Growth, Toxin Content and Production of Dinophysis Norvegica in Cultured Strains Isolated from Funka Bay (Japan)
Abstract
1. Introduction
2. Results
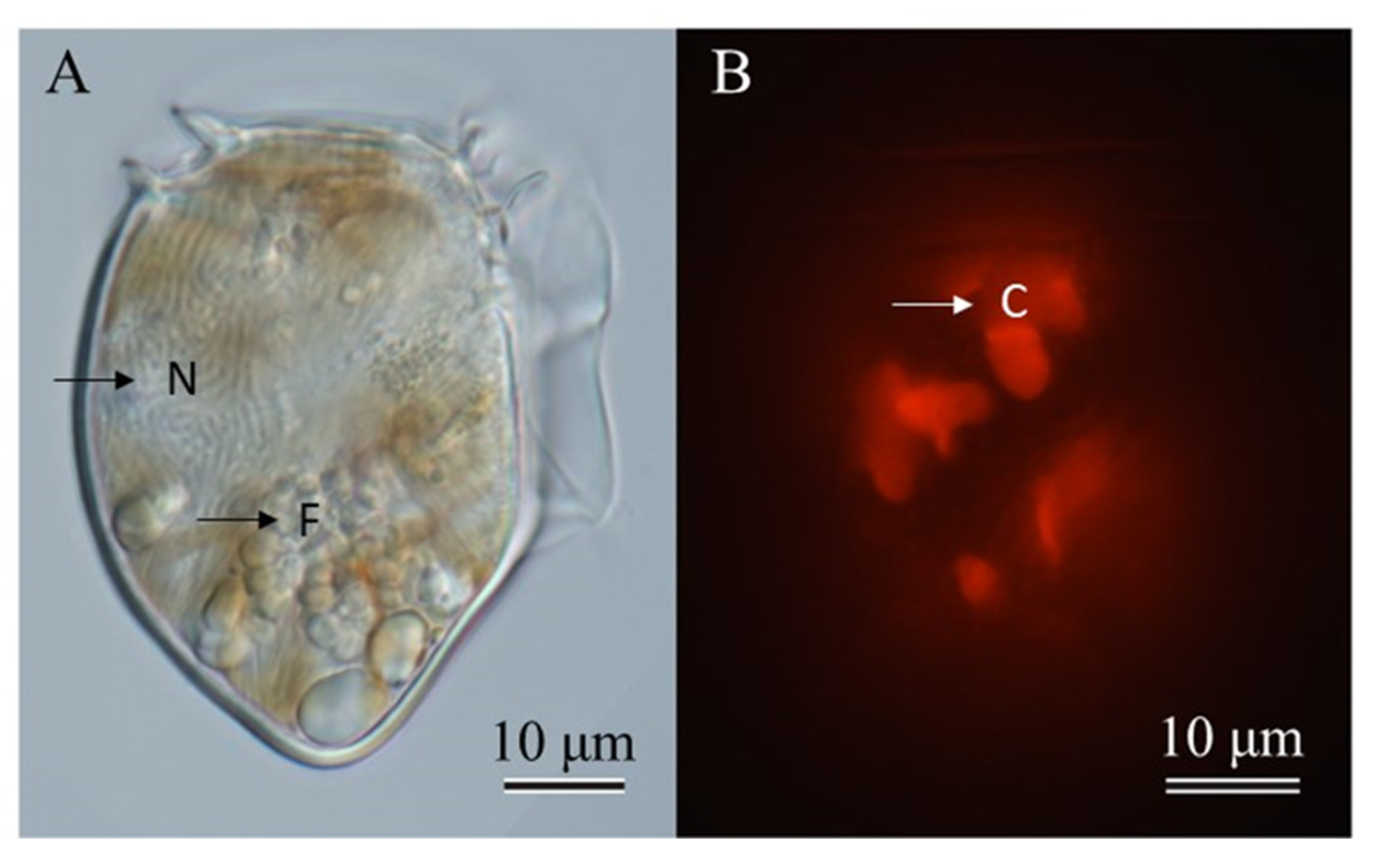
2.1. Species Identification
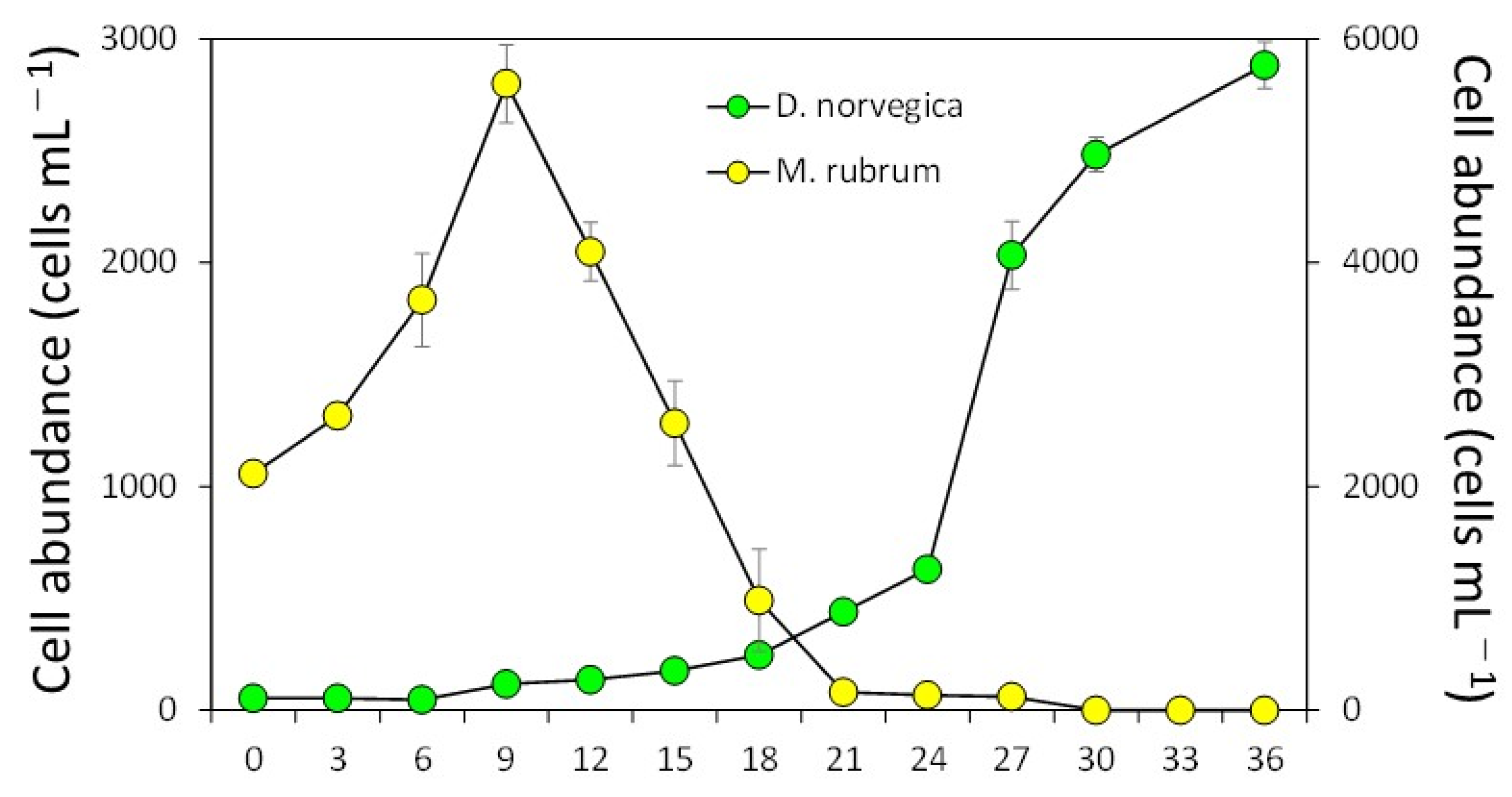
2.2. Feeding Behavior and Growth of Dinophysis Norvegica from Funka Bay in Culture Experiments
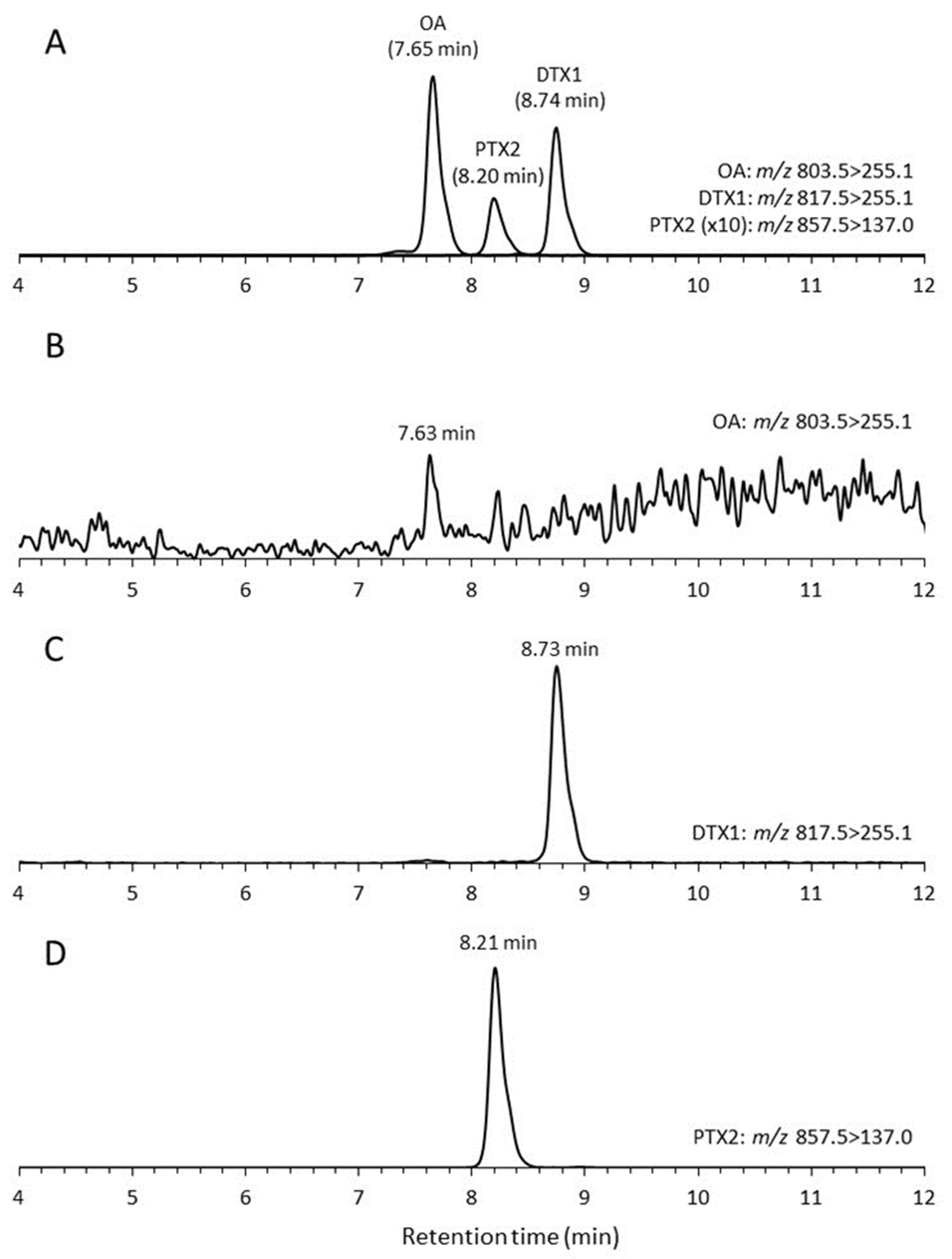
2.3. Toxin Production
3. Discussion
4. Materials and Methods
4.1. Isolation and Establishment of Clonal Cultures
4.2. Growth Experiments
4.3. Sequences of 28S rDNA (D1-D2 Region)
4.4. DSP Toxin Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smayda, T.J. Novel and nuisance phytoplankton blooms in the sea: Evidence for a global epidemic. In Toxic Mar. Plankton; Granéli, E., Sundström, B., Edler, L., Anderson, D.M., Eds.; Elsevier: New York, NY, USA, 1990; pp. 29–40. [Google Scholar]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Van Dolah, F.M. Marine algal toxins: Origins, health effects, and their increased occurrence. Environ. Health Persp. 2000, 108, 133–141. [Google Scholar] [CrossRef]
- Zingone, A.; Oksfeldt Enevoldsen, H. The diversity of harmful algal blooms: A challenge for science and management. Ocean Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Allen, J.I.; Anderson, D.; Burford, M.; Dyhrman, S.; Flynn, K.; Glibert, P.M.; Granéli, E.; Heil, C.; Sellner, K.; Smayda, T.; et al. Global Ecology and Oceanography of Harmful Algal Blooms, Harmful Algal Blooms in Eutrophic Systems; GEOHAB Report 4IOC and SCOR; GEOHAB: Paris, France; Baltimore, MD, USA, 2000; pp. 1–74. [Google Scholar]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 562–584. [Google Scholar] [CrossRef]
- Edwards, M.; Johns, D.G.; Leterme, S.C.; Svendsen, E.; Richardson, A.J. Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr. 2006, 51, 820–829. [Google Scholar] [CrossRef]
- Hégaret, H.; Shumway, S.E.; Wikfors, G.H.; Pate, S.; Burkholder, J.M. Potential transfer of harmful algae through relocation of bivalve molluscs. Mar. Ecol. Prog. Ser. 2008, 361, 169–181. [Google Scholar] [CrossRef]
- Heisler, J.; Gilbert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Moore, S.K.; Trainer, V.L.; Mantua, N.J.; Parker, M.S.; Laws, E.A.; Backer, L.C.; Fleming, L.E. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 2008, 7, S4. [Google Scholar] [CrossRef]
- Roger, A.D.; Laffoley, D. d’A. International Earth System Expert Workshop on Ocean Stresses and Impacts; Summary Report; IPSO: Oxford, UK, 2011; 18p. [Google Scholar]
- Fu, F.X.; Tatters, A.O.; Hutchins, D.A. Global change and the future of harmful algal blooms in the ocean. Mar. Ecol. Prog. Ser. 2012, 470, 207–233. [Google Scholar] [CrossRef]
- Glibert, P.M.; Allen, J.I.; Artioli, Y.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution to climate change: Projections based on model analysis. Glob. Chang. Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Dechroui Bottein, M.-Y.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Burkholder, J.M. Implication of harmful microalgae and heterotrophicdinoflagellates in management of sustainable marine fisheries. Biol. App. 1998, 8, S37–S62. [Google Scholar]
- Yasumoto, T.; Oshima, Y.; Yamaguchi, M. Occurrence of a new type of shellfish poisoning in the Tohoku district. Bull. Jpn. Soc. Sci. Fish. 1978, 44, 1249–1255. [Google Scholar] [CrossRef]
- Lee, J.S.; Igarashi, T.; Fraga, S.; Dahl, E.; Hovgaard, P.; Yasumoto, T. Determination of diarrhetic shellfish toxins in various dinoflagellate species. J. Appl. Phycol. 1989, 1, 147–152. [Google Scholar] [CrossRef]
- Jun, J.H.; Sim, C.S.; Lee, C.O. Cytotoxic compounds from a two-sponge association. J. Nat. Prod. 1995, 58, 1722–1726. [Google Scholar]
- Terao, K.; Ito, E.; Yanagi, T.; Yasumoto, T. Histopatholgical studies on marine toxin poisoning. 1. Ultrastructural changes in the small intestine and liver of suckling mice induced by dinophysistoxin-1 and pectenotoxin-1. Toxicon 1986, 24, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, L.A. A Long-Term Time Series of Dinophysis acuminata Blooms and Associated Shellfish Toxin Contamination in Port Underwood, Marlborough Sounds, New Zealand. Toxins 2019, 11, 7421. [Google Scholar] [CrossRef]
- Maestrini, S.Y. Bloom dynamics and ecophysiology of Dinophysis spp. In Physiological Ecology of Harmful Algal Blooms; Anderson, D.M., Cembella, A.D., Hallegraeff, G.M., Eds.; NATO ASI Series; Springer-Verlag: Berlin, Germany, 1998; Volume G 41, pp. 243–265. [Google Scholar]
- Miles, C.O.; Wilkins, A.L.; Samdal, I.A.; Sandvik, M.; Petersen, D.; Quilliam, M.A.; Naustvoll, L.J.; Jensen, D.J.; Cooney, J.M. A novel pectenotoxin, PTX-12, in Dinophysis spp. and shellfish from Norway. Chem. Res. Toxicol. 2004, 17, 1423–1433. [Google Scholar] [CrossRef]
- Ito, E.; Suzuki, T.; Oshima, Y.; Yasumoto, T. Studies on diarrhetic activity on pectenotoxin-6 in the mouse and rat. Toxicon 2008, 15, 707–716. [Google Scholar] [CrossRef]
- Reguera, B.; Pizarro, G. Planktonic Dinoflagellates Which Produce Polyether Toxins of the Old—DSP Complex. In Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; Taylor & Francis: London, UK, 2008; pp. 257–284. [Google Scholar]
- Tubaro, A.; Sosa, S.; Bornancin, A.; Hungerford, J. Pharmacology and toxicology of diarrheic shellfish toxins. In Seafood and Freshwater Toxins. Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 229–253. [Google Scholar]
- Vilariño, N.; Espiña, B. Pharmacology of pectenotoxins. In Seafood and Freshwater Toxins. Pharmacology, Physiology and Detection, 2nd ed.; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 361–369. [Google Scholar]
- Lawrence, J.; Loreal, H.; Toyofuku, H.; Hess, P.; Iddya, K.; Ababouch, L. Assessment and Management of Biotoxin Risks in Bivalve Molluscs; FAO Fisheries and Aquaculture Technical Paper 551; FAO: Rome, Italy, 2011; p. 337. [Google Scholar]
- Moestrup, Ø.; Akselmann-Cardella, R.; Fraga, S.; Hoppenrath, M.; Iwataki, M.; Komárek, J.; Larsen, J.; Lundholm, N.; Zingone, A. 2009 Onwards. IOC-UNESCO Taxonomic Reference List of Harmful Micro Algae. Available online: http://www.marinespecies.org/hab (accessed on 25 April 2022). [CrossRef]
- Edvardsen, B.; Shalchian-abrizi, K.; Jakobsen, K.S.; Medlin, L.K.; Dahl, E.; Brubak, S.; Paasche, E. Genetic variability and molecular phylogeny of Dinophysis species (Dinophyceae) from Norwegian waters inferred from single cell analyses of rDNA. J. Phycol. 2003, 39, 395–408. [Google Scholar] [CrossRef]
- Hart, M.C.; Green, D.H.; Bresnan, E.; Bolch, C.J. Large ribosomal RNA gene variation and sequence heterogeneity of Dinophysis (Dinophyceae) species from Scottish coastal waters. Harmful Algae 2007, 6, 271–287. [Google Scholar] [CrossRef]
- Raho, N.; Pizarro, G.; Escalera, L.; Reguera, B.; Marín, I. Morphology, toxin composition and molecular analysis of Dinophysis ovum Schütt, a dinoflagelate of the “Dinophysis acuminata complex”. Harmful Algae 2008, 7, 839–848. [Google Scholar] [CrossRef]
- Raho, N.; Rodríguez, F.; Reguera, B.; Marín, I. Are the mitochondrial cox1 and cob genes suitable markers for species of Dinophysis Ehrenberg? Harmful Algae 2013, 28, 64–70. [Google Scholar] [CrossRef]
- Rodríguez, F.; Escalera, L.; Reguera, B.; Rial, P.; Riobó, P.; de Jesús da Silva, T. Morphological variability, toxinology and genetics of the dinoflagellate Dinophysis tripos (Dinophysiaceae, Dinophysiales). Harmful Algae 2012, 13, 26–33. [Google Scholar] [CrossRef]
- Taylor, M.; McIntyre, L.; Ritson, M.; Stone, J.; Bronson, R.; Bitzikos, O.; Rourke, W.; Galanis, E.; Team, O. Outbreak of diarrhetic shellfish poisoning associated with mussels, British Columbia, Canada. Mar. Drugs 2013, 11, 1669–1676. [Google Scholar] [CrossRef]
- Stern, R.F.; Amorim, A.L.; Bresnan, E. Diversity and plastid types in Dinophysis acuminata complex (Dinophyceae) in Scottish waters. Harmful Algae 2014, 39, 223–231. [Google Scholar] [CrossRef]
- Nagai, S.; Sildever, S.; Suzuki, T.; Nishitani, G.; Basti, L.; Kamiyama, T. Successful cultivation and growth characteristics of the dinoflagellate Dinophysis. In Dinoflagellates: Morphology, Life History and Ecological Significance; Suba Rao, D.V., Ed.; Nova Science Publishers: New York, NY, USA, 2020; pp. 129–166. [Google Scholar]
- Sampayo, M.A.de.M. Trying to cultivate Dinophysis spp. In Toxic Phytoplankton Blooms in the Sea; Smayda, T.J., Shimizu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 807–810. [Google Scholar]
- Jacobson, D.M.; Andersen, R.A. The discovery of mixotrophy in photosynthetic species of Dinophysis (Dinophyceae): Light and electron microscopical observations of food vacuoles in Dinophysis acuminata, D. norvegica and two heterotrophic dinophysoid dinoflagellates. Phycologia 1994, 33, 97–110. [Google Scholar] [CrossRef]
- Nishitani, G.; Miyamura, K.; Imai, I. Trying to cultivation of Dinophysis caudata (Dinophyceae) and the appearance of small cells. Plankton Biol. Ecol. 2003, 50, 31–36. [Google Scholar]
- Nishitani, G.; Sugioka, H.; Imai, I. Seasonal distribution of species of the toxic dinoflagellate genus Dinophysis in Maizuru Bay (Japan), with comments on their autofluorescence and attachment of picophytoplankton. Harmful Algae 2002, 1, 253–264. [Google Scholar] [CrossRef]
- Schnepf, E.; Elbrächter, M. Cryptophycean-like double membrane-bound chloroplast in the dinoflagellate, Dinophysis Ehrenb.: Evolutionary, phylogenetic and toxicological implications. Bot. Acta 1988, 101, 196–203. [Google Scholar] [CrossRef]
- Lucas, I.A.N.; Vesk, M. The fine structure of two photosynthetic species of Dinophysis (Dinophysiales, Dinophyceae). J. Phycol. 1990, 26, 345–357. [Google Scholar] [CrossRef]
- Hewes, C.D.; Mitchell, B.G.; Moisan, T.A.; Vernet, M.; Reid, F.M.H. The phycobilin signature of chloroplasts from three dinoflagellate species: A microanalytic study of Dinophysis caudata, D. fortii, and D. acuminata (Dinophysiales, Dinophyceae). J. Phycol. 2002, 34, 945–954. [Google Scholar] [CrossRef]
- Takishita, K.; Koike, K.; Maruyama, T.; Ogata, T. Molecular evidence for plastid robbery (kleptoplastidy) in Dinophysis, a dinoflagellate causing diarrhetic shellfish poisoning. Protist 2002, 153, 293–302. [Google Scholar] [CrossRef]
- Hackett, J.D.; Maranda, L.; Yoon, H.S.; Bhattacharya, D. Phylogenetic evidence for the cryptophyte origin of the plastid of Dinophysis (Dinophysiales, Dinophyceae). J. Phycol. 2003, 39, 440–448. [Google Scholar] [CrossRef]
- Janson, S.; Granéli, E. Genetic analysis of the psbA gene from single cells indicates a cryptomonad origin of the plastid in Dinophysis (Dinophyceae). Phycologia 2003, 42, 473–477. [Google Scholar] [CrossRef]
- Janson, S. Molecular evidence that plastids in the toxin-producing dinoflagellate genus Dinophysis originate from the free-living cryptophyte Teleaulax amphioxeia. Environ. Microbiol. 2004, 6, 1102–1106. [Google Scholar] [CrossRef]
- Park, M.G.; Kim, S.; Kim, H.S.; Myung, G.; Kang, Y.G.; Yih, W. First successful culture of the marine dinoflagellate Dinophysis acuminata. Aquat. Microb. Ecol. 2006, 45, 101–106. [Google Scholar] [CrossRef]
- Nagai, S.; Nitshitani, G.; Tomaru, Y.; Sakiyama, S.; Kamiyama, T. Predation by the toxic dinoflagellate Dinophysis fortii on the ciliate Myrionecta rubra and observation of sequestration of ciliate chloroplasts. J. Phycol. 2008, 44, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Jaén, D.; Mamán, L.; Domínguez, R.; Martín, E. First report of Dinophysis acuta in culture. Harmful Algal News 2009, 39, 1–2. [Google Scholar]
- Riobó, P.; Reguera, B.; Franco, J.M.; Rodríguez, F. First report of the toxin profile of Dinophysis sacculus Stein from LC-MS analysis of laboratory cultures. Toxicon 2013, 76, 221–224. [Google Scholar] [CrossRef]
- Nagai, S.; Suzuki, T.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis tripos (Dinophyceae). Plankton Benthos Res. 2013, 8, 171–177. [Google Scholar] [CrossRef]
- Mafra, L.L., Jr.; Nagai, S.; Uchida, H.; Tavares, C.P.S.; Escobar, B.P.; Suzuki, T. Harmful effects of Dinophysis to the ciliate Mesodinium rubrum: Implications for prey capture. Harmful Algae 2016, 59, 82–90. [Google Scholar] [CrossRef]
- Nishitani, G.; Nagai, S.; Sakiyama, S.; Kamiyama, T. Successful cultivation of the toxic dinoflagellate Dinophysis caudata (Dinophyceae). Plankton Benthos Res. 2008, 3, 78–85. [Google Scholar] [CrossRef]
- Nishitani, G.; Nagai, S.; Takano, Y.; Sakiyama, S.; Baba, K.; Kamiyama, T. Growth characteristics and phylogenetic analysis of the marine dinoflagellate Dinophysis infundibulus (Dinophyceae). Aquat. Microb. Ecol. 2008, 52, 209–221. [Google Scholar] [CrossRef]
- Kim, S.; Kang, Y.G.; Kim, H.S.; Yih, W.; Coats, D.W.; Park, M.G. Growth and grazing responses of the mixotrophic dinoflagellate Dinophysis acuminata as functions of light intensity and prey concentration. Aquat. Microb. Ecol. 2008, 51, 301–310. [Google Scholar] [CrossRef]
- Kim, M.; Nam, S.W.; Shin, W.; Coats, D.W.; Park, M.G. Dinophysis caudata (Dinophyceae) sequesters and retains plastids from the mixotrophic ciliate prey Mesodinium rubrum. J. Phycol. 2012, 48, 569–579. [Google Scholar] [CrossRef]
- Kamiyama, T.; Suzuki, T. Production of dinophysis-1 and pectenotoxin-2 by a culture of Dinophysis acuminata (Dinophyceae). Harmful Algae 2009, 8, 312–317. [Google Scholar] [CrossRef]
- Hackett, J.D.; Tong, M.M.; Kulis, D.M.; Fux, E.; Hess, P.; Bire, R.; Anderson, D.M. DSP toxin production de novo in cultures of Dinophysis acuminata (Dinophyceae) from North America. Harmful Algae 2009, 8, 873–879. [Google Scholar] [CrossRef]
- Kamiyama, T.; Nagai, S.; Suzuki, T.; Miyamura, K. Effect of temperature on production of okadaic acid, dinophysistoxin-1, and pectenotoxin-2 by Dinophysis acuminata in culture experiments. Aquat. Microb. Ecol. 2010, 60, 193–202. [Google Scholar] [CrossRef]
- Tong, M.; Kulis, D.M.; Fux, E.; Smith, J.L.; Hess, P.; Zhou, Q.; Anderson, D.M. The effects of growth phase and light intensity on toxin production by Dinophysis acuminata from northeastern United States. Harmful Algae 2011, 10, 254–264. [Google Scholar] [CrossRef]
- Nagai, S.; Suzuki, T.; Nishikawa, T.; Kamiyama, T. Differences in the production and excretion kinetics of okadaic acid, dinophysistoxin-1, and pectenotoxin-2 between cultures of Dinophysis acuminata and Dinophysis fortii isolated from western Japan. J. Phycol. 2011, 47, 1326–13337. [Google Scholar] [CrossRef] [PubMed]
- Basti, L.; Uchida, H.; Kanamori, M.; Matsushima, R.; Suzuki, T.; Nagai, S. Mortality and pathology of Japanese scallop, Patinopecten (Mizuhopecten) yessoensis, and noble scallop, Mimachlamys nobilis, fed monoclonal culture of PTX-producer, Dinophysis caudata. In Marine and Freshwater Harmful Algae, Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand from 27 to 31 October 2014; MacKenzie, A.L., Ed.; Cawthron Institute, Nelson, New Zealand and International Society for the Study of Harmful Algae: Wellington, New Zealand, 2015; pp. 105–108. [Google Scholar]
- Basti, L.; Uchida, H.; Matsushima, R.; Watanabe, R.; Suzuki, T.; Yamatogi, T.; Nagai, S. Influence of temperature on growth and production of Pectenotoxin-2 by a monoclonal culture of Dinophysis caudata. Mar. Drugs 2015, 13, 7124–7137. [Google Scholar] [CrossRef] [PubMed]
- Basti, L.; Suzuki, T.; Uchida, H.; Kamiyama, T.; Nagai, S. Thermal acclimation affects growth and lipophilic toxin production in a strain of cosmopolitan harmful alga Dinophysis acuminata. Harmful Algae 2018, 73, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Gobler, J.C.; Dohrty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. PNAS 2017, 114, 4975–4980. [Google Scholar] [CrossRef] [PubMed]
- Guillotreau, P.; Le Bihan, V.; Morineau, B.; Pardo, S. The vulnerability of shellfish farmers to HAB events: An optimal matching analysis of closure decrees. Harmful Algae 2021, 101, 101968. [Google Scholar] [CrossRef]
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Reguera, B.; Riobó, P.; Rodríguez, F.; Díaz, P.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Carpenter, E.J.; Janson, S.; Boje, R.; Pollenhne, F.; Chang, J. The dinoflagellate Dinophysis norvegica: Biological and ecological observation in the Baltic Sea. Eur. J. Phycol. 1995, 30, 1–9. [Google Scholar] [CrossRef]
- Okolodkov, Y.B.; Dodge, J.D. Biodiversity and biogeography of planktonic dinoflagellates in the Arctic Ocean. J. Exp. Mar. Biol. Ecol. 1996, 202, 19–27. [Google Scholar] [CrossRef]
- Meyer-Harms, B.; Pollenhe, F. Alloxanthin in Dinophysis norvegica (Dinophysiales, Dinophyceae) from the Baltic Sea. J. Phycol. 1998, 34, 280–285. [Google Scholar] [CrossRef]
- Bresnan, E.; Fryer, R.; Hart, M.; Percy, L. Correlation between Algal Presence in Water and Toxin Presence in Shellfish. Fish. Res. Serv. Contract Rep. 2005, 4, 5. [Google Scholar]
- Jansen, S.; Riser, C.W.; Wassmann, P.; Bathmann, U. Copepod feeding behavior and egg production during a dinoflagellate bloom in the North Sea. Harmful Algae 2006, 5, 102–112. [Google Scholar] [CrossRef]
- Smayda, T.J. Harmful Algal Bloom Communities in Scottish Coastal Waters: Relationship to Fish Farming and Regional Comparisons—A Review; Scottish Executive Environmental Group: Edinburgh, UK, 2006. [Google Scholar]
- Whyte, C.; Swan, S.; Davidson, K. Changing wind patterns linked to unusually high Dinophysis blooms around the Shetland Islands, Scotland. Harmful Algae 2014, 39, 365–373. [Google Scholar] [CrossRef]
- Fabro, E.; Almandoz, G.O.; Ferrario, M.; Tillmann, U.; Cembella, A.; Krock, B. Distribution of Dinophysis species and their association with lipophilic phycotoxins in plankton from the Argentine Sea. Harmful Algae 2016, 59, 31–41. [Google Scholar] [CrossRef]
- Shahi, N.; Nayak, B.B.; Mallik, S.K. Dinophysis norvegica: First report of the toxic temperate water Dinophysis in Manori creek of Mumbai water. Harmful Algae News 2010, 42, 14–15. [Google Scholar]
- Hernández-Becerril, D.U. La Diversidad del Fitoplancton Marino de México. Un Acercamiento Actual. In Planctología Mexicana; Barreiro, M.T., Meave, M.E., Signoret, M., Figueroa, M.G., Eds.; Sociedad Mexicana de Planctología (SOMPAC); Universidad Autónoma Metropolitana: México City, México, 2003; pp. 1–17. [Google Scholar]
- Suba Rao, D.V.; Pan, Y.; Zitko, V.; Bugden, G.; Mackelgan, K. Diarrhetic shellfish poisoning (DSP) associated with a subsurface bloom of Dinophysis norvergica in Bedford Basin, eastern Canada. Mar. Ecol. Prog. Ser. 1993, 97, 117–126. [Google Scholar] [CrossRef]
- Cembella, A.D. Occurrence of okadaic acid, a major diarrhetic shellfish toxin, in natural populations of Dinophysis spp. from the east of North America. J. Appl. Phycol. 1989, 1, 307–310. [Google Scholar] [CrossRef]
- Goto, H.; Igarashi, T.; Watai, M.; Yasumoto, T.; Gomez, O.V.; Valdivia, G.L.; Noren, F.; Gisselson, L.A.; Granéli, E. Worldwide occurrence of pectenotoxins and yessotoxins in shellfish and phytoplankton. In Proceedings of the Ninth International Conference on Harmful Algal Blooms, Tasmania, Australia, 7–11 February 2000; p. 20 (Abstract). [Google Scholar]
- Pimiä, V.; Kankaanpaä, H.; Kononen, K. The first observation of okadaic acid in Mytilus edulis from the Gulf of Finland. Boreal Environ. Res. 1998, 2, 381–385. [Google Scholar]
- Sipiä, V.; Kankaanpaä, H.; Meriluoto, J. The first observation of okadaic acid in flounder in the Baltic Sea. Sarsia 2000, 85, 471–475. [Google Scholar] [CrossRef]
- Deeds, J.R.; Stutts, W.L.; Celiz, M.D.; mac Leod, J.; Hamilton, A.E.; Lewis, B.J.; Miller, D.W.; Kanwit, K.; Smith, J.L.; Kulis, D.M.; et al. Dihydrodinophysistoxin-1 produced by Dinophysis norvegica in the Gulf of Main, USA and its accumulation in shellfish. Toxins 2020, 12, 533. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyazono, A.; Baba, K.; Sugawara, R.; Kamiyama, T. LC-MS/MS analysis of okadaic acid analogues and other lipophilic toxins in single-cell isolates of several Dinophysis species collected in Hokkaido, Japan. Harmful Algae 2009, 8, 233–238. [Google Scholar] [CrossRef]
- Uchida, H.; Watanabe, R.; Matsushima, R.; Oikawa, H.; Nagai, S.; Kamiyma, T.; Baba, K.; Miyazono, A.; Kosaka, K.; Kaga, S.; et al. Toxin profiles of okadaic acid analogues and other lipophilic toxins in Dinophysis from Japanese coastal waters. Toxins 2018, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Balech, E. Some Norwegian Dinophysis species (Dinoflagellata). Sarsia 1976, 61, 75–94. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Fukuyo, Y.; Larsen, J. Taxonomy of harmful dinoflagellates. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; IOC Manuals and Guides No. 33; UNESCO: Paris, France, 1995; pp. 283–317. [Google Scholar]
- IOC-UNESCO. The Harmful Algal Event Database (HAEDAT). Available online: http://haedat.iode.org/index.php (accessed on 18 February 2023).
- Martino, S.; Gianella, F.; Davidson, K. An approach for evaluating the economic impacts of harmful algal blooms: The effects of blooms of toxic Dinophysis spp. on the productivity of Scottish shellfish farms. Harmful Algae 2020, 99, 101912. [Google Scholar] [CrossRef]
- Séchet, V.; Sibat, M.; Billien, G.; Carpentier, L.; Rovillon, G.A.; Raimbault, V.; Malo, F.; Gaillard, S.; Perrière-Rumebe, M.; Hess, P.; et al. Characterization of toxin-producing strains of Dinophysis spp. (Dinophyceae) isolated from French coastal waters, with a particular focus on the D. acuminata-complex. Harmful Algae 2021, 107, 101974. [Google Scholar] [CrossRef]
- Guillard, R.P.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Inveretbrate Animals; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975. [Google Scholar]
- Nagai, S.; Matsuyama, Y.; Oh, S.-J.; Itakura, S. Effect of nutrients and temperature on encystment of the toxic dinoflagellate Alexandrium tamarense (Dinophyceae) isolated from Hiroshima Bay, Japan. Plankton Biol. Ecol. 2004, 51, 103–109. [Google Scholar]
- Guillard, R.R.L.; Kilham, P.; Jackson, T.A. Kinetics of silicon-limited growth in the marine diatom Thalassiosira pesudonana Hasle and Heimdal (=Cyclotella nana Husted). J. Phycol. 1973, 9, 233–237. [Google Scholar]
- Nagai, S.; Yamamoto, K.; Hata, N.; Itakura, S. Study of DNA extraction methods for use in loop-mediated isothermal amplification detection of single resting cysts in the toxic dinoflagellates Alexandrium tamarense and A. catenella. Mar. Genom. 2012, 7, 51–56. [Google Scholar] [CrossRef]
- Scholin, C.A.; Herzog, M.; Sogin, M.; Anderson, D.M. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 1994, 30, 999–1011. [Google Scholar] [CrossRef]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large data sets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.K.T. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Mitsuya, T.; Imai, M.; Yamasaki, M. DSP toxin contents in Dinophysis fortii and scallops collected at Mutsu Bay Japan. J. Appl. Phycolology 1997, 8, 509–515. [Google Scholar] [CrossRef]
- Suzuki, T.; Mitsuya, T.; Matsubara, H.; Yamasaki, M. Determination of pectenotoxin-2 after solid-phase extraction from seawater and from the dinoflagellate Dinophysis fortii by liquid chromatography with electrospray mass spectrometry and ultraviolet detection: Evidence of oxidation of pectenotoxin-2 to pectenotoxin-6 in scallops. J. Chromatography A 1998, 815, 155–160. [Google Scholar]
- Ajani, P.A.; Henriquez-Nunez, H.F.; Verma, A.; Nagai, S.; Uchida, H.; Tesoriero, M.J.; Farrell, H.; Zammit, A.; Brett, S.; Murray, S.A. Mapping the development of a Dinophysis bloom in a shellfish aquaculture area using a novel molecular qPCR assay. Harmful Algae 2022, 116, 102253. [Google Scholar] [CrossRef]
- Suzuki, T.; Quilliam, M. LC-MS/MS analysis of Diarrhetic Shellfish Poisoning (DSP) toxins, okadaic acid and dinophysistoxin analogues, and other lipophilic toxins. Anal. Sci. 2011, 27, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Albinsson, M.E.; Negri, A.P.; Blackburn, S.I.; Bolch, C.J.S. Bacterial Community Affects Toxin Production by Gymnodinium catenatum. PLoS ONE 2014, 9, e104623. [Google Scholar] [CrossRef]


| Strains | Concentration in Culture (ng per mL−1) | Number of Cells | Cell Quota (pg per mL−1) | ||||
|---|---|---|---|---|---|---|---|
| OA | DTX1 | PTX2 | OA | DTX1 | PTX2 | ||
| DN16062021FUN-01 | ND (<0.1) | ND (<0.1) | 137 | 1807 | ND (<0.01) | ND (<0.01) | 75.8 |
| DN16062021FUN-02 | ND (<0.1) | ND (<0.1) | 126 | 2080 | ND (<0.01) | ND (<0.01) | 60.6 |
| DN16062021FUN-03 | ND (<0.1) | ND (<0.1) | 145 | 2333 | ND (<0.01) | ND (<0.01) | 62.1 |
| DN16062021FUN-05 | ND (<0.1) | 1.44 | 375 | 2850 | ND (<0.01) | 0.5 | 131.6 |
| DN16062021FUN-06 | ND (<0.1) | 3.55 | 316 | 3050 | ND (<0.01) | 1.2 | 103.6 |
| DN16062021FUN-07 | ND (<0.1) | ND (<0.1) | 161 | 1057 | ND (<0.02) | ND (<0.02) | 152.4 |
| DN16062021FUN-08 | ND (<0.1) | 0.705 | 132 | 936 | ND (<0.02) | 0.7 | 137.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, S.; Basti, L.; Uchida, H.; Kuribayashi, T.; Natsuike, M.; Sildever, S.; Nakayama, N.; Lum, W.M.; Matsushima, R. Growth, Toxin Content and Production of Dinophysis Norvegica in Cultured Strains Isolated from Funka Bay (Japan). Toxins 2023, 15, 318. https://doi.org/10.3390/toxins15050318
Nagai S, Basti L, Uchida H, Kuribayashi T, Natsuike M, Sildever S, Nakayama N, Lum WM, Matsushima R. Growth, Toxin Content and Production of Dinophysis Norvegica in Cultured Strains Isolated from Funka Bay (Japan). Toxins. 2023; 15(5):318. https://doi.org/10.3390/toxins15050318
Chicago/Turabian StyleNagai, Satoshi, Leila Basti, Hajime Uchida, Takanori Kuribayashi, Masafumi Natsuike, Sirje Sildever, Natsuko Nakayama, Wai Mun Lum, and Ryuji Matsushima. 2023. "Growth, Toxin Content and Production of Dinophysis Norvegica in Cultured Strains Isolated from Funka Bay (Japan)" Toxins 15, no. 5: 318. https://doi.org/10.3390/toxins15050318
APA StyleNagai, S., Basti, L., Uchida, H., Kuribayashi, T., Natsuike, M., Sildever, S., Nakayama, N., Lum, W. M., & Matsushima, R. (2023). Growth, Toxin Content and Production of Dinophysis Norvegica in Cultured Strains Isolated from Funka Bay (Japan). Toxins, 15(5), 318. https://doi.org/10.3390/toxins15050318






