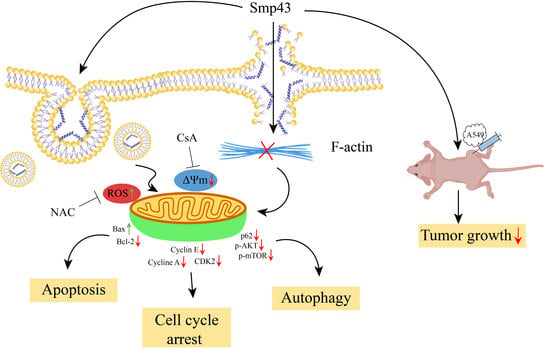
The Potent Antitumor Activity of Smp43 against Non-Small-Cell Lung Cancer A549 Cells via Inducing Membranolysis and Mitochondrial Dysfunction
Abstract
1. Introduction
2. Results
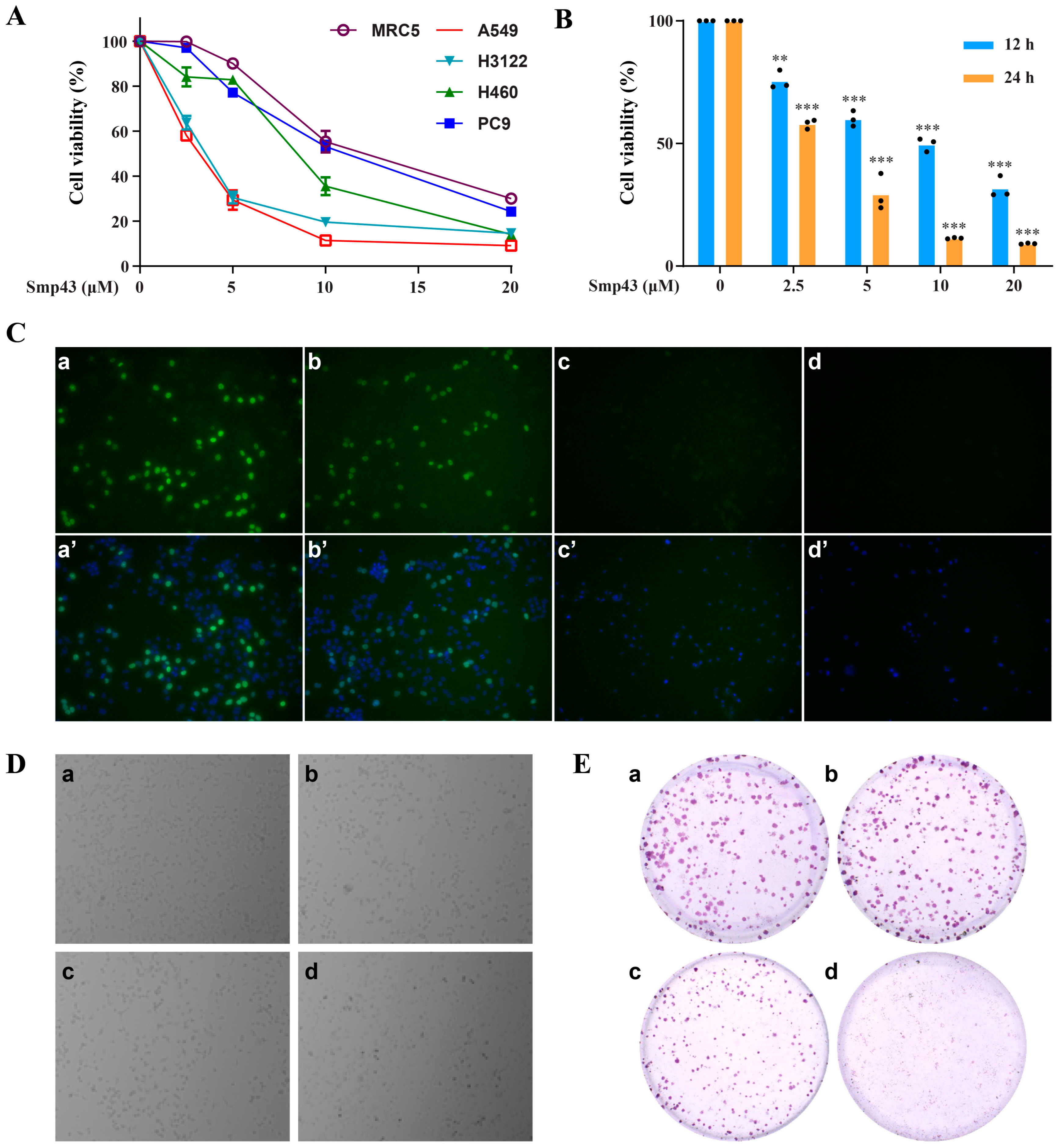
2.1. Smp43 Reduces Cell Viability in Human Lung Cancer
2.2. Smp43 Is Internalized into the A549 Cells via Endocytosis
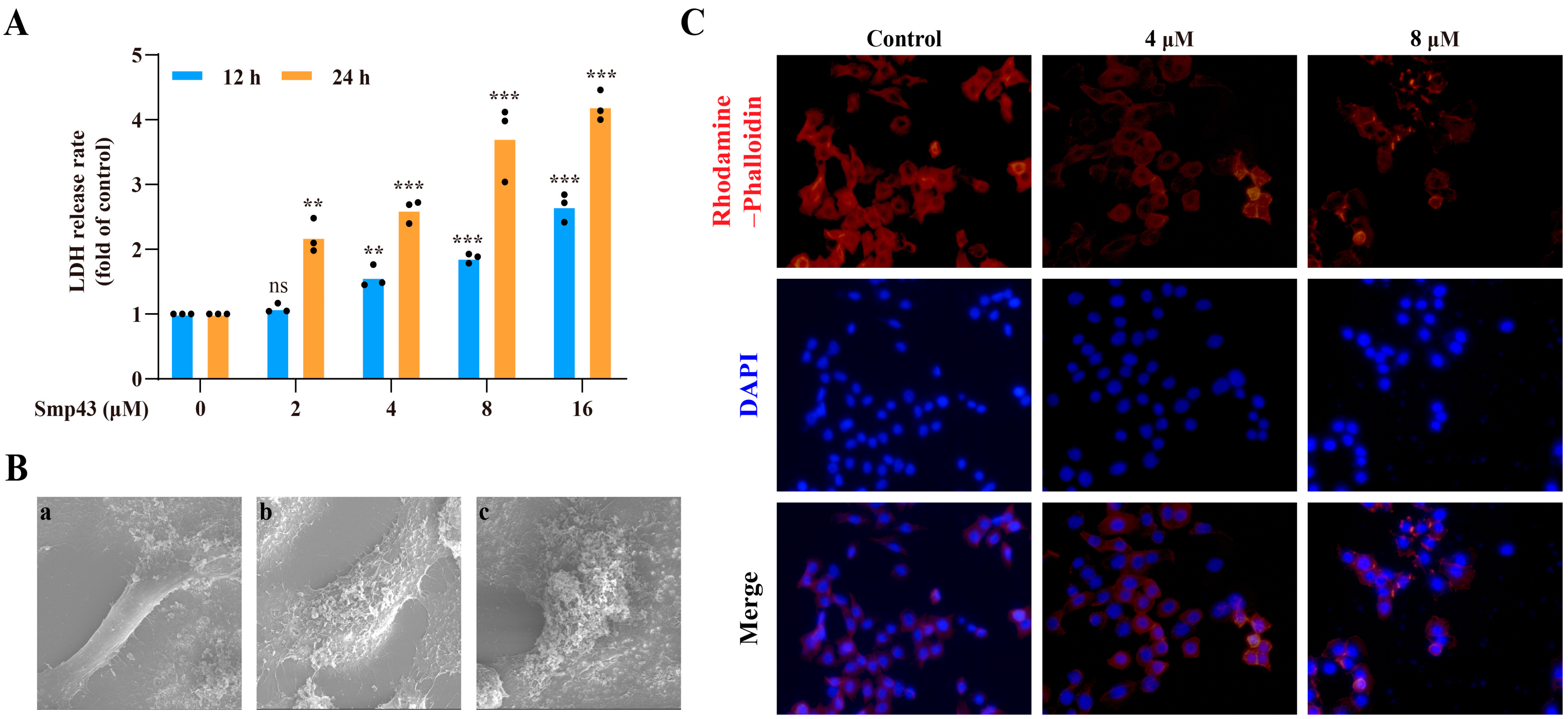
2.3. Smp43 Induces Cell Membrane Disruption and Changes Cell Cytoskeleton Organization
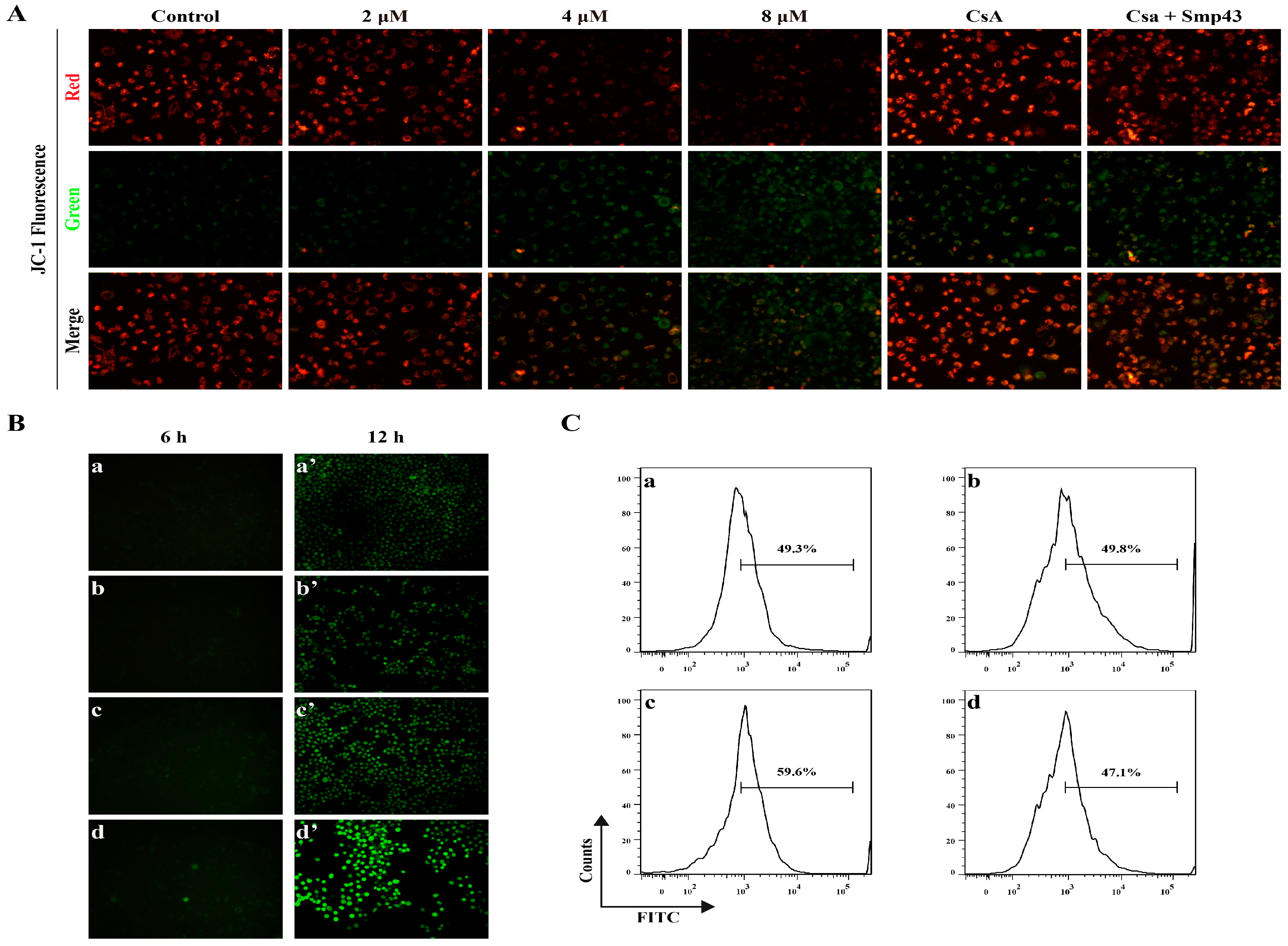
2.4. Smp43 Induces Mitochondrial Damage and ROS Overproduction in A549 Cells
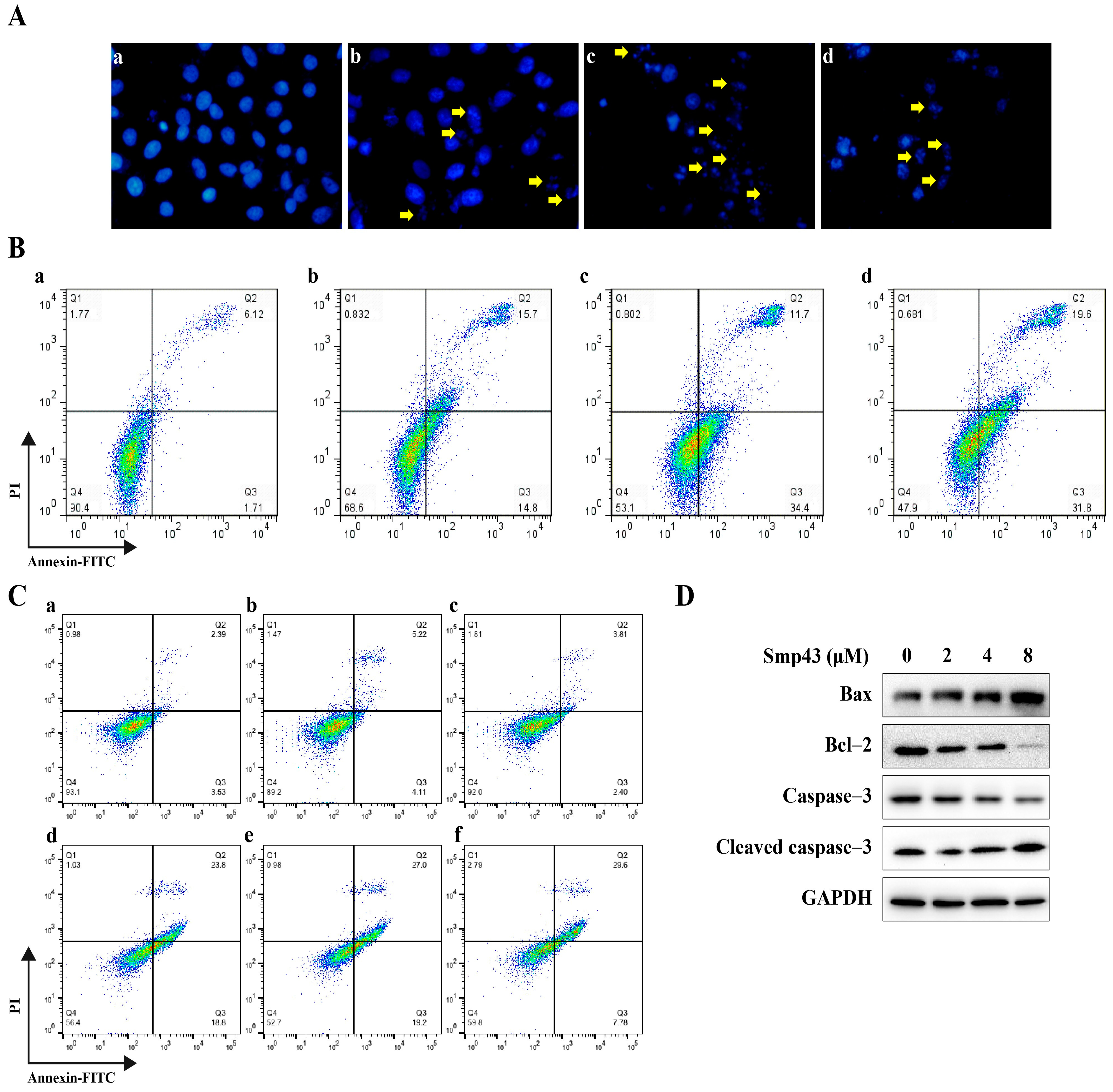
2.5. Smp43 Induces Apoptosis in A549 Cells
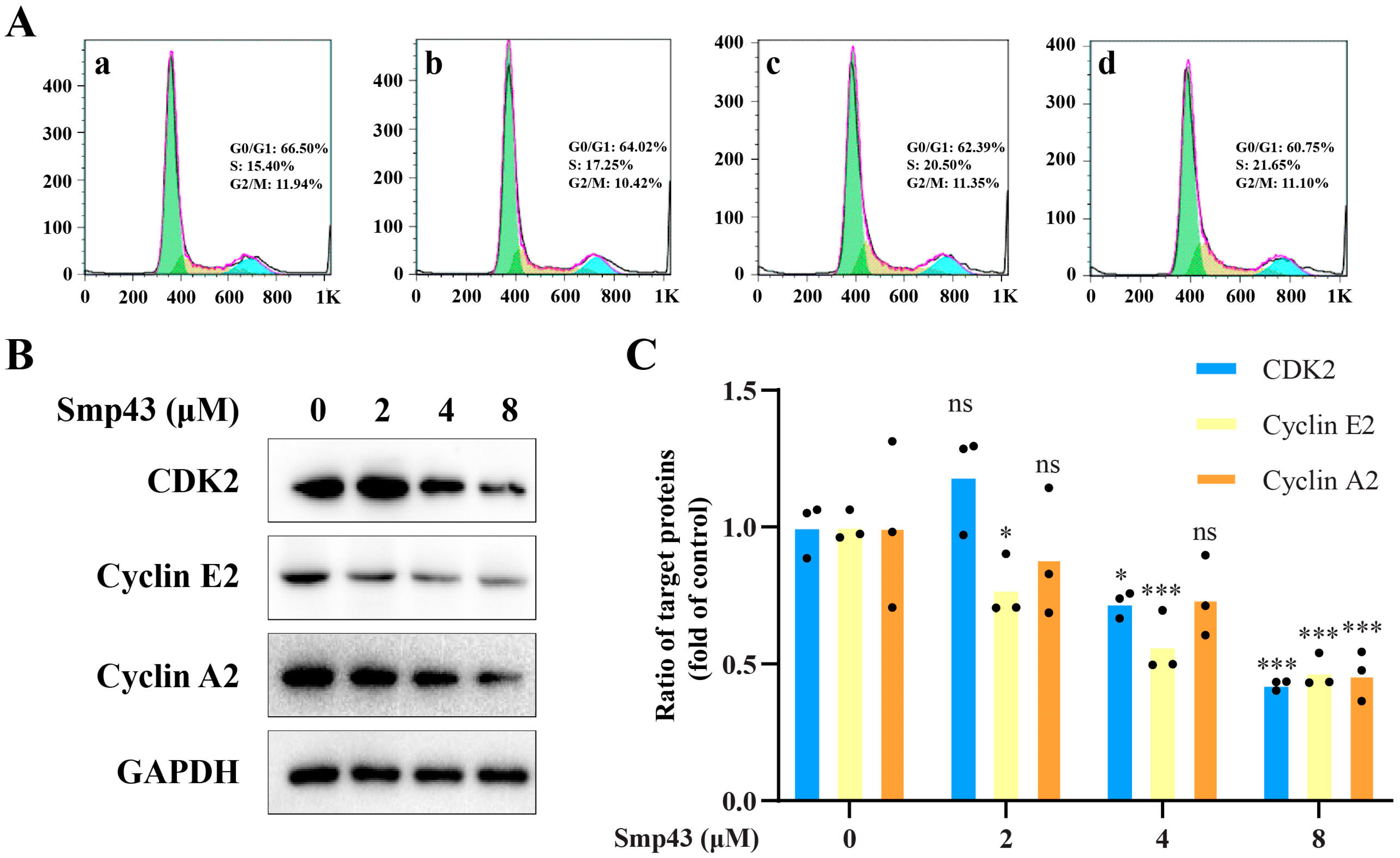
2.6. Smp43 Interferes with Cell Cycle Distribution in A549 Cells
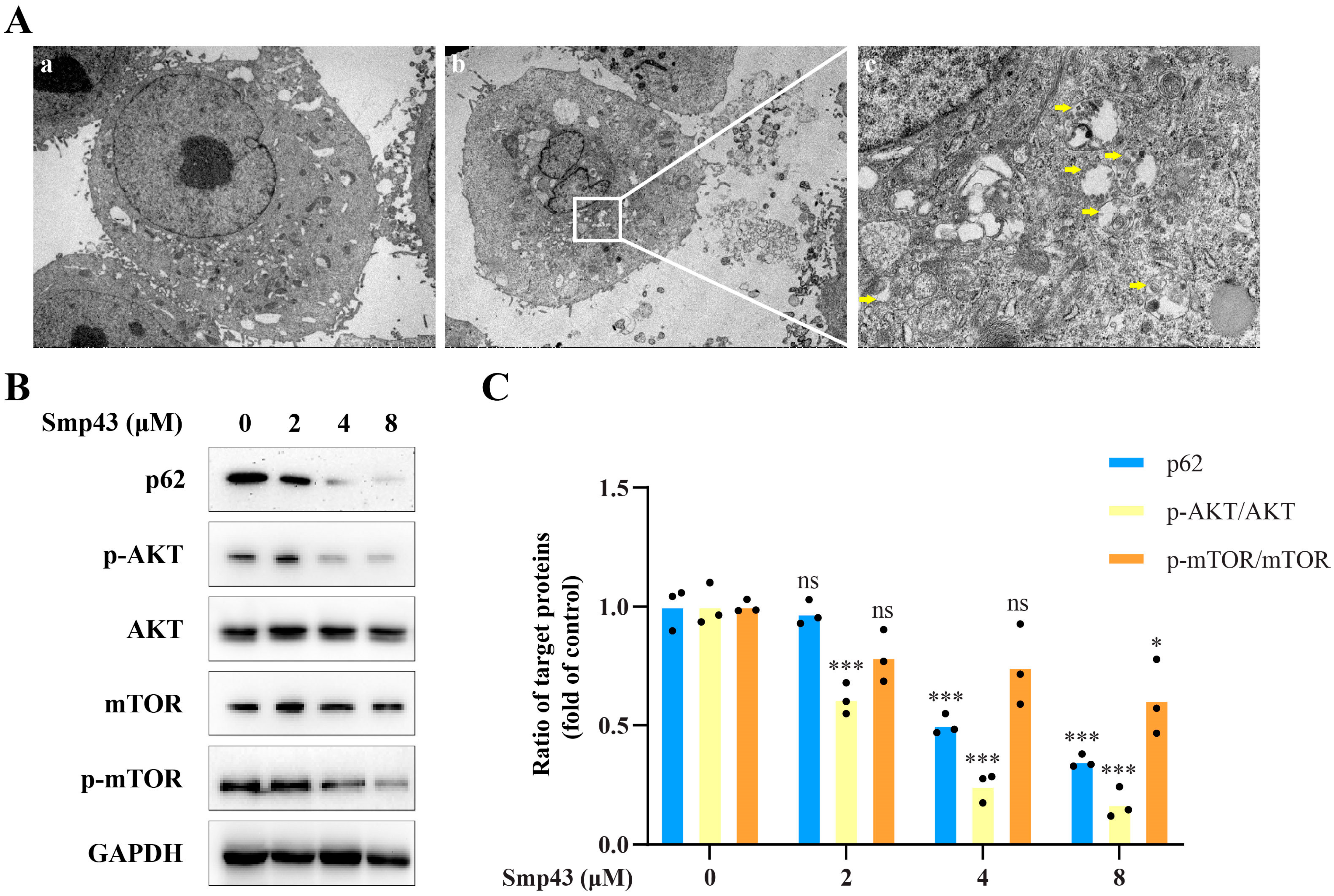
2.7. Smp43 Induces Autophagy in A549 Cells
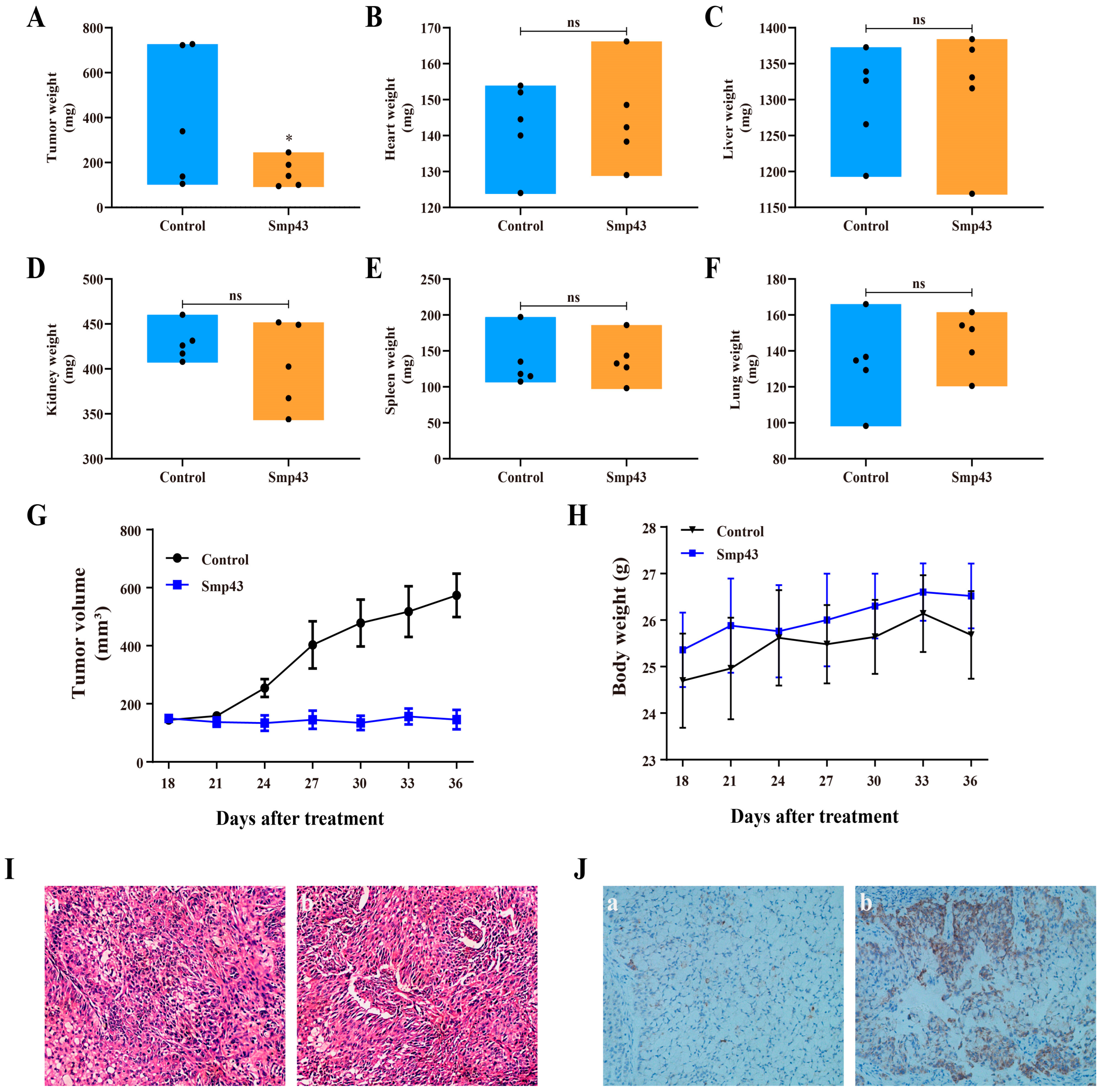
2.8. Smp43 Inhibits Tumor Growth in Mice
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents and Cell Culture
5.2. Animals and Ethics Statement
5.3. Cytotoxicity Assays
5.4. Cell Morphology Evaluation
5.5. Colony Formation Assessment
5.6. Peptide Internalization Assessment
5.7. Zeta Potential Assessment
5.8. LDH Release Assay
5.9. Scanning Electron Microscope Examination
5.10. Fluorescence Microscopy Determination
5.11. Flow Cytometry
5.12. Transmission Electron Microscopy Determination
5.13. Animal Studies
5.14. Western Blot Analysis
5.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| CsA | Cyclosporine A |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscope |
| LDH | Lactate dehydrogenase |
| NAC | N-Acetyl-L-cysteine |
| H&E | Hematoxylin and eosin |
| PBS | Phosphate-buffered saline |
| DCFH-DA | 2′,7′-dichloro-dihydro-fluorescein diacetate |
| SD | Standard deviation |
References
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Fei, S.; Xia, J.; Labropoulou, V.; Swevers, L.; Sun, J. Antimicrobial peptides as potential antiviral factors in insect antiviral immune response. Front. Immunol. 2020, 11, 2030. [Google Scholar] [CrossRef] [PubMed]
- Tornesello, A.L.; Borrelli, A.; Buonaguro, L.; Buonaguro, F.M.; Tornesello, M.L. Antimicrobial peptides as anticancer agents: Functional properties and biological activities. Molecules 2020, 25, 2850. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Diana, J. The dual role of antimicrobial peptides in autoimmunity. Front. Immunol. 2020, 11, 2077. [Google Scholar] [CrossRef]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Guo, R.; Liu, J.; Chai, J.; Gao, Y.; Abdel-Rahman, M.A.; Xu, X. Scorpion peptide Smp24 exhibits a potent antitumor effect on human lung cancer cells by damaging the membrane and cytoskeleton in vivo and in vitro. Toxins 2022, 14, 438. [Google Scholar] [CrossRef]
- Cohen, L.; Moran, Y.; Sharon, A.; Segal, D.; Gordon, D.; Gurevitz, M. Drosomycin, an innate immunity peptide of Drosophila melanogaster, interacts with the fly voltage-gated sodium channel. J. Biol. Chem. 2009, 284, 23558–23563. [Google Scholar] [CrossRef]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Jang, S.H.; Choi, S.Y.; Ryu, P.D.; Lee, S.Y. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur. J. Pharmacol. 2011, 651, 26–32. [Google Scholar] [CrossRef]
- Nguyen, T.; Guo, R.; Chai, J.; Wu, J.; Liu, J.; Chen, X.; Abdel-Rahman, M.A.; Xia, H.; Xu, X. Smp24, a scorpion-venom peptide, exhibits potent antitumor effects against hepatoma HepG2 cells via multi-mechanisms in vivo and in vitro. Toxins 2022, 14, 717. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, X.; Nguyen, T.; Chai, J.; Gao, Y.; Wu, J.; Li, J.; Abdel-Rahman, M.A.; Chen, X.; Xu, X. The strong anti-tumor effect of Smp24 in lung adenocarcinoma A549 cells depends on its induction of mitochondrial dysfunctions and ROS accumulation. Toxins 2022, 14, 590. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, R.A.; Mohallal, M.E.; Mobarak, Y.M.; Ebaid, H.M.; Haywood-Small, S.; Miller, K.; Strong, P.N.; Abdel-Rahman, M.A. Scorpion venom antimicrobial peptides induce caspase-1 dependant pyroptotic cell death. Front. Pharmacol. 2021, 12, 788874. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Yang, W.; Gao, Y.; Guo, R.; Peng, Q.; Abdel-Rahman, M.A.; Xu, X. Antitumor effects of scorpion peptide Smp43 through mitochondrial dysfunction and membrane disruption on hepatocellular carcinoma. J. Nat. Prod. 2021, 84, 3147–3160. [Google Scholar] [CrossRef] [PubMed]
- Poon, G.M.; Gariépy, J. Cell-surface proteoglycans as molecular portals for cationic peptide and polymer entry into cells. Biochem. Soc. Trans. 2007, 35, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu. Rev. Biophys. 2008, 37, 65–95. [Google Scholar] [CrossRef]
- Burke, P.J. Mitochondria, Bioenergetics and apoptosis in cancer. Trends Cancer. 2017, 3, 857–870. [Google Scholar] [CrossRef]
- Boland, M.L.; Chourasia, A.H.; Macleod, K.F. Mitochondrial dysfunction in cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef]
- Thorburn, A.; Thamm, D.H.; Gustafson, D.L. Autophagy and cancer therapy. Mol. Pharmacol. 2014, 85, 830–838. [Google Scholar] [CrossRef]
- Bjørkøy, G.; Lamark, T.; Pankiv, S.; Øvervatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A. Castanho. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Motta, C.; Boiret, N.; Aublet-Cuvelier, B.; Bonhomme, J.; Travade, P. Membrane fluidity and adherence to extracellular matrix components are related to blast cell count in acute myeloid leukemia. Leuk. Lymphoma 1994, 15, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Kolata, G.B. Microvilli: A major difference between normal and cancer cells? Science 1975, 188, 819–820. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Dong, S.; Zhang, L.; Zhao, Y.; Huang, L.; Gong, X.; Wang, H.; Shang, D. Cell surface binding, uptaking and anticancer activity of L-K6, a lysine/leucine-rich peptide, on human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 8293. [Google Scholar] [CrossRef] [PubMed]
- Hall, A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009, 28, 5–14. [Google Scholar] [CrossRef]
- Fife, C.M.; McCarroll, J.A.; Kavallaris, M. Movers and shakers: Cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2014, 171, 507–523. [Google Scholar] [CrossRef]
- Etienne-Manneville, S. Actin and microtubules in cell motility: Which one is in control? Traffic 2004, 5, 470–477. [Google Scholar] [CrossRef]
- Illescas, M.; Peñas, A.; Arenas, J.; Martín, M.A.; Ugalde, C. Regulation of mitochondrial function by the actin cytoskeleton. Front. Cell Dev. Biol. 2021, 9, 795838. [Google Scholar] [CrossRef]
- Gourlay, C.W.; Ayscough, K.R. The actin cytoskeleton: A key regulator of apoptosis and ageing? Nat. Rev. Mol. Cell Biol. 2005, 6, 583–589. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Thompson, M.B.; Scheff, S.W. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 1999, 160, 226–234. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Lee, J.F.; Chen, J.Y. Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT-1080 cells. Mar. Drugs 2011, 9, 1995–2009. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. The roles of autophagy in cancer. Int. J. Mol. Sci. 2018, 19, 3466. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Curcumin induced autophagy anticancer effects on human lung adenocarcinoma cell line A549. Oncol. Lett. 2017, 14, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.X.; Shen, J.; Cheng, A.S.; Lu, L.; Chan, R.L.; Li, Z.J.; Wang, X.J.; Wong, C.C.; Zhang, L.; Ng, S.S.; et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS ONE 2013, 8, e63641. [Google Scholar] [CrossRef] [PubMed]
- Marquez, R.T.; Xu, L. Bcl-2: Beclin 1 complex: Multiple, mechanisms regulating autophagy/apoptosis toggle switch. Am. J. Cancer Res. 2012, 2, 214–221. [Google Scholar]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Yo, Y.T.; Shieh, G.S.; Hsu, K.F.; Wu, C.L.; Shiau, A.L. Licorice and licochalcone-A induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J. Agric. Food Chem. 2009, 57, 8266–8273. [Google Scholar] [CrossRef]
- Yan, W.; Ma, X.; Zhao, X.; Zhang, S. Baicalein induces apoptosis and autophagy of breast cancer cells via inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des. Dev. Ther. 2018, 12, 3961–3972. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.J.; Wang, Y.; Wang, L.; Zhu, M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol. Rep. 2016, 36, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wang, X.; Luo, Y.; Liu, Z.; Tan, W.; Ye, P.; Fu, Z.; Lu, F.; Xiang, W.; Tang, L.; et al. Cantharidin suppresses gastric cancer cell migration/invasion by inhibiting the PI3K/Akt signaling pathway via CCAT1. Chem. Biol. Interact. 2020, 317, 108939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Q.; Yu, Z.Y.; Li, J.; Ouyang, X.N. Gefitinib induces lung cancer cell autophagy and apoptosis via blockade of the PI3K/AKT/mTOR pathway. Oncol. Lett. 2016, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Target Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Luo, X.; Ding, L.; Ye, X.; Zhu, W.; Zhang, K.; Li, F.; Jiang, H.; Zhao, Z.; Chen, Z. An Smp43-Derived Short-Chain α-Helical Peptide Displays a Unique Sequence and Possesses Antimicrobial Activity against Both Gram-Positive and Gram-Negative Bacteria. Toxins 2021, 13, 343. [Google Scholar] [CrossRef]
- Harrison, P.L.; Abdel-Rahman, M.A.; Strong, P.N.; Tawfik, M.M.; Miller, K. Characterisation of three alpha-helical antimicrobial peptides from the venom of Scorpio maurus palmatus. Toxicon 2016, 117, 30–36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Gao, Y.; Nguyen, T.; Chai, J.; Wu, J.; Li, J.; Abdel-Rahman, M.A.; Xu, X.; Chen, X. The Potent Antitumor Activity of Smp43 against Non-Small-Cell Lung Cancer A549 Cells via Inducing Membranolysis and Mitochondrial Dysfunction. Toxins 2023, 15, 347. https://doi.org/10.3390/toxins15050347
Deng Z, Gao Y, Nguyen T, Chai J, Wu J, Li J, Abdel-Rahman MA, Xu X, Chen X. The Potent Antitumor Activity of Smp43 against Non-Small-Cell Lung Cancer A549 Cells via Inducing Membranolysis and Mitochondrial Dysfunction. Toxins. 2023; 15(5):347. https://doi.org/10.3390/toxins15050347
Chicago/Turabian StyleDeng, Ze, Yahua Gao, Tienthanh Nguyen, Jinwei Chai, Jiena Wu, Jiali Li, Mohamed A. Abdel-Rahman, Xueqing Xu, and Xin Chen. 2023. "The Potent Antitumor Activity of Smp43 against Non-Small-Cell Lung Cancer A549 Cells via Inducing Membranolysis and Mitochondrial Dysfunction" Toxins 15, no. 5: 347. https://doi.org/10.3390/toxins15050347
APA StyleDeng, Z., Gao, Y., Nguyen, T., Chai, J., Wu, J., Li, J., Abdel-Rahman, M. A., Xu, X., & Chen, X. (2023). The Potent Antitumor Activity of Smp43 against Non-Small-Cell Lung Cancer A549 Cells via Inducing Membranolysis and Mitochondrial Dysfunction. Toxins, 15(5), 347. https://doi.org/10.3390/toxins15050347