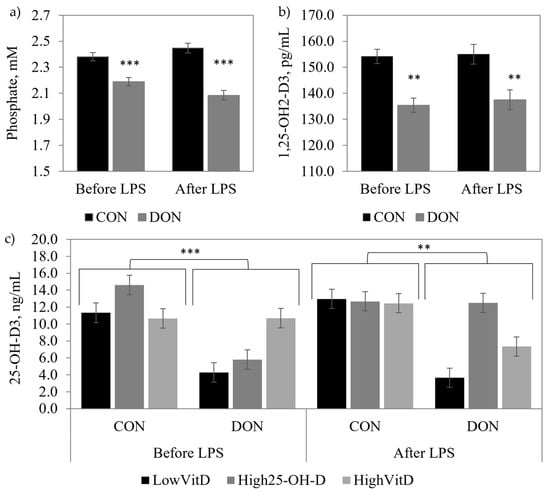
Figure 2.
Effect of DON contamination, vitamin D supplementation, and LPS stimulation on phosphate, 1,25-OH2-D3, and 25-OH-D3 concentrations in the blood of piglets after a repetitive exposure over 21 days. (a) Plasma phosphate before and after LPS stimulation; (b) serum 1,25-OH2-D3 before and after LPS stimulation; and (c) interaction of DON × VitD for plasma 25-OH-D3 before and after LPS stimulation. DON contamination was obtained from naturally contaminated wheat (4.9 mg/kg). The vitamin D supplementations were low vitamin D3 (LowVitD, 200 UI vitamin D3/kg), high vitamin D3 (HighVitD, 2200 UI vitamin D3/kg), or high 25-hydroxyvitamin D3 (High25-OH-D3, 2000 UI in the form of 25(OH)D3/kg [0.05 mg/kg]). One piglet per pen received intraperitoneal LPS injection (20 µg/kg BW (20 µg/kg). CON: control, DON: deoxynivalenol, LPS: lipopolysaccharide, and VitD: vitamin D3, 25-OH-D3: 25-hydroxyl-Vitamin D3. The data are reported as the mean ± SEM. ** p < 0.05; *** p < 0.01.
2.3. Gene Expression
In piglets not injected with LPS, the DON contamination decreased the jejunal VDR gene expression in piglets with HighVitD and High25-OH-D3 supplementations, but it increased LowVitD (DON × VitD; Figure 3A; p < 0.05). Piglets injected with LPS and that were receiving DON-contaminated diets had lower VDR (p < 0.01; Figure 3B) gene expression in the liver compared to the CON piglets. Additionally, DON contamination decreased the renal VDR gene expression in piglets injected with LPS and that were receiving LowVitD and HighVitD. Meanwhile, in piglets fed High25-OH-D3, the opposite results were observed (DON × VitD; Figure 3B; p < 0.05).
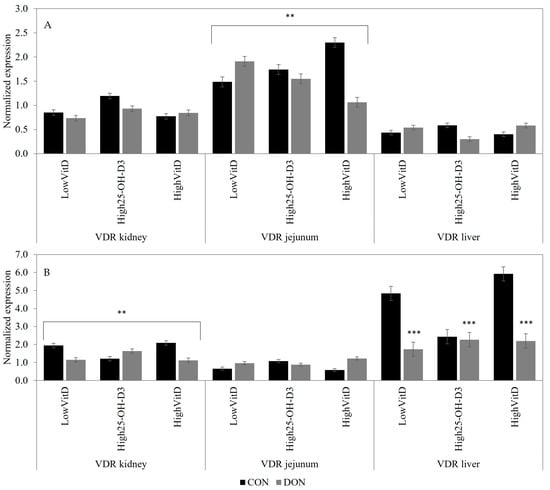
Figure 3.
Effect of DON contamination, vitamin D supplementation, and LPS stimulation on the vitamin D receptor (VDR) gene expression in the kidney, jejunum, and liver after a repetitive exposure over 21 days. (A) VDR gene expression before LPS stimulation; VDR in jejunum showed an interaction between DON × VitD. (B) VDR gene expression after LPS stimulation; VDR in kidney showed an interaction between DON × VitD. VDR in liver was analyzed with a Poisson adjustment. DON contamination was obtained from naturally contaminated wheat (4.9 mg/kg). The vitamin D supplementations were low vitamin D3 (LowVitD, 200 UI vitamin D3/kg), high vitamin D3 (HighVitD, 2200 UI vitamin D3/kg), or high 25-hydroxyvitamin D3 (High25-OH-D3, 2000 UI in the form of 25(OH)D3/kg [0.05 mg/kg]). One piglet per pen received intraperitoneal LPS injection (20 µg/kg BW (20 µg/kg). CON: control, DON: deoxynivalenol, LPS: lipopolysaccharide, and VitD: vitamin D3, 25-OH-D3: 25-hydroxyl-Vitamin D3. The data are reported as the mean ± SEM. ** p < 0.05; *** p < 0.01.
In the jejunum tissue, the DON contamination downregulated the SLC8A1 (p < 0.05) and SLC20A2 (p = 0.05) gene expression compared to the CON group when the piglets did not receive LPS injection (Figure 4A). In unchallenged LPS piglets, the DON contamination decreased jejunal Klotho gene expression, but the reduction was more pronounced in piglets fed with HighVitD3 and High25-OH-D3 supplementation when compared to LowVitD3 (DON × VitD; Figure 4A; p < 0.05). In piglets receiving LPS injection, the DON contamination tended to increase the jejunal SLC20A2 gene expression in piglets fed LowVitD3 diets (DON × VitD; Figure 4B; p = 0.10). In piglets receiving LPS injection, the High25-OH-D3 supplementation in CON diets also increased the jejunal TRPV6 gene expression compared to LowVitD3 and HighVitD3, but in piglets fed DON-contaminated feed, the opposite result was observed (DON × VitD; Figure 4B; p = 0.05).
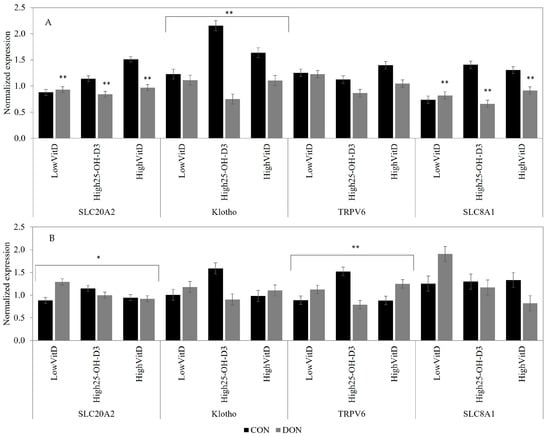
Figure 4.
Effect of DON contamination, vitamin D supplementation, and LPS stimulation on gene expression in the jejunum after a repetitive exposure over 21 days. (A) Gene expression before LPS stimulation where Klotho showed an interaction between DON × VitD. (B) Gene expression after LPS stimulation where the SLC20A2 and TRPV6 showed an interaction between DON × VitD. SLC8A1 was analyzed with a Poisson adjustment. DON contamination was obtained from naturally contaminated wheat (4.9 mg/kg). The vitamin D supplementations were low vitamin D3 (LowVitD, 200 UI vitamin D3/kg), high vitamin D3 (HighVitD, 2200 UI vitamin D3/kg), or high 25-hydroxyvitamin D3 (High25-OH-D3, 2000 UI in the form of 25(OH)D3/kg [0.05 mg/kg]). One piglet per pen received intraperitoneal LPS injection (20 µg/kg BW (20 µg/kg). CON: control, DON: deoxynivalenol, LPS: lipopolysaccharide, VitD: vitamin D3, 25-OH-D3: 25-hydroxyl-Vitamin D3., SLC20A2: sodium-dependent phosphate transporter 2, Klotho: fibroblast growth factor 23 coreceptor, SLC8A: sodium/calcium exchanger 1, and TRPV6: transient receptor potential vanilloid 6. The data are reported as the mean ± SEM. * p < 0.10; ** p < 0.05.
In the liver, only CYP2R1 was evaluated, and its expression was reduced by the DON contamination in piglets injected with LPS (p < 0.05; Figure 5A). In the kidney, the DON contamination tended to downregulate the CYP27B1 gene expression in piglets not receiving LPS injection (p = 0.10; Figure 5A). In piglets stimulated with an LPS injection, the CYB27B1 gene expression was higher in LowVitD3 and HighVitD3 than HighVit25-OH-D3 in the CON diet, but in the DON-contaminated diets, the opposite result was observed (DON × VitD; Figure 5B; p = 0.04). DON contamination reduced the renal Klotho gene expression in piglets not receiving LPS injection (p < 0.01; Figure 5A). Klotho gene expression was also downregulated by the DON contamination in piglets injected with LPS, but this effect tended to be reduced in piglets fed a High25-OH-D3-supplemented diet (DON × VitD; Figure 5B; p = 0.08).
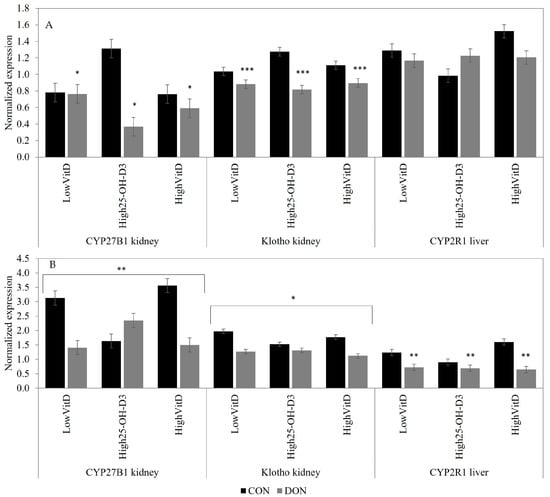
Figure 5.
Effect of DON contamination, vitamin D supplementation, and LPS stimulation on the P- and vitamin-D-related gene expression in the kidney and liver after a repetitive exposure over 21 days. (A) Gene expression before LPS stimulation. (B) Gene expression after LPS stimulation where the renal CYP27B1 and Klotho expressions showed an interaction between DON × VitD. CYP27B1 was analyzed with a Poisson adjustment. The DON contamination was obtained from naturally contaminated wheat (4.9 mg/kg). The vitamin D supplementations were low vitamin D3 (LowVitD, 200 UI vitamin D3/kg), high vitamin D3 (HighVitD, 2200 UI vitamin D3/kg), or high 25-hydroxyvitamin D3 (High25-OH-D3, 2000 UI in the form of 25(OH)D3/kg [0.05 mg/kg]). One piglet per pen received intraperitoneal LPS injection (20 µg/kg BW (20 µg/kg). CON: control, DON: deoxynivalenol, LPS: lipopolysaccharide, VitD: vitamin D3, 25-OH-D3: 25-hydroxyl-Vitamin D3, CYP27B1: 1α-Hydroxylase, Klotho: fibroblast growth factor 23 coreceptor, and CYP2R1: 25-Hydroxylase. The data are reported as mean ± SEM. * p < 0.10; ** p < 0.05; *** p < 0.01.
In piglets not stimulated by LPS, the DON contamination downregulated the SLC8A1 (p < 0.05), CALB1 (p < 0.05), S100G (p < 0.01), and TRPV5 (p = 0.06) gene expressions in the kidney (Figure 6A). When stimulating the LPS condition, piglets fed DON-contaminated feed had lower SLC8A1 (p < 0.01) and tended to have lower CALB1 (p = 0.10) gene expression when compared to the CON group (Figure 6B). Piglets receiving LowVitD3 and HighVitD3 supplementation tended to have a higher S100G gene expression than High25-OH-D3 in piglets fed a CON diet, but an opposite effect was observed in piglets fed a DON-contaminated diet (DON × VitD; Figure 6B; p = 0.10). Vitamin D supplementation did not impact the expression of Ca, p, and vitamin-D-related genes in the kidney.
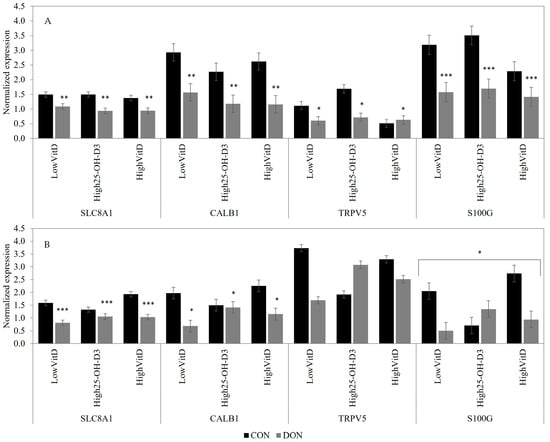
Figure 6.
Effect of DON contamination, vitamin D supplementation, and LPS stimulation on Ca-related gene expression in the kidney after a repetitive exposure over 21 days. (A) Gene expression before LPS stimulation. (B) Gene expression after LPS stimulation where S100G tended to show an interaction between DON × VitD. CALB1 and S100G were analyzed with a Poisson adjustment. DON contamination was obtained from naturally contaminated wheat (4.9 mg/kg). The vitamin D supplementations were low vitamin D3 (LowVitD, 200 UI vitamin D3/kg), high vitamin D3 (HighVitD, 2200 UI vitamin D3/kg), or high 25-hydroxyvitamin D3 (High25-OH-D3, 2000 UI in the form of 25(OH)D3/kg [0.05 mg/kg]). One piglet per pen received intraperitoneal LPS injection (20 µg/kg BW (20 µg/kg). CON: control, DON: deoxynivalenol, LPS: lipopolysaccharide, VitD: vitamin D3, 25-OH-D3: 25-hydroxyl-Vitamin D3, SLC8A1: sodium/calcium exchanger 1, CALB-1: Calbindin D28K, TRPV5: transient receptor potential vanilloid 5, and S100G: Calbindin D9K. The data are reported as the mean ± SEM. * p < 0.10; ** p < 0.05; *** p < 0.01.
3. Discussion
No effect of vitamin D supplementation alone was observed in this study. However, vitamin D supplementation sometimes modified the response to DON in piglets that were or were not challenged with LPS.
3.1. Impact of DON Contamination and Vitamin D Supplementation
In this study, DON was the only mycotoxin present at a significant amount (DON, 4.9 mg/kg from naturally contaminated wheat), while other mycotoxins such as Aflatoxins, Zearalenone, Fuminosine, Ochratoxin, and T-2 were present in negligible amounts (<1.0 ppb, <0.03 ppm, <0.1 ppm, <0.003 ppm, and <0.06 ppm, respectively). The DON-contaminated diet reduced ADFI and ADG by 36% without affecting feed efficiency. These findings are consistent with previous studies, which have shown that DON contamination induces an anorexic effect in pigs [10,36,37], which can be explained by several mechanisms and thus reduces the gains due to the reduction in energy and protein intake. One of the mechanisms that induce anorexia is linked to the neurotoxic effect of DON on plasma serotonin (5-HT) secretion, which is involved in emesis, nausea, and appetite control [38]. Recently, Wang et al. [39] observed an increased 5-HT secretion in all studied regions of the piglet brain when they were fed a DON-contaminated feed at a concentration of 2.2 mg/kg for 60 days. Generally, a 5-HT increase is partly regulated by the Ca-sensing receptor (CaSR) in the parathyroid gland [40], which is also a Ca and P metabolism regulator.
This link with Ca and P homeostasis and DON may explain the increased bone mineralization per BW observed in the DON treatment groups. In addition, DON contamination clearly reduces the concentrations of 25-OH-D3 and 1,25-OH2-D3, phosphate, and the three other metabolites associated with Ca and P metabolism. For example, 1,25-OH2-D3 regulates bone remodeling proteins, which maintain the balance between degradation by osteoclasts and the formation by osteoblasts in the bone matrix [41]. In fact, calcitriol acts to maintain Ca homeostasis by stimulating bone resorption and osteoclastogenesis. Osteoblasts increase the release of the receptor activator of nuclear factor kappaB ligand (RANKL), which increases the production and action of osteoclasts [42]. A reduced calcitriol level would decrease the osteoclast production and the bone resorption process. Results from the gene expression in the kidney support a DON effect on Ca metabolism, which is consistent with the BMC results. The CALB1 gene responsible for Ca renal reabsorption [43]—the S100G gene expression that carries out Ca transport [44]—is SCL8A1, and it is responsible for Ca entry in the cell [45]. TRPV5 is responsible for the Ca reabsorption from renal tubules [43] that were all decreased by DON contamination. The jejunal SLC8A1 gene was also downregulated by DON contamination. However, the plasma Ca concentration was not modified by DON contamination. Contrary to our results, Le Thanh et al. [8] observed an increased Ca retention and digestibility, as well as a reduced Ca excretion when piglets weighing 6 kg received a diet contaminated with approximately 4 mg/kg of DON. This increase in Ca and P absorption could be explained by an increase in transcellular and paracellular passages. DON generally decreases the electrical resistance (TEER) of the intestinal barrier and therefore increases its permeability, in addition to increasing the paracellular passage [46]. Le Thanh et al. [8] measured the Ca balance from 9–14 days after beginning the consumption of a DON-contaminated diet in comparison to 21 days in our experiment. The increased permeability of the gut membrane by DON may have induced a high entry of Ca, causing hypercalcemia that was compensated by calcitonin regulations, thus leading to a downregulation of the genes related to the Ca renal and intestinal absorptions observed in the current study.
In the case of P-related genes, the Klotho gene expression in the kidney and jejunum, the coreceptor to FGF23, was decreased by DON contamination. FGF23 is a hypophosphatemic hormone that modulates vitamin D bioactivation [19] and inhibits PTH production in the parathyroid glands [47]. The Klotho expression decrease could be due to the low vitamin D levels induced by DON as the high vitamin D status can upregulate Klotho expression [48]. Jejunal SLC20A2 gene expression, which is responsible for the intestinal absorption of P [49], was decreased by DON contamination. It was previously observed that the P urinary excretion was reduced by DON contamination, but the P digestibility was not modified [8].
In theory, vitamin D supplementation, especially in the bioactive form 25-OH-D3, is expected to modify the born turnover process and increase the vitamin D status in CON pigs [42]. In fact, 25-OH-D3 is hydroxylated directly into calcitriol and is more efficiently absorbed, whereas vitamin D3 needs to be transformed into 25-OH-D3 in the liver beforehand [16,50]. However, there was no effect of vitamin D supplementation on the bone mineral content, regardless of its form. The CON treatment met the industry recommendations for vitamin D for feed, but the Ca levels were higher than expected in the present study, which could partly explain the absence of a significant effect from vitamin D3 and 25-OH-D3 supplementation on the BMC and blood concentrations of 25-OH-D3 and 1,25-OH2-D3. Other authors have also observed no effect in the two forms of vitamin D supplementation on bone mineralization [51,52], while some have shown an effect on serum 25-OH-D3 [53] in pigs. In the jejunum, vitamin D supplementation increased the VDR gene expression, which should increase the paracellular transport of vitamin D, P, and Ca [54], but it did not impact blood parameters. In human subjects, an increased Ca intake with a high vitamin D intake caused a decrease in serum 25-OH-D3 [55]. In the DON-contaminated pigs, a decrease in plasma 25-OH-D3 and serum calcitriol concentrations was observed, and adding vitamin D3 or 25-OH-D3 to the diet did not change this result. The DON contamination tended to decrease CYP27B1 gene expression in the kidney, which should have been upregulated in response to decreased serum calcitriol [16]. Vitamin D, through the bioactive calcitriol, is known to increase Ca and P intestinal absorption and Ca renal reabsorption [44,56]. Although the apparent total tract digestibility of Ca and P was not measured, none of the evaluated genes related to Ca and P absorption and reabsorption were modified by the vitamin D supplementation. This suggests that vitamin D does not impact the Ca, P, and vitamin D metabolism responses to DON contamination. Thus, DON probably decreased the blood levels of 25-OH-D3 and 1,25-OH2-D3 indirectly as a consequence of the modified Ca metabolism response and increased bone mineralization. DON contamination may also act on the enzyme 24-Hydroxylase, responsible for the calcitriol degradation into 24,25-OH2-D3 [16], which would partly explain why 25-OH-D3 and calcitriol levels remain low even when vitamin D supplementation is added. However, the gene CYP24A1 coding for 24-Hydroxylase was not evaluated.
3.2. Impact of DON and Vitamin D Supplement under LPS Challenge
An inflammatory stimulation can occur when piglets are exposed to LPS that is produced by Gram-negative bacteria. Previous studies have shown that an acute LPS challenge with DON exposure increases the response of the innate immune system and systemic circulation [34]. However, the additional stimulation of LPS was not always observed in DON-contaminated pigs. One study found that pigs fed a 3.1 mg/kg DON-contaminated feed and receiving intravenous LPS (7.5 μg/kg of BW) showed no difference in plasma TNF-α and IL-6 concentrations compared to the control group with LPS [31], while another study showed no difference for multiple parameters (aspartate-aminotransferase, albumin, γ-glutamyltransferase) with a similar experimental design [57]. An objective of the current study was thus to evaluate the effect of DON and vitamin D supplements on the Ca, P, and vitamin D metabolisms during an acute inflammatory response induced by LPS.
As described above, DON contamination has induced hypophosphatemia and hypovitaminosis D, as well as reduced the gene expression related to Ca and P reabsorption in the kidney; meanwhile, bone mineralization was increased regardless of whether vitamin D3 or 25-OH-D3 was added. A plausible explanation is that the increased intestinal barrier permeability caused by DON [46] induced the Ca afflux, leading to hypercalcemia. The pigs responded by reducing their efficiency to reabsorb and resorb bone Ca to reduce calcemia since Ca metabolism is very tightly regulated [58]. Interestingly, piglets stimulated with LPS also had reduced plasma Ca compared to those not receiving a LPS injection (9.6% plasma Ca, P = 0.01, results not shown). This was also observed in dogs stimulated with a 2 μg/kg intravenous LPS injection for 12 h [59]. Holowaychuk et al. [59] observed a decrease in serum ionic Ca (iCa) between 4 h–12 h, and the PTH concentration was increased between 4 h–24 h in response to the hypocalcemia. However, 25-OH-D3 concentration started decreasing after 2 h following the LPS challenge. The hypovitaminosis D was in accordance with the iCa decrease in this study, which partly explains the hypocalcemia caused by LPS stimulation [59]. In 12–14 week-old pigs stimulated with a 10 μg/kg intravenous LPS injection, Carlstedt et al. [60] also observed a marked decrease (−17.4%) in iCa within 2 h after LPS injection. Additionally, DON contamination reduced the expression of some genes related to Ca absorption in the jejunum (SLC8A1) and kidney (TRPV5), but—following LPS stimulation—they did not differ. This may be related to the induced hypocalcemia.
Therefore, the LPS challenge may have shifted the systemic response from the hypercalcemia caused by DON to a hypocalcemia response induced by LPS. Additionally, neither vitamin D3 nor 25-OH-D3 supplementation increased the low vitamin D status caused by the DON challenge without LPS stimulation. However, following the LPS challenge, 25-OH-D3 supplementation tended to increase the 25-OH-D3 concentration in piglets fed a DON-contaminated diet. Moreover, following the LPS challenge, the response of CYP27B1 and VDR in the kidney to DON was significant but depended on vitamin D supplementation. The 25-OH-D3 supplementation increased both renal CYP27B1 and VDR for the piglets receiving DON contamination and LPS challenges, while the opposite response was observed for CON and VitD3. CYP27B1 carries out the second hydroxylation that converts 25-OH-D3 into calcitriol. The latter participates in the anti-inflammatory response [61], and its biological effects are mediated by its binding to VDR [62,63]. Additionally, the VDR in the liver was decreased by DON during the LPS challenge, suggesting that DON contamination may increase inflammatory conditions through a modulation of the VDR expression. These results showed the beneficial effect of 25-OH-D3 to induce a vitamin D response in cases of low Ca status.
4. Conclusions
In summary, chronic repetitive exposure over 21 days to DON contamination (4.9 mg/kg) induced anorexia, which decreased the growth performance of piglets. The DON increased bone mineralization per kg of body weight, as well as reduced the circulating 25-OH-D3, calcitriol, and phosphate concentrations. As Ca is tightly regulated, the circulating Ca level was maintained, but active intestinal and renal Ca absorption was negatively regulated by DON contamination, thus supporting the hypercalcemia hypothesis. Intestinal P absorption was also negatively regulated by the DON challenge. The concentrations of 25-OH-D3 and calcitriol were not increased by their dietary supplementation during the DON challenge, which is associated with the modified Ca metabolism response. After the LPS-induced inflammatory stimulation, feeding a 25-OH-D3 supplementation increased the gene expression related to 1,25-(OH)2-D3 production and its activity during the DON challenge. Therefore, 25-OH-D3 supplementation could increase the production of calcitriol, which has anti-inflammatory properties, to face the combined LPS and DON challenge in pigs.
5. Materials and Methods
5.1. Animals and Feeding Trial
This experiment was performed at the Centre de Recherche en Sciences Animales de Deschambault (Quebec, Canada), and followed the guidelines of the Canadian Council on Animal Care (2009). The protocol (2018-057) was approved by the Institutional Animal Care Committee. All diets fulfilled the NRC requirements (2012 [64]; Table A1), and the supplementation level of vitamin D3 was added according to the values proposed by Isabel et al. [65]. Sixty-six castrated male piglets ([Yorkshire × Landrace] × Duroc) weaned at 21 days of age (6.4 ± 0.89 kg) were distributed in 33 pens (2 piglets/pen) depending on their weight at weaning. They were kept in 3 complete blocks with 6 repetitions and 3 incomplete blocks with 5 repetitions depending on the treatment assigned according to the randomization method [66]. The piglets received one of the following six treatments in a 2 × 3 factorial design: control treatments (CON) and DON-contaminated treatments (DON, 4.9 mg/kg from naturally contaminated wheat, Aflatoxins < 1.0 ppb, Zearalenone < 0.03 ppm, Fuminosin < 0.1 ppm, Ochratoxin < 0.003 ppm, T-2 < 0.06) for six pens each. The mycotoxin profile was determined in a commercial laboratory by LC/MS/MS (Actlabs Agriculture, Ancaster, ON, Canada) to confirm that DON was the predominant mycotoxin and that other mycotoxins were present in negligible concentrations. Those treatments were supplemented with low vitamin D3 (LowVitD, 200 UI vitamin D3/kg; DSM, Belvidere, NJ, USA) or with high vitamin D3 (HighVitD, 2200 UI vitamin D3/kg, DSM) or high 25-hydroxyvitamin D3 (High25-OH-D3, 2000 UI in the form of 25(OH)D3/kg [0.05 mg/kg, Hy-D®, DSM)]). The CON, DON, and CON+HighVitD were in complete blocks with 6 repetitions. The CON+High25-OH-D3, DON+ HighVitD, and DON+ High25-OH-D3 were in incomplete blocks with 5 repetitions. Upon their arrival, the piglets were fed with a commercial diet (Agri-Marché, St-Isidore, QC, Canada) for a seven-day adaptation period, and then received the experimental diets for 21 days. Piglets were weighed at the beginning and the end of the trial, and feed intake was evaluated after 7 days and 21 days per pen. One piglet per pen received intraperitoneal LPS injection (20 µg/kg BW, Sigma-Aldrich Canada, Oakville, ON, Canada) to induce an acute inflammatory reaction 3 h prior to euthanasia, which was performed with a non-penetrating captive bolt stunner. Blood samples were taken from each piglet 21 days before they were euthanized and 3 h after the LPS injection. Liver, intestinal mucosa, and kidney tissue were then collected. The bone mineral content (BMC) of each piglet was then evaluated after euthanasia with dual-energy X-ray absorptiometry (DXA; Discovery W, Hologic, MA, USA).
5.2. Laboratory Analysis
5.2.1. Blood Analysis
Blood samples were taken from all piglets by jugular venipuncture (BD Canada, Mississauga, ON, Canada). Plasma and serum samples were centrifuged at 2000× g at 4 °C for 15 min. Plasma and serum samples were kept frozen at −20 °C until analysis. Serum concentrations of DON, DOM-1 [8], phosphate, calcium, and magnesium (Mg) (BioAssay Systems, Hayward, CA, USA) were evaluated. Concentrations of 25-OH-D3 (Signalway Antibody, College Park, MD, USA) and 1,25-OH2-D3 (BioVendor, Brno, Czech Republic) were evaluated in plasma and serum, respectively, by the sandwich ELISA method.
5.2.2. Gene Expression Analysis
Tissue samples of the liver, jejunum mucosa, and kidney were taken to assess the gene expressions related to the P, Ca, and vitamin D metabolisms. They were snap-frozen in liquid nitrogen directly after collection. The mRNA gene expression was quantified according to the method of Lessard et al. [36]. The tissue samples (50 mg) were then homogenized in 1 mL of TRIzol © (Thermo Fisher Scientific, Carlsbad, CA, USA). Then, 200 μL of chloroform was added and samples were centrifuged for 15 min at 12,000× g at 4 °C. The aqueous phase was conducted in a new tube, and 500 μL of isopropanol was added. After centrifugation for 10 min at 12,000× g at 4 ℃, the isopropanol was removed from the tubes and 75% ethanol was added for a 5 min centrifugation at 7500× g at 4 °C. Fifty μL of DNase free water was added to dilute the pellet. The extracted RNA concentration and integrity were assessed with the NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA), as well as by nucleic acid electrophoresis with an Agilent 2100 Bioanalyzer instrument (Agilent, Santa Clara, CA, USA). Reverse transcription was performed with the qScript Flex (Qiagen Beverly Inc., Cummings, MA, USA), with a 1 μg/μL mRNA concentration. The relative mRNA abundance of genes known to be involved in the vitamin D, Ca, and P metabolisms was quantified using real-time qPCR analyses. Table 2 provides the complete list of selected genes. The qPCR was performed with 10 μL of PerfeCTa® SYBR® Green FastMix® (Quanta Bioscience Inc., Gaithersburg, MD, USA), 1 μL of cDNA, 1 μL of designed primers (Table A2), and 8 μL of RNAse free water using the Lightcycler 480 (Roche, Basel, Switzerland). The PCR cycling conditions were 10 min at 95 °C, followed by 50 cycles at 10 s at 57 °C or 58 °C for primer annealing depending on the gene, and 20 s at 72 °C for the primer extension. A melting curve step was added at 72 °C for 10 s and 94 °C for 5 measures. A relative standard curve was established by serial dilutions of a cDNA pool to determine the mRNA expression levels.
In the first place, the vitamin D receptor (VDR) gene expression was evaluated in all tissues. In the liver, only the CYP2R1 (25-hydroxylase activates vitamin D3 into 25-OH-D3 in liver) gene expression was also evaluated as the liver does not participate in most of the important regulations for Ca and P absorption. In the kidney and jejunum mucosa, the SLC20A2 (Na-Pi type III transporter, intestinal and kidney absorption of P), Klotho (FGF23 coreceptor), CALB1 (Calbindin-1, Ca kidney transportation), SLC8A1 (Na2+/Ca2+ 1 exchanger, transfers Ca to blood circulation), and S100G (Calbindin D9K, Ca transportation) gene expressions were assessed. The TRPV6 (transient receptor potential vanilloid 6, transcellular intestinal Ca absorption) gene expression was evaluated in the jejunum. The CYP27B1 (1α-hydroxylase, hydroxylation of 25-OH-D3 into 1-25-(OH)2-D3) and TRPV5 (transient receptor potential vanilloid 5, entry channel of Ca into kidney) gene expressions were evaluated in the kidney. The gene expressions were then normalized with three housekeeping genes, GAPDH, β-Actin, and HPRT.
5.3. Statistical Analyses
Growth performance including the average daily feed intake (ADFI), average daily gain (ADG), and the gain-to-feed ratio (G:F) were analyzed in a factorial 2 × 3 design. DON contamination and Vitamin D supplement were set as the fixed effects, and a pen of 2 piglets as the experimental unit with a mixed ANOVA were used on the Minitab software (Minitab20, LLC, State College, PA, USA). For plasma and serum concentrations, and the gene expressions in tissue, a 2 × 3 (DON contamination × Vitamin D supplement as fixed effects) factorial design was used to assess the effects of DON and vitamin D, as well as their interactions before and after LPS injection with a piglet as the experimental unit. Those data were evaluated using a Glimmix procedure on SAS (SAS studio 2021, SAS Inst. Inc. Cary, NC, USA). A p-value that was less than 0.05 indicated a significant difference, whereas a p-value less than 0.10 indicated a statistical trend.
Author Contributions
Conceptualization, F.G. and Y.C.; methodology, F.G. and Y.C.; validation, F.G. and Y.C.; formal analysis, B.S., M.-P.L.M. and F.G.; investigation, B.S.; resources, F.G. and M.-P.L.M.; writing—original draft preparation, B.S., M.-P.L.M. and F.G.; writing—review and editing, B.S., Y.C., M.-P.L.M. and F.G.; visualization, B.S., M.-P.L.M. and F.G.; supervision, B.S., M.-P.L.M. and F.G.; project administration, F.G. and M.-P.L.M.; funding acquisition, F.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DSM and the Natural Sciences and Engineering Research Council of Canada (grant number RDC 514177-2017).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board of the Institutional Animal Care Committee (protocol code 057–2018; 24 April 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding authors upon request.
Acknowledgments
We gratefully acknowledge the technical support of Dominic Gagné, Isabelle Lachance, Annie Pelletier, Annick Rioux, Ferial Amira Slim, and Isabelle Gilbert from the Department of Animal Sciences, Université Laval (QC, Canada). We also gratefully acknowledge the assistance of co-workers at the Centre de Recherche en Sciences Animales de Deschambault.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Composition of the experimental diets.
Table A1.
Composition of the experimental diets.
| Control a | Control | Control | DON | DON | DON | |
|---|---|---|---|---|---|---|
| +HighVitD | +High25OHD | +HighVitD | +High25OHD | |||
| Ingredient (%) | ||||||
| Control wheat | 50.00 | 50.00 | 50.00 | |||
| Contaminated wheat | 50.00 | 50.00 | 50.00 | |||
| Corn | 14.06 | 14.06 | 14.06 | 14.06 | 14.06 | 14.06 |
| Soybean meal | 20.29 | 20.29 | 20.29 | 20.29 | 20.29 | 20.29 |
| Hamlet Soy Protein (HP300) | 3.03 | 3.03 | 3.03 | 3.03 | 3.03 | 3.03 |
| Choice white fat | 2.05 | 2.05 | 2.05 | 2.05 | 2.05 | 2.05 |
| Whey powder | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| Monocalcium phosphate | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 |
| Limestone | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 | 1.16 |
| Vitamin and mineral premix y,z | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Lysine-HCl | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| DL-Methionine | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| L-Threonine | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Calculated composition (%) | ||||||
| Calcium | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| Phosphorus digestible | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 |
| Analyzed composition | ||||||
| Calcium, % | 0.94 | 0.84 | 0.94 | 1.05 | 1.20 | 1.27 |
| Phosphorus, % | 0.65 | 0.58 | 0.64 | 0.60 | 0.76 | 0.61 |
| Vitamin D, IU/kg | 226 | 1802 | 209 | 170 | 2444 | 232 |
| 25-OH-D3 | --- | --- | 2156 | --- | --- | 2864 |
| Deoxynivalenol, mg/kg | 0.11 | 0.10 | 0.18 | 5.16 | 4.69 | 4.98 |
a Control diet (0.11 mg/kg DON), Control+HighVitD diet (0.10 mg/kg DON), Control+High25-OH-D3 diet (0.18 mg/kg DON), DON-contaminated diet (5.16 mg/kg DON), DON+HighVitD diet (4.69 mg/kg DON), DON+High25-OH-D3 diet (4.98 mg/kg DON). z Provided per kilogram of diet: vitamin A palmitate 2000 IU; vitamin D3 200 IU; vitamin E acetate 16 IU; menadione sodium bisulphite 3.75 mg; thiamine HCl 1.0 mg; riboflavin 3.5 mg; niacin 30.0 mg; calcium pantothenate 15.0 mg; pyridoxine HCl 7.0 mg; biotin 0.5 mg; choline bitartrate 375 mg; and vitamin B12 25.0 µg. y Provided per kilogram of diet: Zn (as zinc carbonate) 100 mg; Fe (as ferric citrate) 100 mg; Cu (as cupric carbonate) 25 mg; I (as potassium iodate) 0.28 mg; Mn (as manganous carbonate) 46 mg; and Se (as sodium selenite) 0.30 mg.

Table A2.
Primer sequences used for qPCR.
Table A2.
Primer sequences used for qPCR.
| Gene | Primer Sequence (5′-3′) | Product Size, bp | Genebank Accession No. |
|---|---|---|---|
| Vitamin D | |||
| CYP2R1 | (F) TTCATCCCTTCTTGACTCCAAC | 201 | XM_003480731 |
| (R) TTTATCTGTCACCTGTCACCAC | |||
| VDR | (F) AGGCTTCTTCAGACGGAGCAT | 143 | NM_001097414.1 |
| (R) ACTCCTTCATCATGCCGATGT | |||
| CYP27B1 | (F) TGGGCTCTCTATGAACTCTCTC | 157 | DQ295065.1 |
| (R) GTCTTAGCACTTCCTTGACCAC | |||
| Phosphorus | |||
| Klotho | (F) ACGCGGAACATGACGTACAG | 121 | XM_013989399.1 |
| (R) CCTGCAAGGCGATGGAGAT | |||
| SLC20A2 | (F) GTGCACCTGCTCTTCCACTTC | 106 | XM_005657658.1 |
| (R) ACAAAGCTACCAGAGGACCAATG | |||
| Calcium | |||
| S100G | (F) GAAGGAGGAGCTGAAGCAACTG | 139 | NM_214140.2 |
| (R) CACTAACACCTGGAATTCTTCAAAAC | |||
| CALB-1 | (F) TGGATCAGTATGGGCAAAGAGA | 133 | NM_001130226.1 |
| (R) GTCTTCATGAATTCCTCACAGGACTT | |||
| TRPV5 | (F) GCTGCGAGTACGTCGCTATGT | 86 | XM_003484001.2 |
| (R) CAGAGGGCTGTTTCTCAGAGAGA | |||
| SLC8A1 | (F) AATTGCTAGAGCTACTGTGTATTTCG | 136 | FJ268730.1 |
| (R) ATTGGGCTTCTTTATGGTTATTTCT | |||
| TRPV6 | (F) TGGGTGTCCCAAAGTCCAAG | 95 | XM_013985575.1 |
| (R) ACTCCCTCCTCCTCCCAAAT | |||
| Reference | |||
| GAPDH | (F) CCCCAACGTGTCGGTTGT | 91 | XM_021091114.1 |
| (R) CTCGGACGCCTGCTTCAC | |||
| β-Actin | (F) CATCACCATCGGCAACGA | 128 | XM_003357928.4 |
| (R) GGATGTCGACGTCGCACTT | |||
| HPRT | (F) TTGTGGTAGGCTATGCCCTTGACT | 117 | NM_001032376 |
| (R) CTCAACTTGAACTCTCCTCTTAGG |
References
- 5 Key Areas to Tackle High Swine Feed Costs. Available online: https://porkcheckoff.org/news/5-key-areas-to-tackle-high-swine-feed-costs/ (accessed on 15 August 2022).
- Woyengo, T.A.; Beltranena, E.; Zijlstra, R.T. Nonruminant Nutrition Symposium: Controlling feed cost by including alternative ingredients into pig diets: A review. J. Anim. Sci. 2014, 92, 1293–1305. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Döll, S.; Schrickx, J.A.; Dänicke, S.; Fink-Gremmels, J. Interactions of deoxynivalenol and lipopolysaccharides on cytokine excretion and mRNA expression in porcine hepatocytes and Kupffer cell enriched hepatocyte cultures. Toxicol. Lett. 2009, 190, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dwivedi, P.D.; Pandey, H.P.; Das, M. Role of oxidative stress in Deoxynivalenol induced toxicity. Food Chem. Toxicol. 2014, 72, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, deoxynivalenol and zearalenone in feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh, B.V.; Lessard, M.; Chorfi, Y.; Guay, F. The efficacy of anti-mycotoxin feed additives in preventing the adverse effects of wheat naturally contaminated with Fusarium mycotoxins on performance, intestinal barrier function and nutrient digestibility and retention in weanling pigs. Can. J. Anim. Sci. 2015, 95, 197–209. [Google Scholar] [CrossRef]
- Reddy, K.E.; Lee, W.; Jeong, J.y.; Lee, Y.; Lee, H.-J.; Kim, M.S.; Kim, D.-W.; Yu, D.; Cho, A.; Oh, Y.K.; et al. Effects of deoxynivalenol- and zearalenone-contaminated feed on the gene expression profiles in the kidneys of piglets. Asian-Australas. J. Anim. Sci. 2018, 31, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Serviento, A.M.; Brossard, L.; Renaudeau, D. An acute challenge with a deoxynivalenol-contaminated diet has short- and long-term effects on performance and feeding behavior in finishing pigs. J. Anim. Sci. 2018, 96, 5209–5221. [Google Scholar] [CrossRef]
- Accensi, F.; Pinton, P.; Callu, P.; Abella-Bourges, N.; Guelfi, J.-F.; Grosjean, F.; Oswald, I.P. Ingestion of low doses of deoxynivalenol does not affect hematological, biochemical, or immune responses of piglets1. J. Anim. Sci. 2006, 84, 1935–1942. [Google Scholar] [CrossRef]
- Becker, C.; Reiter, M.; Pfaffl, M.W.; Meyer, H.H.D.; Bauer, J.; Meyer, K.H.D. Expression of immune relevant genes in pigs under the influence of low doses of deoxynivalenol (DON). Mycotoxin Res. 2011, 27, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Le Thanh, B.V.; Lemay, M.; Bastien, A.; Lapointe, J.; Lessard, M.; Chorfi, Y.; Guay, F. The potential effects of antioxidant feed additives in mitigating the adverse effects of corn naturally contaminated with Fusarium mycotoxins on antioxidant systems in the intestinal mucosa, plasma, and liver in weaned pigs. Mycotoxin Res. 2016, 32, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Mo, Y.; Li, J.; Yang, J.; Wang, J.; Karrow, N.A.; Wu, H.; Sun, L. Ferroptosis is involved in deoxynivalenol-induced intestinal damage in pigs. J. Anim. Sci. Biotechnol. 2023, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Combs, G.F. Chapter 6—Vitamin D. In The Vitamins, 4th ed.; Combs, G.F., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 139–180. [Google Scholar]
- Flynn, A. The role of dietary calcium in bone health. Proc. Nutr. Soc. 2003, 62, 851–858. [Google Scholar] [CrossRef]
- Fukumoto, S. Phosphate metabolism and vitamin D. BoneKEy Rep. 2014, 3, 497. [Google Scholar] [CrossRef]
- Nussey, S.; Whitehead, S.A. The parathyroid glands and vitamin D. In Endocrinology: An Integrated Approach, 1st ed.; BIOS Scientific Publishers: Oxford, UK; London, UK, 2001; pp. 171–215. [Google Scholar]
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Jurutka, P.W. The role of vitamin D in the FGF23, klotho, and phosphate bone-kidney endocrine axis. Rev. Endocr. Metab. Disord. 2012, 13, 57–69. [Google Scholar] [CrossRef]
- van de Graaf, S.F.J.; Boullart, I.; Hoenderop, J.G.J.; Bindels, R.J.M. Regulation of the epithelial Ca2+ channels TRPV5 and TRPV6 by 1α,25-dihydroxy Vitamin D3 and dietary Ca2+. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 303–308. [Google Scholar] [CrossRef]
- Zou, D.; Wu, W.; He, Y.; Ma, S.; Gao, J. The role of klotho in chronic kidney disease. BMC Nephrol. 2018, 19, 285. [Google Scholar] [CrossRef]
- Sergeev, I.N.; Kravchenko, L.V.; Piliia, N.M.; Batukhanov, A.B.; Sobolev, V.S.; Kuz’mina, E.E.; Iakushina, L.M.; Spirichev, V.B.; Tutel’ian, V.A. The effect of the trichothecene mycotoxin deoxynivalenol (vomitoxin) on calcium homeostasis, vitamin D metabolism and receptors in rats. Vopr. Med. Khim. 1990, 36, 26–29. [Google Scholar] [PubMed]
- McCue, M.; Reichert, J.L.; Crenshaw, T.D. Impact of dietary vitamin D3 supplements in nursery diets on subsequent growth and bone responses of pigs during an immune challenge. J. Anim. Sci. 2019, 97, 4895–4903. [Google Scholar] [CrossRef]
- Witschi, A.-K.M.; Liesegang, A.; Gebert, S.; Weber, G.M.; Wenk, C. Effect of source and quantity of dietary vitamin D in maternal and creep diets on bone metabolism and growth in piglets1. J. Anim. Sci. 2011, 89, 1844–1852. [Google Scholar] [CrossRef] [PubMed]
- Burild, A.; Lauridsen, C.; Faqir, N.; Sommer, H.M.; Jakobsen, J. Vitamin D3 and 25-hydroxyvitamin D3 in pork and their relationship to vitamin D status in pigs. J. Nutr. Sci. 2016, 5, e3. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.A.; Etheve, S.; Heegaard, P.M.H.; Skovgaard, K.; Mary, A.L.; Litta, G.; Lauridsen, C. Influence of vitamin D metabolites on vitamin D status, immunity and gut health of piglets. Vet. Immunol. Immunopathol. 2023, 257, 110557. [Google Scholar] [CrossRef]
- Pinton, P.; Braicu, C.; Nougayrede, J.-P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol Impairs Porcine Intestinal Barrier Function and Decreases the Protein Expression of Claudin-4 through a Mitogen-Activated Protein Kinase-Dependent Mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef]
- Klunker, L.R.; Kahlert, S.; Panther, P.; Diesing, A.K.; Reinhardt, N.; Brosig, B.; Kersten, S.; Dänicke, S.; Rothkötter, H.J.; Kluess, J.W. Deoxynivalenol and E.coli lipopolysaccharide alter epithelial proliferation and spatial distribution of apical junction proteins along the small intestinal axis. J. Anim. Sci. 2013, 91, 276–285. [Google Scholar] [CrossRef]
- Kullik, K.; Brosig, B.; Kersten, S.; Valenta, H.; Diesing, A.K.; Panther, P.; Reinhardt, N.; Kluess, J.; Rothkötter, H.J.; Breves, G.; et al. Interactions between the Fusarium toxin deoxynivalenol and lipopolysaccharides on the in vivo protein synthesis of acute phase proteins, cytokines and metabolic activity of peripheral blood mononuclear cells in pigs. Food Chem. Toxicol. 2013, 57, 11–20. [Google Scholar] [CrossRef]
- Dänicke, S.; Brosig, B.; Kahlert, S.; Panther, P.; Reinhardt, N.; Diesing, A.-K.; Kluess, J.; Kersten, S.; Valenta, H.; Rothkötter, H.-J. The plasma clearance of the Fusarium toxin deoxynivalenol (DON) is decreased in endotoxemic pigs. Food Chem. Toxicol. 2012, 50, 4405–4411. [Google Scholar] [CrossRef]
- Renner, L.; Kahlert, S.; Tesch, T.; Bannert, E.; Frahm, J.; Barta-Böszörményi, A.; Kluess, J.; Kersten, S.; Schönfeld, P.; Rothkötter, H.-J.; et al. Chronic DON exposure and acute LPS challenge: Effects on porcine liver morphology and function. Mycotoxin Res. 2017, 33, 207–218. [Google Scholar] [CrossRef]
- Dänicke, S.; Valenta, H.; Ganter, M.; Brosig, B.; Kersten, S.; Diesing, A.-K.; Kahlert, S.; Panther, P.; Kluess, J.; Rothkötter, H.-J. Lipopolysaccharides (LPS) modulate the metabolism of deoxynivalenol (DON) in the pig. Mycotoxin Res. 2014, 30, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.; Pestka, J.J. LPS priming potentiates and prolongs proinflammatory cytokine response to the trichothecene deoxynivalenol in the mouse. Toxicol. Appl. Pharmacol. 2006, 211, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Lessard, M.; Savard, C.; Deschene, K.; Lauzon, K.; Pinilla, V.A.; Gagnon, C.A.; Lapointe, J.; Guay, F.; Chorfi, Y. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine. Food Chem. Toxicol. 2015, 80, 7–16. [Google Scholar] [CrossRef]
- Andretta, I.; Kipper, M.; Lehnen, C.R.; Hauschild, L.; Vale, M.M.; Lovatto, P.A. Meta-analytical study of productive and nutritional interactions of mycotoxins in growing pigs. Animal 2012, 6, 1476–1482. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Bates, M.A.; Bursian, S.J.; Flannery, B.; Zhou, H.-R.; Link, J.E.; Zhang, H.; Pestka, J.J. Peptide YY3–36 and 5-Hydroxytryptamine Mediate Emesis Induction by Trichothecene Deoxynivalenol (Vomitoxin). Toxicol. Sci. 2013, 133, 186–195. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Cao, L.; Zhu, L.; Zhang, Y.; Chu, X.; Zhu, D.; Rahman, S.u.; Peng, C.; Feng, S.; et al. Mechanism of deoxynivalenol-induced neurotoxicity in weaned piglets is linked to lipid peroxidation, dampened neurotransmitter levels, and interference with calcium signaling. Ecotoxicol. Environ. Saf. 2020, 194, 110382. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, H.-R.; Bursian, S.J.; Link, J.E.; Pestka, J.J. Calcium-Sensing Receptor and Transient Receptor Ankyrin-1 Mediate Emesis Induction by Deoxynivalenol (Vomitoxin). Toxicol. Sci. 2016, 155, 32–42. [Google Scholar] [CrossRef]
- Sims, N.A.; Martin, T.J. Coupling the activities of bone formation and resorption: A multitude of signals within the basic multicellular unit. BoneKEy Rep. 2014, 3, 481. [Google Scholar] [CrossRef]
- Goltzman, D. Functions of vitamin D in bone. Histochem. Cell Biol. 2018, 149, 305–312. [Google Scholar] [CrossRef]
- Suzuki, Y.; Landowski, C.P.; Hediger, M.A. Mechanisms and Regulation of Epithelial Ca2+ Absorption in Health and Disease. Annu. Rev. Physiol. 2008, 70, 257–271. [Google Scholar] [CrossRef]
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Khananshvili, D. The SLC8 gene family of sodium-calcium exchangers (NCX)—Structure, function, and regulation in health and disease. Mol. Asp. Med. 2013, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of deoxynivalenol and its acetylated and modified forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef] [PubMed]
- Clinkenbeard, E.L.; White, K.E. Systemic Control of Bone Homeostasis by FGF23 Signaling. Curr. Mol. Biol. Rep. 2016, 2, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.; Xing, C.; Hu, M.C. Alpha Klotho and phosphate homeostasis. J. Endocrinol. Investig. 2014, 37, 1121–1126. [Google Scholar] [CrossRef]
- Forster, I.C.; Hernando, N.; Biber, J.; Murer, H. Phosphate transporters of the SLC20 and SLC34 families. Mol. Aspects Med. 2013, 34, 386–395. [Google Scholar] [CrossRef]
- Lütke-Dörhoff, M.; Schulz, J.; Westendarp, H.; Visscher, C.; Wilkens, M.R. Dietary supplementation of 25-hydroxycholecalciferol as an alternative to cholecalciferol in swine diets: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 1288–1305. [Google Scholar] [CrossRef]
- O’Doherty, J.V.; Gahan, D.A.; O’Shea, C.; Callan, J.J.; Pierce, K.M. Effects of phytase and 25-hydroxyvitamin D3 inclusions on the performance, mineral balance and bone parameters of grower–finisher pigs fed low-phosphorus diets. Animal 2010, 4, 1634–1640. [Google Scholar] [CrossRef]
- Regassa, A.; Adhikari, R.; Nyachoti, C.M.; Kim, W.K. Effects of 25-(OH)D3 on fecal Ca and P excretion, bone mineralization, Ca and P transporter mRNA expression and performance in growing female pigs. J. Environ. Sci. Health B 2015, 50, 293–299. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Chung, T.K.; Jung, Y.J.; Kim, I.H. Dietary 25(OH)D3 supplementation to gestating and lactating sows and their progeny affects growth performance, carcass characteristics, blood profiles and myogenic regulatory factor-related gene expression in wean-finish pigs. Anim. Biosci. 2022, 35, 461–474. [Google Scholar] [CrossRef]
- Fujita, H.; Sugimoto, K.; Inatomi, S.; Maeda, T.; Osanai, M.; Uchiyama, Y.; Yamamoto, Y.; Wada, T.; Kojima, T.; Yokozaki, H.; et al. Tight Junction Proteins Claudin-2 and -12 Are Critical for Vitamin D-dependent Ca2+ Absorption between Enterocytes. Mol. Biol. Cell 2008, 19, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Jorde, R.; Grimnes, G. Increased calcium intake is associated lower serum 25-hydroxyvitamin D levels in subjects with adequate vitamin D intake: A population-based observational study. BMC Nutr. 2020, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Lieben, L.; Masuyama, R.; Carmeliet, G. Vitamin D endocrine system and the intestine. BoneKEy Rep. 2014, 3, 496. [Google Scholar] [CrossRef] [PubMed]
- Stanek, C.; Reinhardt, N.; Diesing, A.-K.; Nossol, C.; Kahlert, S.; Panther, P.; Kluess, J.; Rothkötter, H.-J.; Kuester, D.; Brosig, B.; et al. A chronic oral exposure of pigs with deoxynivalenol partially prevents the acute effects of lipopolysaccharides on hepatic histopathology and blood clinical chemistry. Toxicol. Lett. 2012, 215, 193–200. [Google Scholar] [CrossRef] [PubMed]
- González-Vega, J.C.; Stein, H.H. Calcium Digestibility and Metabolism in Pigs. Asian-Australas. J. Anim. Sci. 2014, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Holowaychuk, M.K.; Birkenheuer, A.J.; Li, J.; Marr, H.; Boll, A.; Nordone, S.K. Hypocalcemia and Hypovitaminosis D in Dogs with Induced Endotoxemia. J. Vet. Intern. Med. 2012, 26, 244–251. [Google Scholar] [CrossRef]
- Carlstedt, F.; Eriksson, M.; Kiiski, R.; Larsson, A.; Lind, L. Hypocalcemia during porcine endotoxemic shock: Effects of calcium administration. Crit. Care Med. 2000, 28, 2909–2914. [Google Scholar] [CrossRef]
- Xu, S.; Chen, Y.-H.; Tan, Z.-X.; Xie, D.-D.; Zhang, C.; Xia, M.-Z.; Wang, H.; Zhao, H.; Xu, D.-X.; Yu, D.-X. Vitamin D3 pretreatment alleviates renal oxidative stress in lipopolysaccharide-induced acute kidney injury. J. Steroid Biochem. Mol. Biol. 2015, 152, 133–141. [Google Scholar] [CrossRef]
- Zúñiga, S.; Firrincieli, D.; Housset, C.; Chignard, N. Vitamin D and the vitamin D receptor in liver pathophysiology. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 295–302. [Google Scholar] [CrossRef]
- Dong, B.; Zhou, Y.; Wang, W.; Scott, J.; Kim, K.; Sun, Z.; Guo, Q.; Lu, Y.; Gonzales, N.M.; Wu, H.; et al. Vitamin D Receptor Activation in Liver Macrophages Ameliorates Hepatic Inflammation, Steatosis, and Insulin Resistance in Mice. Hepatology 2020, 71, 1559–1574. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; p. 420.
- Isabel, B.; Rey, A.; López, B. Optimum vitamin nutrition in pigs. In Optimum Vitamin Nutrition—In the Production of Quality Animal Foods; DSM Nutritional Products Limited; 5M Publishing: Sheffield, UK, 2012; pp. 243–308. [Google Scholar]
- Kim, B.G.; Lindemann, M.D. A spreadsheet method for experimental animal allotment. J. Anim. Sci. 2007, 85, 112. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).