Abstract
An automated method was developed for differentiating closely related B. cereus sensu lato (s.l.) species, especially biopesticide Bacillus thuringiensis, from other human pathogens, B. anthracis and B. cereus sensu stricto (s.s.). In the current research, four typing methods were initially compared, including multi-locus sequence typing (MLST), single-copy core genes phylogenetic analysis (SCCGPA), dispensable genes content pattern analysis (DGCPA) and composition vector tree (CVTree), to analyze the genomic variability of 23 B. thuringiensis strains from aizawai, kurstaki, israelensis, thuringiensis and morrisoni serovars. The CVTree method was the best option to be used for typing B. thuringiensis strains since it proved to be the fastest method, whilst giving high-resolution data about the strains. In addition, CVTree agrees well with ANI-based method, revealing the relationship between B. thuringiensis and other B. cereus s.l. species. Based on these data, an online genome sequence comparison resource was built for Bacillus strains called the Bacillus Typing Bioinformatics Database to facilitate strain identification and characterization.
Keywords:
Bacillus thuringiensis; B. cereus sensu stricto; B. anthracis; phylogenetic analysis; composition vector tree Key Contribution:
The work reported here provides a simple means for researchers to characterize new strains of B. cereus group and establish their relationship with other Bacillus strains. This will greatly facilitate the risk assessment of strains that can potentially be used as biopesticides and, in addition, can provide information about the likely biotechnological potential of a given strain based on its similarity to other strains with known and potentially useful characteristics.
1. Introduction
The Bacillus cereus group, which is also known as B. cereus sensu lato (s.l.), is a subdivision of the genus Bacillus. Currently the B. cereus group contains 22 closely related pathogenic and non-pathogenic species [1]. The pathogenic status of B. cereus group members is not homogeneous as they contain strains used as probiotics in animal nutrition, such as B. toyonensis [2], and also the deadly dangerous pathogen, B. anthracis, the virulent pathogen of mammals and causative agent of anthrax [3]. B. thuringiensis has been used successfully as an important biological insecticide due to the pesticidal proteins produced by these bacteria, which are toxic against different insect orders, such as Lepidoptera, Coleoptera and Diptera [4,5]. B. cereus sensu stricto (s.s.) is an opportunistic pathogen that can poison human food [6]. B. cytotoxycus is also a pathogenic species that was responsible for a severe foodborne outbreak [7]. On the other hand, the majority of the remaining members of the B. cereus group are mostly non-pathogenic species that are not or are rarely associated with illnesses in humans or other animals, such as B. mycoides or B. weihenstephanensis. The significant impact on human health, agriculture, and the food industry shown by B. cereus group members supports the need for an efficient typing and classification system of isolates within the group.
Various typing methods have been used for the identification and taxonomic characterization of B. cereus s.l. species, including traditional phenotypic tests, such as flagellar H serotyping [8] and analyzing the presence or absence of virulence plasmids [9], colonial morphology [10], and psychrophilic and thermotolerant abilities [11], as well as genotyping methods, including the analysis of single genes (16S rDNA, gyrB and plcR) [12,13], amplified fragment length polymorphisms (AFLP) [14], and multi-locus sequence typing (MLST) based on five to seven housekeeping gene sequences [15]. Although these methods provide insights into the genetic diversity and phylogenetic relationships among species classified as B. cereus s.l., problems still remain in distinguishing between organisms that are capable of causing illness or death from those used in industries [16,17]. For example, the biopesticide, B. thuringiensis, and human pathogens, B. anthracis and B. cereus s.s., are mainly differentiated on the basis of their production of different virulence factors, whose genes are located predominantly on plasmids that can be easily transferred or lost [18]. Furthermore, various studies also have demonstrated that these three species are closely genetically related, which has led to the proposal that they should be recognized as a single species [19,20]. Although B. thuringiensis-based products have been considered as safe insecticides [21], the ability to discriminate B. thuringiensis from the human pathogenic B. anthracis and B. cereus s.s. species is essential for the registration of B. thuringiensis biological control agents with a Generally Regarded as Safe (GRAS) status.
With the rapid development of genome sequencing technology, bacterial genome sequencing has become a convenient and feasible analysis technique. Thus, strain typing methods based on whole-genome sequences have become more attractive, such as those based on average nucleotide identity (ANI) [22] and digital DNA–DNA hybridization (dDDH) [23]. However, for both methods, the similarity between B. thuringiensis- and B. cereus s.s.-type strains is greater than the species boundary cutoffs [24]. Carroll et al. found that the current species definitions of this group led to overlapping genomospecies clusters at the conventional 95 ANI genomospecies threshold and proposed a nomenclatural framework, which accounted for genomospecies using resolvable genomospecies clusters obtained at ≈92.5 ANI [25]. According to the framework, the B. cereus group consists of eight published genomospecies (B. cereus s.s., B. mosaicus, B. mycoides, B. pseudomycoides, B. paramycoides, B. toyonensis, B. cytotoxicus and B. luti), four previously proposed genomospecies (“B. bingmayongensis”, “B. gaemokensis”, “B. manliponensis” and “B. clarus”), and six new ones, referred to as “Unknown B. cereus group Species 13–18”. The former species, B. cereus s.s. and B. thuringiensis, were assigned to the B. cereus s.s. genomospecies. B. anthracis were assigned to the B. mosaicus genomospecies, with six other former species B. albus, B. mobilis, B. pacificus, B. paranthracis, B. tropicus and B. wiedmannii. Additionally, B. mycoides, B. weihenstephanensis, B. proteolyticus, and B. nitratireducens were assigned to the B. mycoides genomospecies. The author also released software BTyper3 (version 3.1.1; Structural and Computational Biology Unit, European Molecular Biology Laboratory, Heidelberg, Germany; available from https://github.com/lmc297/BTyper3, accessed on 13 March 2021), which can be used to assign B. cereus s.l. isolates to taxonomic units within this proposed framework. However, this tool requires complex computing resources and some programming ability, which means that it may not be user-friendly for fast-paced and large-scale data analysis by microbiologists who are unfamiliar with the Linux command line environment. Developing an automated and low-resource-consuming system is essential for differentiating between closely related B. cereus s.l. isolates.
Composition vector tree (CVTree) analysis is an alignment-free method based on subsequences of a defined length (named k-strings). Using the CVTree algorithm, the genome sequences are cut into small k-strings, and each organism is represented by a composition vector (CV), which is calculated using the difference between the frequencies of k-strings and the prediction frequencies via the Markov model. Then, the similarity between the two genomes is measured according to the cosine of the angle between two CVs [26]. Finally, dissimilarity matrices are generated for phylogenetic studies. This CVTree analysis has been effectively applied to several phylogenetic studies of different datasets, including archaea [27], prokaryotes [28], fungi [29], chloroplast [30] and DNA viruses [31]. However, this method has not been previously applied to B. cereus s.l. group strains. The aim of this study was to find the most suitable method for differentiating between strains within the B. cereus group, with a particular emphasis on B. thuringiensis subspecies/genomospecies. The method of achieving the differentiation of B. thuringiensis strains into serovars was developed on the basis of flagellar antigens by de Barjac and Bonnefoi [32], and it has been the main strategy for typing B. thuringiensis isolates [8]. Here, four typing methods (MLST, single-copy core genes phylogenetic analysis (SCCGPA), dispensable genes content pattern analysis (DGCPA) and CVTree) were compared using 23 antiserum standard B. thuringiensis strains from aizawai, kurstaki, israelensis, thuringiensis and morrisoni serovars. The CVTree was selected to evaluate the subtyping of 182 antiserum standard B. thuringiensis strains from 86 different serovars. The analysis was then extended to 2359 B. cereus s.l. strains. Finally, a public user-friendly web server—the Bacillus Typing Bioinformatics Database (BTBIDB)—which uses CVTree that would allow users to analyze the phylogenetic relationship of their strains with those in our database, was built. This study resulted in the description of an operational typing technique that shows a high level of confidence and that will be useful to assist in the future genomic analysis of B. thuringiensis strains and other closely related B. cereus s.l. species.
2. Results
2.1. Evaluation of Different Phylogenetic Analysis Methods for Typing B. thuringiensis Strains
In this study, the unweighted pair group method with arithmetic mean (UPGMA) phylogenetic analysis was applied to 23 antiserum standard B. thuringiensis strains from five different serovars, and the convenience and the efficiency of four different typing approaches, MLST, SCCGPA, DGCPA and CVTree, were compared. First, the strain typing resolutions of the four methods were compared. Although antigen serotyping represents a crude typing system, all four genomic methods roughly agreed with antigen serotyping, revealing five main clusters corresponding to the different serovars (Figure 1). From this analysis, it was clear that the CVTree and DGCPA methods had a greater discriminatory potential than MLST and SCCGPA did, since the distances among individual strains was larger using these former typing methods.
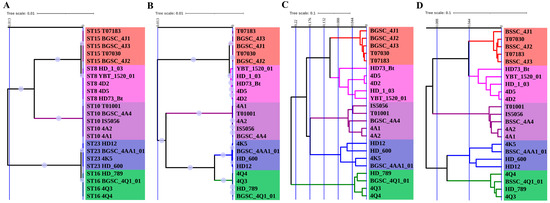
Figure 1.
Phylogenetic analysis of 23 B. thuringiensis strains based on four typing methods. (A), multi-locus sequence typing (MLST); (B), single-copy core genes phylogenetic analysis (SCCGPA); (C), dispensable genes content pattern analysis (DGCPA); (D), composition vector tree (CVTree). Bootstrap support values were calculated from 1000 replicates for MLST and SCCGPA, but they were not applied for DGCPA and CVTree. Bootstrap values over 0.5 are indicated by filled purple circles on the branch. Colors of the branches and strain label background represent different serovars, red for aizawai, magenta for kurstaki, purple for thuringiensis, blue for morrisoni and green for israelensis. The blue vertical bars indicate the dissimilarity coefficient.
Then, the efficiency of three genome-based methods, SCCGPA, DGCPA and CVTree, were evaluated from the perspective of time and resource consumption. Since all three methods used genomes as input data, we only compared the elapsed time in three dry lab steps, i.e., preparing the input data, calculating the dissimilarity matrix and inferring a phylogenetic tree based on the dissimilarity matrix. In the input data preparation step, CVTree directly used amino acid sequence data; so, no more processing was needed. Both SCCGPA and DGCPA needed 60,300 s to run the blast-all program (integrated into the PGAP software: version 1.2.1; Beijing Institute of Genomics, Chinese Academy of Sciences; Beijing, China) to recognize and extract the required data from genomes. In this study, 3653 single core gene clusters and 5626 dispensable gene clusters were recognized from the 23 genomes.
For the dissimilarity matrix calculation step, CVTree took 45 s. For SCCGPA, the MAFFT program took 4020 s to produce a multiple alignment file, and PHYLIP took 4800 s to produce the dissimilarity matrix; then, trimAI took less than a second to trim and produce the multiple alignment file. For DGCPA, an in-house Perl script took five seconds to produce a dissimilarity matrix.
Finally, for all methods just two seconds were required to build the trees.
In total, CVTree took 47 s to complete the process, while DGCPA and SCCGPA took 16.75 and 19.20 h, respectively (Table 1), showing that traditional sequence alignment-based methods are time-consuming. CVTree avoids sequence alignment by using k-string counts of all protein products encoded in a genome, and this alignment-free methodology was significantly faster than the other methods were.

Table 1.
Time consumption comparisons of three methods of genome comparison.
2.2. CVTree Method for Bacillus thuringiensis Strain Typing
A total of 182 antiserum standard B. thuringiensis strains from 86 different serovars were used as a model to evaluate the subtyping ability of CVTree to distinguish between isolates of the same species and to better assess the relationship between the genome sequence and serovar (Figure 2). Forty-seven strains from 16 serovars were sequenced by the staff in our laboratory, and the remaining one hundred and thirty-five strains from 84 serovars were obtained from the NCBI GenBank database. Details about each of the 47 newly sequenced genomes are provided in Table S1, which shows the genome size (base pairs), the predicted number of genes, CDS and tRNAs. These 47 genomes varied in size, ranging from 5.12–6.79 Mb, with the number of genes ranging from 5276–7121, indicating substantial genomic diversity within the newly sequenced B. thuringiensis strains.
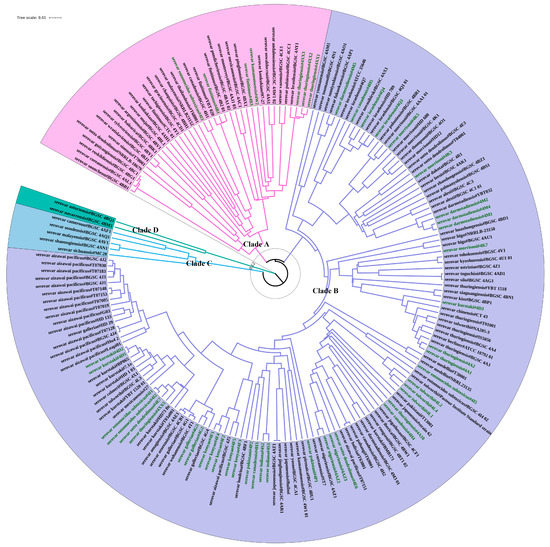
Figure 2.
Phylogenetic analysis of 182 antiserum standard B. thuringiensis strains using CVTree. This tree was obtained using CVTree with k-string = 6 and PHYLIP. Colors of strain label backgrounds represent different clades. Strains with their names in green represent 47 newly sequenced BGSC B. thuringiensis strains.
The CVTree analysis of these 182 B. thuringiensis strains identified four major Clades (Clade A–Clade D) with dissimilarity coefficients values between 0.114–0.128 (Figure 2). These four Clades are in accordance with the distribution previously described by Zheng et al. on the basis of single-copy core genes sequence similarity [33]. The most common serovars were assigned to the largest Clade, B, including all strains previously classified as kurstaki, israelensis, aizawai, morrisoni, darmstadiensis, tolworthi, kenyae and galleriae serovars. Clade B also included most of the strains belonging to the thuringiensis (75%, n = 12), canadensis (80%, n = 5), entomocidus (80%, n = 5) and sotto (80%, n = 5) serovars. Clade B is considered to be the “Thuringiensis” [33] or “B. kurstaki and Bc” [18] clade. All five B. thuringiensis serovar finitimus strains were assigned to Clade A. Clades C and D represent two small clades containing some unique serovars (cameroun, seoulensis, malayensis, shanongiensis and sichuansis for Clade C, and asturiensis and navarrensis for Clade D). In most cases, strains with the same serovar were assigned into the same Clade, often to the same branch; yet, there are some ambiguous strains assigned to different positions of the tree, indicating either higher variation within these strains or incorrect serovar typing [15].
2.3. CVTree Method for Differentiating Bacillus thuringiensis from Other Closely Related B. cereus s.l. Species
To establish if our CVTree analysis correlates with Carroll et al.’s analysis, genomic information about 2359 strains belonging to 20 former B. cereus s.l. species were obtained from GenBank to perform this comparison (Table S2). These 2359 strains included 2055 strains used in study of Carroll et al. A high level of correlation was observed between CVTree and the ANI-based method, revealing 12 robust and well-separated genomospecies clusters. In addition, six putative novel genomospecies (from xiii to xviii) classified by Carroll et al. were also identified in our CVTree analysis (Figure S1). In the case of the novel genomospecies, xiv (identified as Unknown Species 14 by Carroll et al.), it was found that all nine strains in this group clustered with B. luti genomospecies. The ANI value between these nine strains and the closest B. luti-type strain, TD41, ranged from 91.3056 to 92.0269.
To determine the classification ability of CVTree, 239 B. cereus s.l. strains, whose former species definitions were consistent with the genomospecies definitions, were selected and combined with the 182 antiserum standard B. thuringiensis strains analyzed above (Figure 3). Colored strain names on a grey background reflect the four primary clade distribution of 182 B. thuringiensis strains discussed above (magenta for Clade A, green for Clade B, blue for Clade C and purple for Clade D). All strains in Clade A, the “Anthracis” Clade clustered with the B. mosaicus genomospecies. Within this clade, fifteen B. thuringiensis strains clustered with B. anthracis, seven clustered with B. paranthracis, two clustered with B. pacificus, four clustered with B. tropicus, six clustered with B. wiedmannii and four clustered with B. albus. All the strains in Clade B, the “Thuringiensis” Clade, clustered with B. cereus s.s. genomospecies. All five strains in Clade C clustered with B. toyonensis genomospecies. Two Clade D strains clustered with B. mycoides genomospecies. Among the 47 newly sequenced B. thuringiensis strains, 7 clustered with B. mosaicus genomospecies, and the remaining 40 clustered with B. cereus s.s.
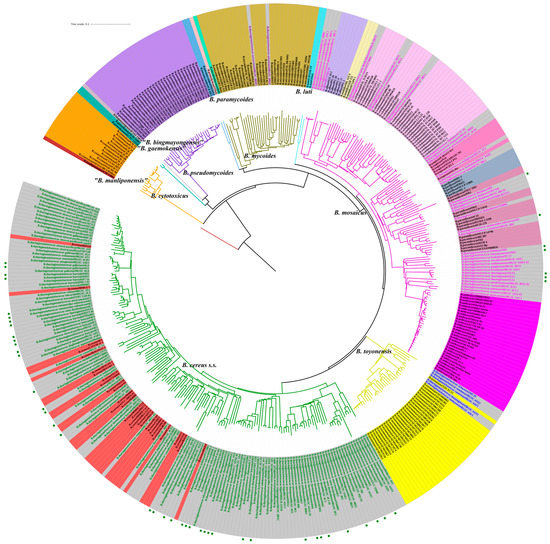
Figure 3.
Phylogenetic analysis of 421 B. cereus s.l. strains using CVTree. This tree was obtained using CVTree with k-string = 6. Colors of strain label backgrounds represent previously designated species names. Colors of branches represent different clusters based on CVTree analysis where appropriate are labelled with the names of previously designated genomospecies classified by Carroll et al. For B. thuringiensis strains (grey background), the color of the text reflects the four primary clades identified in Figure 2: magenta for Clade A, green for Clade B, blue for Clade C and purple for Clade D. The outermost green squares represent the 47 newly sequenced BGSC B. thuringiensis strains. B. manliponensis#JCM_15802 was treated as an outgroup for which phylogeny was rooted.
2.4. BTBIDB Web Server for B. cereus s.l. Phylogeny
The author of CVTree constructed an effective web server, CVTree 4 (http://cvtree.online/v4/prok/, accessed on 25 August 2021) [34], which is capable of comparing the tree branching orders using systematics at all taxonomic ranks from domains down to species and strains. However, this web server is focused on studies of phylogeny and taxonomy on a large scale. In this work, in order to facilitate the particular phylogeny study of B. cereus s.l. strains via CVTree, a free web server (BTBIDB, Bacillus Typing Bioinformatics Database, https://btbidb.com, accessed on 15 September 2021) was built. This web server possesses a comprehensive database of 4216 genomes from 136 Bacillus species (Table S3), including 2359 B. cereus s.l. strains and the 47 newly sequenced BGSC B. thuringiensis strains analyzed above. Via the “Testing Your Strain” section of BTBIDB, a logged-in user can quickly analyze the taxonomic placement of their own strains among selected genomes from the server’s inbuilt database. A detailed user manual for the web server can be found in the “Tutorial” section of BTBIDB.
3. Discussion
Horizontal gene transfer within the B. cereus group has been previously shown [18,33] and complicates the taxonomic classification of these bacteria, which has traditionally been defined by their phenotypic characteristics encoded in plasmid DNA. In particular, B. thuringiensis is defined by the possession of horizontally mobile genes encoding pesticidal proteins that are responsible for the production of crystalline inclusions within bacteria. However, several strains designated as B. thuringiensis neither carry insecticidal genes, nor produce visible crystals [33]. For example, 4H1, 4AY1 and 4BA1 strains, which lack crystal inclusions, were located in Clade A via the CVTree analysis in this work and were assigned to B. mosaicus genomospecies by Carroll et al., suggesting the incorrect classification of these strains. It is still challenging to distinguish between B. thuringiensis and B. cereus s.s. (Figure 3 and Figure S1), as others have observed [35].
The authors of several recent studies tried to solve the species–phenotype incongruences observed in the distribution of B. thuringiensis and B. cereus groups using genome-based taxonomic frameworks [24,25,36]. Via an adjustment of the ANI genomospecies threshold, Carroll et al. proposed an explicit, standardized framework for the taxonomic classification of B. cereus group, which accounted for both phylogenomic diversity and phenotypic heterogeneities. Using the SCCGPA approach, Zheng et al. analyzed the population structure of B. cereus group and found that most of B. thuringiensis strains were clustered into two main clades, with Clade 1 being the “Anthracis” clade and Clade 2 being the “Thuringiensis” clade. It is interesting to compare our CVTree result with the works of Zheng et al. and Carroll et al. A high degree of similarity was observed when the three output trees were compared. Exactly the same strains that we labeled as members of Clades C and D were included in Clades 3 and 4 in Zheng et al.’s work. In our CVTree, Clade A was subdivided into two main branches, which corresponded to Clade 1.1 and Clade 1.2 in Zheng et al.’s work, with the same strains grouped in these branches. Additionally, all strains in Clade A, the “Anthracis” Clade, clustered with the B. mosaicus genomospecies defined in Carroll et al.’s work. Regarding Clade B, our analysis showed that this is subdivided into two main branches, with one of them representing the strains that Zheng et al. cataloged as Clade 2.2. The other branch of our Clade B tree was subdivided into two branches that corresponded to two independent Clades, 2.1 and 2.3, in Zheng et al.’s work. All the strains in Clade B, the “Thuringiensis” clade, clustered with B. cereus s.s. genomospecies, as defined in Carroll et al.’s work. Additionally, we also found that B. thuringiensis strains belonging to Clade 2.3 in Zheng et al.’s work and were clustered with B. cereus s.s. strains, and strains belonging to Clades 2.1 and 2.2 formed standalone B. thuringiensis Clades lacking any B. cereus s.s. strains (Figure 3). These data confirm that CVTree is a method that gives results comparable to those of SCCGPA and ANI.
On a larger scale, the B. cereus group, also called the ‘Cereus clade’, and the ‘Subtilis clade’ together form two large distinct Clades of the genus Bacillus. Except for these two Clades, the Bacillus genus also contained many other divergent species, which exhibit extensive polyphyly and share very little in common with one another. By reconstructing multiple genomic-scale phylogenetic trees, Gupta et al. identified different monophyletic Clades of Bacillus species and a number of molecular markers that are specific and distinguishing characteristics of different Bacillus species Clades [37]. They found that in addition to the Cereus and Subtilis Clades, most of the Bacillus species consistently formed novel distinct Clades, which should be recognized as novel genera. Thus, in this study, we also compared our CVTree output with that of Gupta et al. This comparison is not intended to contribute to the debate about the classification of these Bacillus species, but rather to verify the typing ability of CVTree on a larger scale. Once again, CVTree has accurately revealed two main monophyletic Clades, ‘Cereus clade’ and ‘Subtilis clade’, as well as other novel distinct Clades proposed by Gupta et al. As shown in Figure S2, CVTree strongly supported the groupings of the Cereus Clade, which is grouped based on the phylogenetic and molecular distinctness in Gupta et al., including the ‘Core Cereus Clade’, and the other, more branching Panaciterrae and Luciferensis Clades. The novel species, B. rhizoplanae [38], was also grouped into the ‘Core Cereus Clade’.
Methodologically, genomic DNA sequences provide a number of ways to distinguish between different strains, including the analysis of core gene similarities (SCCGPA), the presence or absence of dispensable genes (DGCPA) [39] and the overall similarity between two genome sequences (ANI) [40]. For SCCGPA and DGCPA, both methods sample an enormously greater proportion of the genome than MLST does, but they still do not use whole-genome data. Among the initial 23 antiserum standard B. thuringiensis genomes analyzed in this work, core genes and dispensable genes account for 59.38–69.09% and 29.31–38.18% of the total number of genes, respectively. By contrast, both ANI and CVTree use whole-genome information. CVTree differs from the ANI method in that it uses all identified protein-encoding genes, and in terms of methodology it is an alignment-free method based on K-length peptide counting. The alignment-free methodology makes using CVTree significantly faster than using other methods is. Moreover, although the length of the peptide can be varied for prokaryotes, a value of five or six is recommended [41]. In this study, it was found that a value of 6 gave results consistent with previous phylogenetic analyses, and k-string = 6 was set during CVTree analysis.
4. Conclusions
In summary, the CVTree approach proved to be a quick and reliable method for determining the relationships between B. thuringiensis and other closely related B. cereus s.l. species. It provides data that are consistent with other genomic phylogeny approaches. More importantly a free, web-based tool—the Bacillus Typing Bioinformatics Database (https://btbidb.com, accessed on 15 September 2021)—which allows users to compare their own strains against a comprehensive database, was built. This server will be useful for researchers with a specific interest in taxonomy or to help other researchers interested in the characterization of risk assessment of specific strains within this group.
5. Materials and Methods
5.1. Bacterial Strains and Data Resources
In this work, genomic data from 4216 strains belonging to 136 different Bacillus species, including 2359 B. cereus s.l. strains, were collected (Tables S2 and S3). These included 2359 B. cereus s.l. strains, such as 955 B. cereus s.s., 540 B. thuringiensis, 259 B. anthracis, 214 B. toyonensis, 154 B. wiedmannii, 110 B. pseudomycoides, 67 B. mycoides, 14 B. cytotoxicus, 14 B. paranthracis, 7 B. albus, 6 B. pacificus, 6 B. tropicus, 3 B. mobilis, 2 B. paramycoides, 2 B. luti, 2 B. gaemokensis, 1 B. manliponensis, 1 B. bingmayongensis, 1 B. proteolyticus and 1 B. nitratireducens. Except for forty-seven B. thuringiensis strains newly sequenced in our laboratory, as described below, the rest were collected from the NCBI GenBank database (including finished annotated genomes and assemblies, ftp://ftp.ncbi.nlm.nih.gov/genomes/genbank/bacteria/, accessed on 15 January 2021). These strains were named by combining their species types, serovar types and original strain names, such as B. thuringiensis#serovar_kurstaki#BMB171. One B. weihenstephanensis strain (B. mycoides#WSBC_10204) was assigned as B. mycoides species in the NCBI GeneBank Database. Because two species were reported to be closely related, this assignment was not changed here. QUAST [42] was performed to evaluate genome assemblies via computing the number of contigs, largest contig length, GC content and N50 length (Table S3).
5.2. Genome Sequencing and Assembly
For genome sequencing, strains were grown overnight on LB agar plates. The cells were harvested, and 100 mg of Bt cells was washed with ddH2O, and their DNA was extracted as previously described [43]. DNA samples were stored at −20 °C until sequencing. Sequencing was performed using an Illumina HiSeq 2500 sequencer (Illumina, San Diego, CA, USA). The reads were cleaned by removing those with Ns or more than 20% low-quality bases, and 1 Gb 2 × 100 bp pair-end clean reads for each strain were obtained. SPAdes (version 3.13.1) [44] with default settings was employed for genome assembly. Genome assemblies were then submitted to the NCBI database for genome annotation via the NCBI Prokaryotic Genome Annotation Pipeline [45].
5.3. Evaluation of Phylogenetic Analysis Methods
In order to evaluate the convenience and efficiency of different approaches for the phylogenetic analysis of strains, 23 B. thuringiensis strains that belonged to five common serovars (aizawai, kurstaki, israelensis, thuringiensis and morrisoni) were randomly selected to perform MLST, SCCGPA, DGCPA and CVTree analyses. All dry lab analyses were performed using an LENOVO x3850 X6-[6241S1C]-/00FN846 server running CentOS Linux release 7.5.1804, with 4 × 2.00 GHz Intel(R) Xeon(R) processors and 16 GB of 1600 Mhz DDR3 RAM, and one thread was used.
Input data were prepared as follows: For the MLST approach, seven housekeeping gene sequences (glpF, gmK, ilvD, pta, pur, pycA and tpi) from the selected 23 B. thuringiensis strains were identified by nucleotide sequencing based on the polymerase chain reaction (PCR) [15] or extracted from genome sequences using BTyper (version 2.3.3) [46]. For SCCGPA and DGCPA, the pan-genome analysis pipeline (PGAP version 1.2.1) [47] was employed to identify core and dispensable genes using an identity threshold of 50%; core genes were defined as those present in all strains, and dispensable genes were those shared partially among strains [48]. Finally, for CVTree amino acid sequence data were directly used. The same annotated genomes with clearly described coding sequences (CDS) of proteins were used.
Dissimilarity matrices were calculated as follows: For MLST and SCCGPA, the nucleotide sequences of selected genes were concatenated, and then multiple alignments were performed using MAFFT (version 7.427) [49]. TrimAI (version 1.2rev59) [50] was used to remove the poorly aligned regions of multiple sequence alignments and produce a multiple alignment file in PHYLIP format for subsequence analysis. The dissimilarity matrix between strains was counted using SEQBOOT and DNAdist (PROTdist for protein sequence) routines within PHYLIP (version 3.698) [51]. Bootstrap tests in the MLST and SCCGPA analyses were performed with 1000 replicates. For DGCPA analysis, the dispensable gene profile was recorded based on whether a particular locus was present (scored as 1) or absent (scored as 0) in the genome. The dissimilarity matrix between strains was counted based on the difference of each locus using an in-house Perl script. Finally, for CVTree analysis, the dissimilarity matrix between strains was calculated based on the CV types of each strain using the CVTree standalone version, with k-string = 6 [41].
For phylogenetic analysis, UPGMA is a simple agglomerative hierarchical clustering method used to produce a dendrogram from a dissimilarity matrix, assuming that the distances from the root to each branch tip are equal [52]. With an orderly branch length, this tree provides a describable and quantifiable evaluation method of strain typing, and the evolutionary distances between strains are represented as the values of their dissimilarity coefficients, corresponding to the tree scale, where a larger dissimilarity coefficient indicates a greater distance between strains. In this study, UPGMA phylogenetic trees were constructed based of the dissimilarity matrix using the NEIGHBOR program of PHYLIP (version 3.698) [51]. All tree files were annotated using an online tool, iTOL (Interactive Tree of Life, https://itol.embl.de/, accessed on 18 March 2021) [53].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins15060393/s1. Table S1: 182 B. thuringiensis strains used in this study; Table S2: 2359 B. cereus s.l. strains used in this study; Figure S1: Phylogenetic analysis of 2359 B. cereus s.l. strains using CVTree. This tree was obtained using CVTree with k-string = 6. Colors of strain label backgrounds represent previously designated species names. Colors of branches represent different clusters based on the CVTree analysis. Colors of the outermost circle represent different genomospecies classified by Carroll et al., with white representing strains not included in that study. B. manliponensis#JCM_15802 was treated as an outgroup on which the phylogeny was rooted; Table S3: 4216 B. cereus s.l. built-in strains in BTBIDB web server; Figure S2: Phylogenetic analysis of Bacillaceae species, including all named Bacillus species and species from related genera, using CVTree. This tree was obtained using CVTree with k-string = 6. Except for Cereus Clade and Subtilis Clade, species from other Bacillaceae clades/genera that were proposed by Gupta et al. are compressed. Non-validly described species in the tree are shown within quotation marks: ‘species name’.
Author Contributions
Conceptualization, N.C. and J.Z.; methodology, K.W., C.S., A.B., M.S. and N.C.; investigation, K.W. and C.S.; formal analysis, K.W. and C.S.; writing—original draft preparation, H.Z. and K.W.; writing—review and editing, C.S., A.B., M.S., H.Z., N.C. and J.Z.; supervision, J.Z.; funding acquisition, K.W. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 32102295 and No. 31872935).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Genome sequences of all strains used in this study were available in GenBank under the accession numbers shown in Tables S1–S3.
Acknowledgments
We would like to thank Luke Tinsley for his help in compiling the group of strains used in the 2359 strains analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, Y.; Du, J.; Lai, Q.; Zeng, R.; Ye, D.; Xu, J.; Shao, Z. Proposal of nine novel species of the Bacillus cereus group. Int. J. Syst. Evol. Microbiol. 2017, 67, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Abou-Kassem, D.E.; Elsadek, M.F.; Abdel-Moneim, A.E.; Mahgoub, S.A.; Elaraby, G.M.; Taha, A.E.; Elshafie, M.M.; Alkhawtani, D.M.; Abd El-Hack, M.E.; Ashour, E.A. Growth, carcass characteristics, meat quality, and microbial aspects of growing quail fed diets enriched with two different types of probiotics (Bacillus toyonensis and Bifidobacterium bifidum). Poult. Sci. 2021, 100, 84–93. [Google Scholar] [CrossRef]
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.; Liu, S. Anthrax pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef]
- Shan, Y.; Shu, C.; He, K.; Cheng, X.; Geng, L.; Xiang, W.; Zhang, J. Characterization of a novel insecticidal protein Cry9Cb1 from Bacillus thuringiensis. J. Agric. Food Chem. 2019, 67, 3781–3788. [Google Scholar] [CrossRef]
- Wang, K.; Shu, C.; Zhang, J. Effective bacterial insecticidal proteins against coleopteran pests: A review. Arch. Insect Biochem. Physiol. 2019, 102, e21558. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef]
- Guinebretière, M.H.; Auger, S.; Galleron, N.; Contzen, M.; De Sarrau, B.; De Buyser, M.L.; Lamberet, G.; Fagerlund, A.; Granum, P.E.; Lereclus, D.; et al. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus Group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 2013, 63, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Lecadet, M.M.; Frachon, E.; Dumanoir, V.C.; Ripouteau, H.; Hamon, S.; Laurent, P.; Thiéry, I. Updating the H-antigen classification of Bacillus thuringiensis. J. Appl. Microbiol. 1999, 86, 660–672. [Google Scholar] [CrossRef]
- Reyes-Ramírez, A.; Ibarra, J.E. Plasmid patterns of Bacillus thuringiensis type strains. Appl. Env. Microbiol. 2008, 74, 125–129. [Google Scholar] [CrossRef]
- Di Franco, C.; Beccari, E.; Santini, T.; Pisaneschi, G.; Tecce, G. Colony shape as a genetic trait in the pattern-forming Bacillus mycoides. BMC Microbiol. 2002, 2, 33. [Google Scholar] [CrossRef]
- Lechner, S.; Mayr, R.; Francis, K.P.; Prüss, B.M.; Kaplan, T.; Wiessner-Gunkel, E.; Stewart, G.S.; Scherer, S. Bacillus weihenstephanensis sp. nov. is a new psychrotolerant species of the Bacillus cereus group. Int. J. Syst. Bacteriol. 1998, 48, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Tsen, H.Y. Discrimination of Bacillus cereus and Bacillus thuringiensis with 16S rRNA and gyrB gene based PCR primers and sequencing of their annealing sites. J. Appl. Microbiol. 2010, 92, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Kim, J.W.; Kim, J.M.; Kim, W.; Chung, S.I.; Kim, I.J.; Kook, Y.H. Population structure of the Bacillus cereus group as determined by sequence analysis of six housekeeping genes and the plcR gene. Infect Immun. 2004, 72, 5253–5261. [Google Scholar] [CrossRef]
- Hill, K.K.; Ticknor, L.O.; Okinaka, R.T.; Asay, M.; Blair, H.; Bliss, K.A.; Laker, M.; Pardington, P.E.; Richardson, A.P.; Tonks, M. Fluorescent amplified fragment length polymorphism analysis of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis isolates. Appl. Env. Microbiol. 2004, 70, 1068–1080. [Google Scholar] [CrossRef]
- Wang, K.; Shu, C.; Soberón, M.; Bravo, A.; Zhang, J. Systematic characterization of Bacillus Genetic Stock Center Bacillus thuringiensis strains using Multi-Locus Sequence Typing. J. Invertebr. Pathol. 2018, 155, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Navarrete, R.; Sanchis, V. Evolutionary processes and environmental factors underlying the genetic diversity and lifestyles of Bacillus cereus group bacteria. Res. Microbiol. 2017, 168, 309–318. [Google Scholar] [CrossRef]
- Carroll, L.M.; Cheng, R.A.; Wiedmann, M.; Kovac, J. Keeping up with the Bacillus cereus group: Taxonomy through the genomics era and beyond. Crit. Rev. Food Sci. Nutr. 2022, 62, 7677–7702. [Google Scholar] [CrossRef]
- Méric, G.; Mageiros, L.; Pascoe, B. Lineage-specific plasmid acquisition and the evolution of specialized pathogens in Bacillus thuringiensis and the Bacillus cereus group. Mol. Ecol. 2018, 27, 1524–1540. [Google Scholar] [CrossRef]
- Helgason, E.; Okstad, O.A.; Caugant, D.A.; Johansen, H.A.; Fouet, A.; Mock, M.; Hegna, I.; Kolstø, A.B. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—One species on the basis of genetic evidence. Appl. Env. Microbiol. 2000, 66, 2627–2630. [Google Scholar] [CrossRef]
- Zwick, M.E.; Joseph, S.J.; Didelot, X.; Chen, P.E.; Bishop-Lilly, K.A.; Stewart, A.C.; Willner, K.; Nolan, N.; Lentz, S.; Thomason, M.K. Genomic characterization of the Bacillus cereus sensu lato species: Backdrop to the evolution of Bacillus anthracis. Genome Res. 2012, 22, 1512–1524. [Google Scholar] [CrossRef]
- Raymond, B.; Federici, B.A. In defense of Bacillus thuringiensis, the safest and most successful microbial insecticide available to humanity—A response to EFSA. FEMS Microbiol. Ecol. 2017, 93, fix084. [Google Scholar] [CrossRef]
- Arahal, D.R. Whole-genome analyses: Average nucleotide identity. Method Microbiol. 2014, 41, 103–122. [Google Scholar]
- Auch, A.F.; Von Jan, M.; Klenk, H.P.; Göker, M. Digital DNA-DNA hybridization for microbial species delineation by means of genome-to-genome sequence comparison. Stand Genom. Sci. 2010, 2, 117–134. [Google Scholar] [CrossRef]
- Baek, I.; Lee, K.; Goodfellow, M.; Chun, J. Comparative genomic and phylogenomic analyses clarify relationships within and between Bacillus cereus and Bacillus thuringiensis: Proposal for the recognition of two Bacillus thuringiensis genomovars. Front. Microbiol. 2019, 10, 1978. [Google Scholar] [CrossRef]
- Carroll, L.M.; Wiedmann, M.; Kovac, J. Proposal of a taxonomic nomenclature for the Bacillus cereus group which reconciles genomic definitions of bacterial species with clinical and industrial phenotypes. mBio 2020, 11, e00034-20. [Google Scholar] [CrossRef]
- Qi, J.; Wang, B.; Hao, B.L. Whole proteome prokaryote phylogeny without sequence alignment: A K-string composition approach. J. Mol. Evol. 2004, 58, 1–11. [Google Scholar] [CrossRef]
- Zuo, G.; Zhao, X.; Hao, B. Phylogeny and Taxonomy of Archaea: A Comparison of the Whole-Genome-Based CVTree Approach with 16S rRNA Sequence Analysis. Life 2015, 5, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Z.; Hao, B.L. Composition vector approach to whole-genome-based prokaryotic phylogeny: Success and foundations. J. Biotechnol. 2010, 149, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Z.; Gao, L.; Hao, B. A fungal phylogeny based on 82 complete genomes using the composition vector method. BMC Evol. Biol. 2009, 9, 195. [Google Scholar] [CrossRef]
- Chu, K.H.; Qi, J.; Yu, Z.G.; Anh, V. Origin and phylogeny of chloroplasts revealed by a simple correlation analysis of complete genomes. Mol. Biol. Evol. 2004, 21, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Qi, J. Whole genome molecular phylogeny of large dsDNA viruses using composition vector method. BMC Evol. Biol. 2007, 7, 41. [Google Scholar] [CrossRef]
- De Barjac, H.; Bonnefoi, A. Essai de classification biochimique et sérologique de 24 souches de Bacillus du type B. Thuringiensis. Entomophaga 2006, 7, 5–31. [Google Scholar] [CrossRef]
- Zheng, J.; Gao, Q.; Liu, L.; Liu, H.; Wang, Y.; Peng, D.; Ruan, L.; Raymond, B.; Sun, M. Comparative genomics of Bacillus thuringiensis reveals a path to specialized exploitation of multiple invertebrate hosts. mBio 2017, 8, e00822-17. [Google Scholar] [CrossRef]
- Zuo, G. CVTree: A Parallel Alignment-free Phylogeny and Taxonomy Tool based on Composition Vectors of Genomes. Genom. Proteom. Bioinf. 2021, 19, 662–667. [Google Scholar] [CrossRef]
- Raymond, B.; Wyres, K.L.; Sheppard, S.K.; Ellis, R.J.; Bonsall, M.B. Environmental factors determining the epidemiology and population genetic structure of the Bacillus cereus group in the field. PLoS Pathog. 2010, 6, e1000905. [Google Scholar] [CrossRef]
- Carroll, L.M.; Cheng, R.A.; Kovac, J. No assembly required: Using BTyper3 to assess the congruency of a proposed taxonomic framework for the Bacillus cereus group with historical typing methods. Front. Microbiol. 2020, 11, 580691. [Google Scholar] [CrossRef]
- Gupta, R.S.; Patel, S.; Saini, N.; Chen, S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: Description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the Subtilis and Cereus clades of species. Int. J. Syst. Evol. Microbiol. 2020, 70, 5753–5798. [Google Scholar] [CrossRef] [PubMed]
- Kämpfer, P.; Lipski, A.; McInroy, J.A.; Clermont, D.; Criscuolo, A.; Glaeser, S.P. Bacillus rhizoplanae sp. nov. from maize roots. Int. J. Syst. Evol. Microbiol. 2022, 72, 005450. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Ehrlich, G.D.; Hu, F.Z. Pan-genome analysis provides much higher strain typing resolution than multi-locus sequence typing. Microbiology 2009, 156, 1060–1068. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Zuo, G.; Li, Q.; Hao, B. On K-peptide length in composition vector phylogeny of prokaryotes. Comput. Biol. Chem. 2014, 53, 166–173. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Li, Y.; Shu, C.; Shan, Y.; Geng, L.; Song, F.; Zhang, J. Complete genome sequence of Bacillus thuringiensis Bt185, a potential soil insect biocontrol agent. J. Integr. Agric. 2017, 16, 749–751. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020, 70, e102. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Carroll, L.M.; Kovac, J.; Miller, R.A.; Wiedmann, M. Rapid, high-throughput identification of anthrax-causing and emetic Bacillus cereus group genome assemblies via BTyper, a computational tool for virulence-based classification of Bacillus cereus group isolates by using nucleotide sequencing data. Appl. Env. Microbiol. 2017, 83, e01096-17. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, J.; Yang, J.; Sun, S.; Xiao, J.; Yu, J. PGAP: Pan-genomes analysis pipeline. Bioinform 2012, 28, 416–418. [Google Scholar] [CrossRef] [PubMed]
- Tettelin, H.; Masignani, V.; Cieslewicz, M.J.; Donati, C.; Medini, D.; Ward, N.L.; Angiuoli, S.V.; Crabtree, J.; Jones, A.L.; Durkin, A.S.; et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. USA 2005, 102, 13950–13955. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinform 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. PHYLIP-phylogeny inference package (Version 3.2). Cladistics 1989, 5, 164–166. [Google Scholar]
- Michener, C.D.; Sokal, R.R. A quantitative approach to a problem in classification. Evolution 1957, 11, 130–162. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, 242–245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).