Practical Application of a Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—Relevance to Anti-Müllerian Hormone and Serum Amyloid A Clarified from a Two-Year Survey
Abstract
1. Introduction
2. Results
2.1. Long-Term (2 Years) Monitoring of ZEN in a JB Breeding Cattle Herd
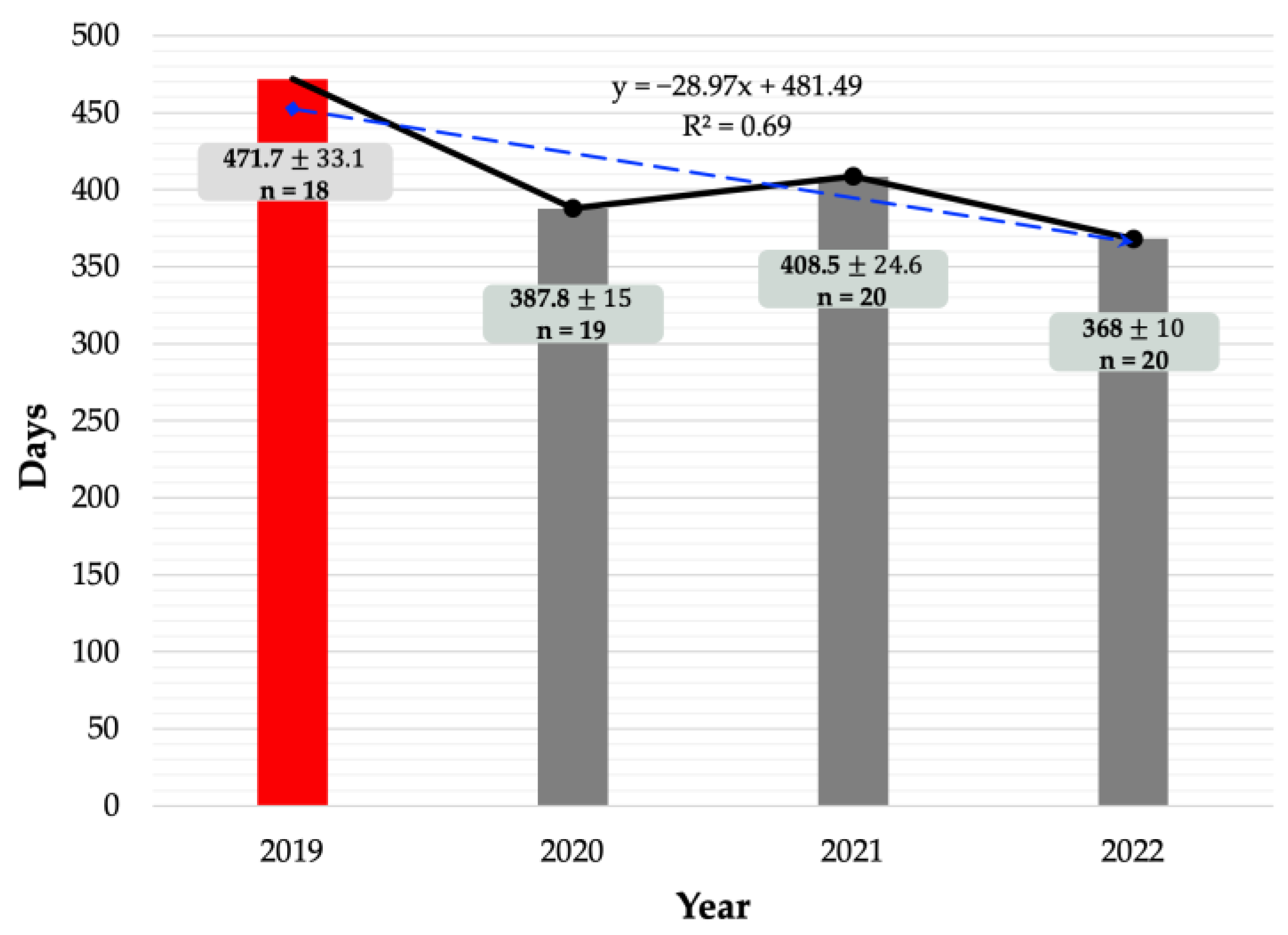
2.2. Calving Intervals as a Reproductive Indicator
3. Discussion
4. Conclusions
5. Materials and Methods
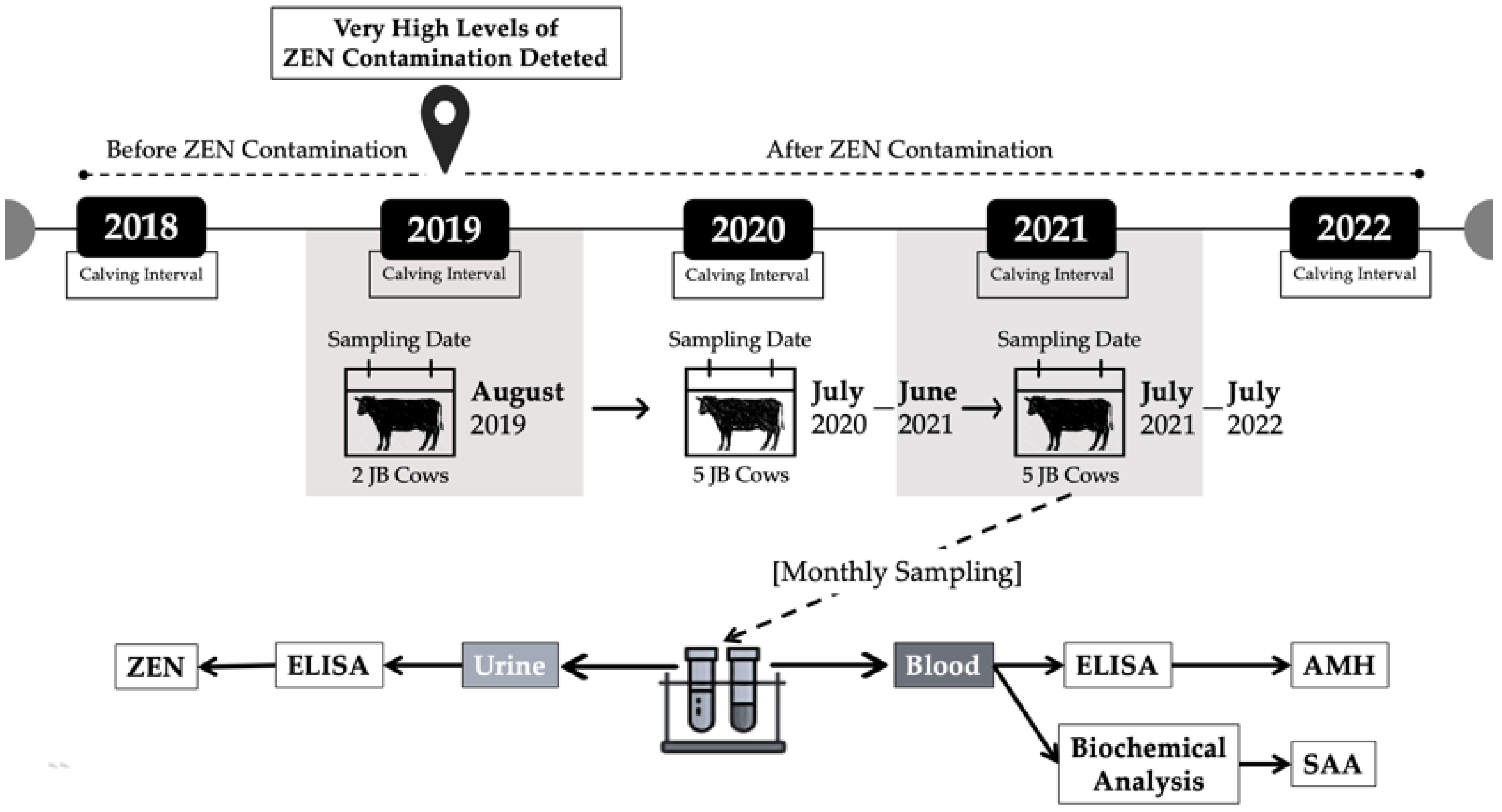
5.1. ZEN Monitoring and Reproductive Performance Evaluation
5.2. Analysis Methods of ZEN, AMH, and SAA
5.3. Feeding Management
5.4. Data Management and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of The Widely Cited FAO Estimate of 25. Crit. Rev. Food Sci. Nutr 2019, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Jalilzadeh-Amin, G.; Dalir-Naghadeh, B.; Ahmadnejad-Asl-Gavgani, M.; Fallah, A.A.; Mousavi Khaneghah, A. Prevalence and Concentration of Mycotoxins in Animal Feed in the Middle East and North Africa (MENA): A Systematic Review and Meta-Analysis. Toxins 2023, 15, 214. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; González-Peñas, E. Co-Occurrence of Mycotoxins in Feed for Cattle, Pigs, Poultry, and Sheep in Navarra, a Region of Northern Spain. Toxins 2023, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.; Valente, D.; Carolino, N.; Dinis, H.; Sousa, R.; Duarte, S.C.; Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A. Occurrence of Zearalenone in Dairy Farms—A study on The Determinants of Exposure and Risk Assessment. Toxicon 2023, 225, 107051. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-Occurrence in Animal Feed-Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin Occurrence in Commodities, Feeds and Feed Ingredients Sourced in The Middle East and Africa. Food Addit Contam Part B Surveill 2011, 4, 168–179. [Google Scholar] [CrossRef]
- Abbes, J.; Belgacem, H.; Ezzdini, K.; Wahhab, M.; Abbes, S. Zearalenone Nephrotoxicity: DNA Fragmentation, Apoptotic Gene Expression and Oxidative Stress Protected by Lactobacillus plantarum MON03. Toxicon 2020, 175, 28–35. [Google Scholar] [CrossRef]
- Gromadzka, K.; Waskiewicz, A.; Chelkowski, J.; Golinski, P. Zearalenone and Its Metabolites: Occurrence, Detection, Toxicity and Guidelines. World Mycotoxin J. 2008, 1, 209–220. [Google Scholar] [CrossRef]
- Castelló, P.L.; Sacco, M.A.; Aquila, I.; Cortés, J.C.M.; García, C.J. Evaluation of Zearalenones and Their Metabolites in Chicken, Pig and Lamb Liver Samples. Toxins 2022, 14, 782. [Google Scholar] [CrossRef]
- Widodo, O.S.; Etoh, M.; Kokushi, E.; Uno, S.; Yamato, O.; Pambudi, D.; Okawa, H.; Taniguchi, M.; Lamid, M.; Takagi, M. Practical Application of Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—The Relationship between Monthly Anti-Müllerian Hormone and Serum Amyloid A Concentrations. Toxins 2022, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, H.; Takagi, M.; Kawamura, O.; Taniguchi, C.; Nakamura, M.; Chuma, T.; Uno, S.; Kokushi, E.; Matsumoto, D.; Tshering, C.; et al. Natural Contamination of Dietary Rice Straw with Zearalenone and Urinary Zearalenone Concentrations in A Cattle Herd. J. Anim. Sci. 2012, 90, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Uno, S.; Kokushi, E.; Shiga, S.; Mukai, S.; Kuriyagawa, T.; Takagaki, K.; Hasunuma, H.; Matsumoto, D.; Okamoto, K.; et al. Measurement of Urinary Zearalenone Concentrations for Monitoring Natural Feed Contamination in Cattle Herds-On Farm Trials. J. Anim. Sci. 2011, 89, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, K.P.; Jeyakumar, S.; Mall, S.; Vedamurthy, G.V.; Kumaresan, A.; Devadasn, M.J.; Kataktalware, M.A.; Arul, D.; Das, D.N.; Manimaran, A. Anti-Müllerian Hormone as An Endocrine Biomarker of Reproductive Longevity and Assessment of Single Nucleotide Polymorphisms in AMH Gene of Bos Indicus Breeds of Cattle. Reprod Domest Anim. 2022, 57, 1450–1464. [Google Scholar] [CrossRef]
- Umer, S.; Zhao, S.J.; Sammad, A.; Sahlu, B.W.; Pang, Y.W.; Zhu, H. AMH: Could It Be Userd as A Biomarker for Fertility and Superovulation in Domestic Animals? Genes 2019, 10, 1009. [Google Scholar] [CrossRef]
- Mossa, F.; Ireland, J.J. Physiology and Endocrinology Symposium: Anti-Müllerian Hormone: A Biomarker for The Ovarian Reserve, Ovarian Function, and Fertility in Dairy Cows. J. Anim. Sci. 2019, 97, 1446–1455. [Google Scholar] [CrossRef]
- Mossa, F.; Jimenez-Krassel, F.; Scheetz, D.; Weber-Nielsen, M.; Evans, A.C.O.; Ireland, J.J. Anti-Müllerian Hormone (AMH) and Fertility Management in Agricultural Species. Reproduction 2017, 154, 1–11. [Google Scholar] [CrossRef]
- Bazzano, M.; Marchegiani, A.; Troisi, A.; McLean, A.; Laus, F. Serum Amyloid A as a Promising Biomarker in Domestic Animals’ Reproduction: Current Knowledge and Future Perspective. Animals 2022, 12, 589. [Google Scholar] [CrossRef]
- Eckersall, P.D.; Bell, R. Acute Phase Proteins: Biomarkers of Infection and Inflammation in Veterinary Medicine. Vet J. 2010, 185, 23–27. [Google Scholar] [CrossRef]
- Chan, J.P.; Chang, C.C.; Hsu, W.L.; Liu, W.B.; Chen, T.H. Association of Increased Serum Acute-Phase Protein Concentrations with Reproductive Performance in Dairy Cows with Postpartum Metritis. Vet Clin Pathol. 2010, 39, 72–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, B.; Wang, M.; Tong, J.; Pan, J.; Wang, N.; Gong, P.; Long, M. Selenium Protects Against Zearalenone-Induced Oxidative Stress and Apoptosis in The Mouse Kidney by Inhibiting Endoplasmic Reticulum Stress. Oxid Med Cell Longev. 2020, 6059058. [Google Scholar] [CrossRef] [PubMed]
- Fink-Gremmels, J. The Role of Mycotoxins in The Health and Performance of Dairy Cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Weaver, H.A.; Kurtz, H.T.; Behrens, J.C.; Robinson, T.S.; Seguin, B.E.; Bates, F.Y.; Mirocha, J.C. Effect of Zearalenone of Dairy Cows. Am. J. Vet. Res. 1986, 47, 659–662. [Google Scholar]
- Weaver, H.A.; Kurtz, H.T.; Behrens, J.C.; Robinson, T.S.; Seguin, B.E.; Bates, F.Y.; Mirocha, J.C. Effects of Zearalenone on The Fertility of Virgin Dairy Heifers. Am. J. Vet. Res. 1986, 47, 1395–1397. [Google Scholar]
- Guerre, P. Mycotoxin and Gut Microbiota Interactions. Toxins 2020, 12, 769. [Google Scholar] [CrossRef]
- Fushimi, Y.; Takagi, M.; Hasunuma, H.; Uno, S.; Kokushi, E.; Watanabe, U.; Liu, J.; Marey, M.A.; Miyamoto, A.; Otoi, T.; et al. Application of Mycotoxin Adsorbent to Cattle Feed Contaminated with Zearalenone: Urinary Zearalenone Excretion and Association with Anti-Müllerian Hormone. World Mycotoxin J. 2014, 7, 267–378. [Google Scholar] [CrossRef]
- Koizumi, M.; Kadokawa, H. Positive Correlations of Age and Parity with Plasma Anti-Müllerian Hormone Concentrations in Japanese Black Cows. J. Reprod. Dev. 2017, 63, 205–209. [Google Scholar] [CrossRef]

| ZEN | AMH | SAA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | |
| ZEN (lag 1-month) | –0.227 | 0.401 | 0.611 | 0.336 | 0.171 | 0.145 | 0.001 | 0.002 | 0.238 |
| ZEN (lag 2-month) | 0.545 | 0.229 | 0.098 | –0.247 | 0.098 | 0.086 | 0.001 | 0.001 | 0.094 |
| AMH (lag 1-month) | 1.116 | 0.833 | 0.272 | 0.214 | 0.356 | 0.59 | –0.002 | 0.004 | 0.646 |
| AMH (lag 2-month) | 2.021 | 0.825 | 0.092 | 0.64 | 0.353 | 0.167 | 0.002 | 0.004 | 0.71 |
| SAA (lag 1-month) | –61.186 | 54.531 | 0.344 | 5.901 | 23.325 | 0.817 | –0.603 | 0.274 | 0.115 |
| SAA (lag 2-month) | –54.915 | 39.886 | 0.262 | 35.217 | 17.061 | 0.131 | 0.35 | 0.201 | 0.179 |
| ∆ZEN | ∆AMH | ∆SAA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | |
| ∆ZEN (lag 1-month) | –0.22 | 0.463 | 0.652 | 0.188 | 0.177 | 0.331 | –0.001 | 0.002 | 0.6 |
| ∆AMH (lag 1-month) | 0.699 | 1.198 | 0.581 | –0.142 | 0.459 | 0.768 | –0.008 | 0.005 | 0.184 |
| ∆SAA (lag 1-month) | –63.11 | 62.435 | 0.351 | –9.712 | 23.925 | 0.699 | –0.455 | 0.283 | 0.159 |
| ZEN | AMH | SAA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | |
| ZEN (lag 1-month) | 5.75 × 10−3 | 1.30 × 10−1 | 0.965 | –0.012 | 0.021 | 0.582 | –1.48 × 10−5 | 2.66 × 10−4 | 0.956 |
| ZEN (lag 2-month) | –6.12 × 10−2 | 1.27 × 10−1 | 0.632 | –0.042 | 0.021 | 0.049 * | 3.01 × 10−4 | 2.59 × 10−4 | 0.252 |
| AMH (lag 1-month) | 9.98 × 10−1 | 8.80 × 10−1 | 0.264 | 0.41 | 0.144 | 0.007 ** | –1.47 × 10−3 | 1.80 × 10−3 | 0.42 |
| AMH (lag 2-month) | 1.16 × 100 | 9.21 × 10−1 | 0.216 | 0.299 | 0.151 | 0.054 | 3.58 × 10−3 | 1.89 × 10−3 | 0.065 |
| SAA (lag 1-month) | 1.53 × 10 | 7.20 × 10 | 0.833 | 1.814 | 11.759 | 0.878 | 5.49 × 10−3 | 1.47 × 10−1 | 0.711 |
| SAA (lag 2-month) | –1.02 × 102 | 7.26 × 10 | 0.17 | 12.499 | 11.859 | 0.298 | 2.38 × 10−1 | 1.49 × 10−1 | 0.117 |
| ∆ZEN | ∆AMH | ∆SAA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | Estimate | Std. Error | p-Value | |
| ∆ZEN (lag 1-month) | –0.441 | 0.137 | 0.003 ** | –0.003 | 0.019 | 0.855 | 2.3 × 10−5 | 2.7 × 10−4 | 0.933 |
| ∆ZEN (lag 2-month) | –0.242 | 0.129 | 0.067 | –0.045 | 0.018 | 0.016 * | 3.5 × 10−4 | 2.6 × 10−4 | 0.179 |
| ∆AMH (lag 1-month) | 1.725 | 1.049 | 0.109 | –0.428 | 0.145 | 0.005 ** | –0.002 | 0.002 | 0.266 |
| ∆AMH (lag 2-month) | 1.662 | 1.064 | 0.126 | –0.199 | 0.147 | 0.184 | 0.002 | 0.002 | 0.475 |
| ∆SAA (lag 1-month) | 27.007 | 80.606 | 0.739 | –7.057 | 11.111 | 0.529 | –0.527 | 0.161 | 0.002 ** |
| ∆SAA (lag 2-month) | –70.452 | 81.193 | 0.391 | 5.148 | 11.192 | 0.648 | 0.057 | 0.163 | 0.728 |
| Calving Interval | n | Mean | SEM | p-Value | Calving Interval | n | Mean | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Pre-ZEN monitoring (2019) | 18 | 471.7 | 33.1 | 0.007 * | Pre-ZEN monitoring (2019) | 18 | 471.7 | 33.1 | 0.005 * |
| Post-ZEN monitoring (2020, 2021, 2022) | 59 | 388.2 | 10.3 | Post-ZEN monitoring (2022) | 20 | 368.3 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widodo, O.S.; Pambudi, D.; Etoh, M.; Kokushi, E.; Uno, S.; Yamato, O.; Taniguchi, M.; Lamid, M.; Takagi, M. Practical Application of a Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—Relevance to Anti-Müllerian Hormone and Serum Amyloid A Clarified from a Two-Year Survey. Toxins 2023, 15, 317. https://doi.org/10.3390/toxins15050317
Widodo OS, Pambudi D, Etoh M, Kokushi E, Uno S, Yamato O, Taniguchi M, Lamid M, Takagi M. Practical Application of a Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—Relevance to Anti-Müllerian Hormone and Serum Amyloid A Clarified from a Two-Year Survey. Toxins. 2023; 15(5):317. https://doi.org/10.3390/toxins15050317
Chicago/Turabian StyleWidodo, Oky Setyo, Dhidhi Pambudi, Makoto Etoh, Emiko Kokushi, Seiichi Uno, Osamu Yamato, Masayasu Taniguchi, Mirni Lamid, and Mitsuhiro Takagi. 2023. "Practical Application of a Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—Relevance to Anti-Müllerian Hormone and Serum Amyloid A Clarified from a Two-Year Survey" Toxins 15, no. 5: 317. https://doi.org/10.3390/toxins15050317
APA StyleWidodo, O. S., Pambudi, D., Etoh, M., Kokushi, E., Uno, S., Yamato, O., Taniguchi, M., Lamid, M., & Takagi, M. (2023). Practical Application of a Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—Relevance to Anti-Müllerian Hormone and Serum Amyloid A Clarified from a Two-Year Survey. Toxins, 15(5), 317. https://doi.org/10.3390/toxins15050317






