Free and Modified Mycotoxins in Organic and Conventional Oats (Avena sativa L.) Grown in Scotland
Abstract
1. Introduction
2. Results
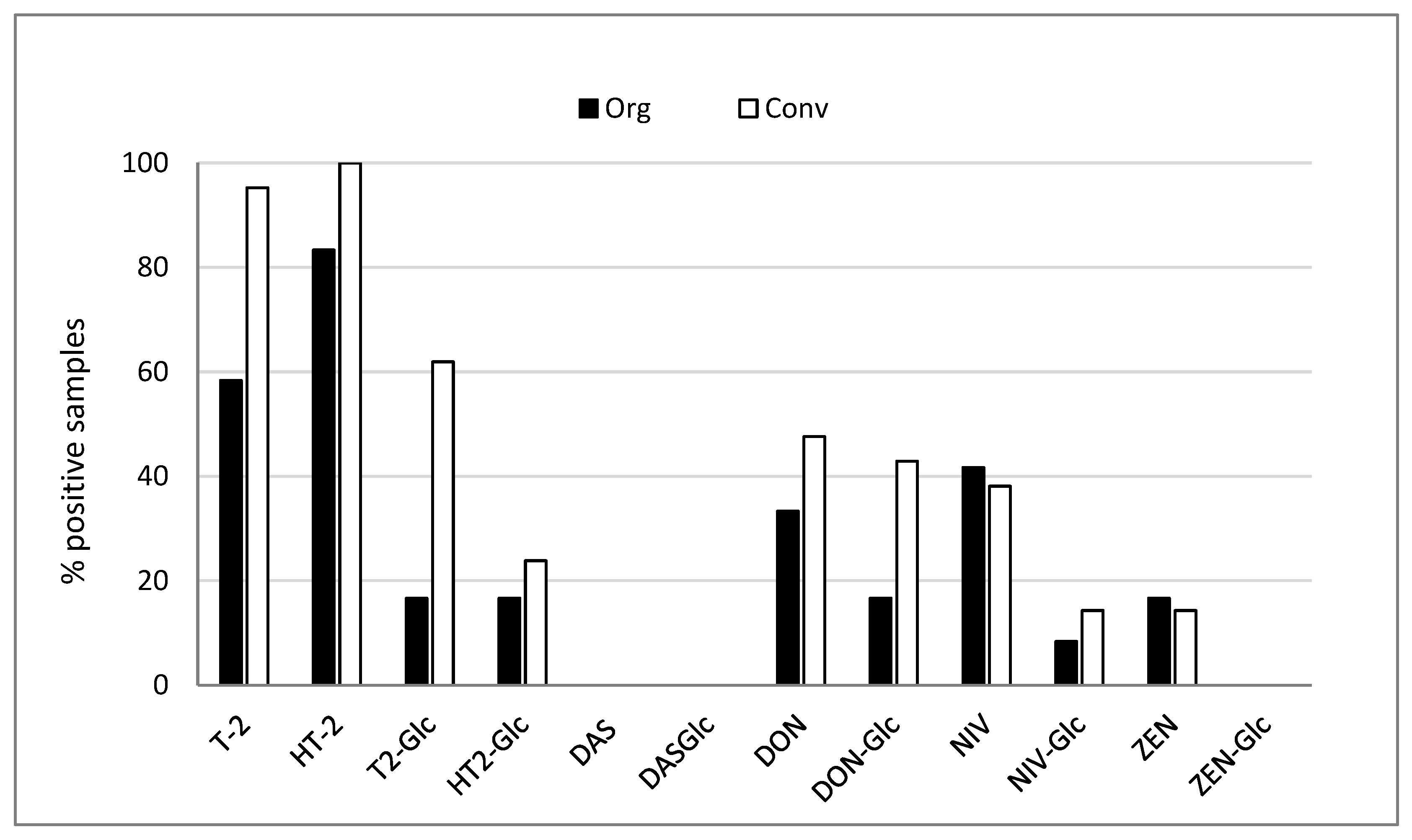
2.1. Prevalence of Free and Modified Mycotoxins in Organic and Conventional Oats
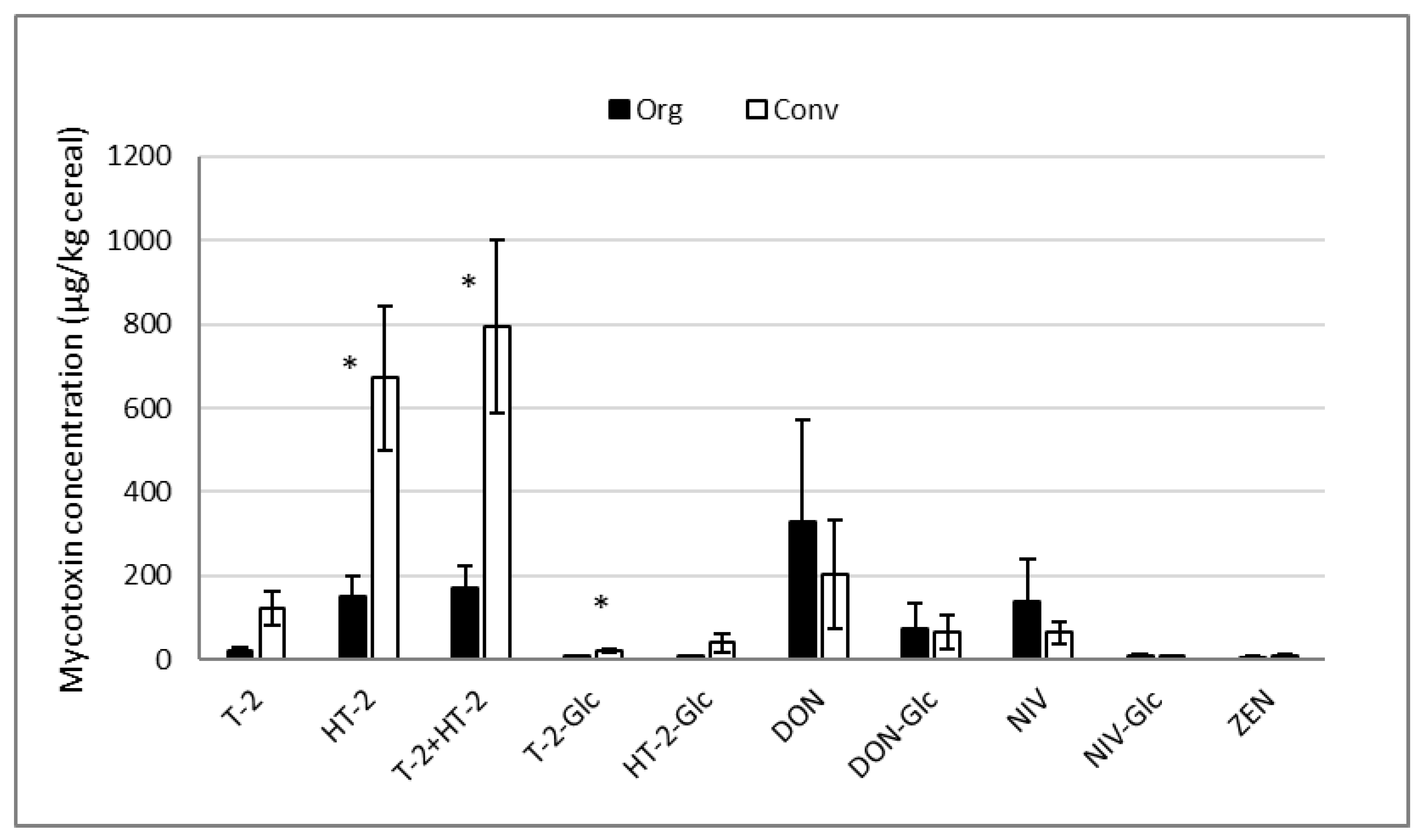
2.2. Concentrations of Free and Modified Mycotoxins in Organic and Conventional Oats
2.3. Co-Occurrence of Free Mycotoxins in Organic and Conventional Oats
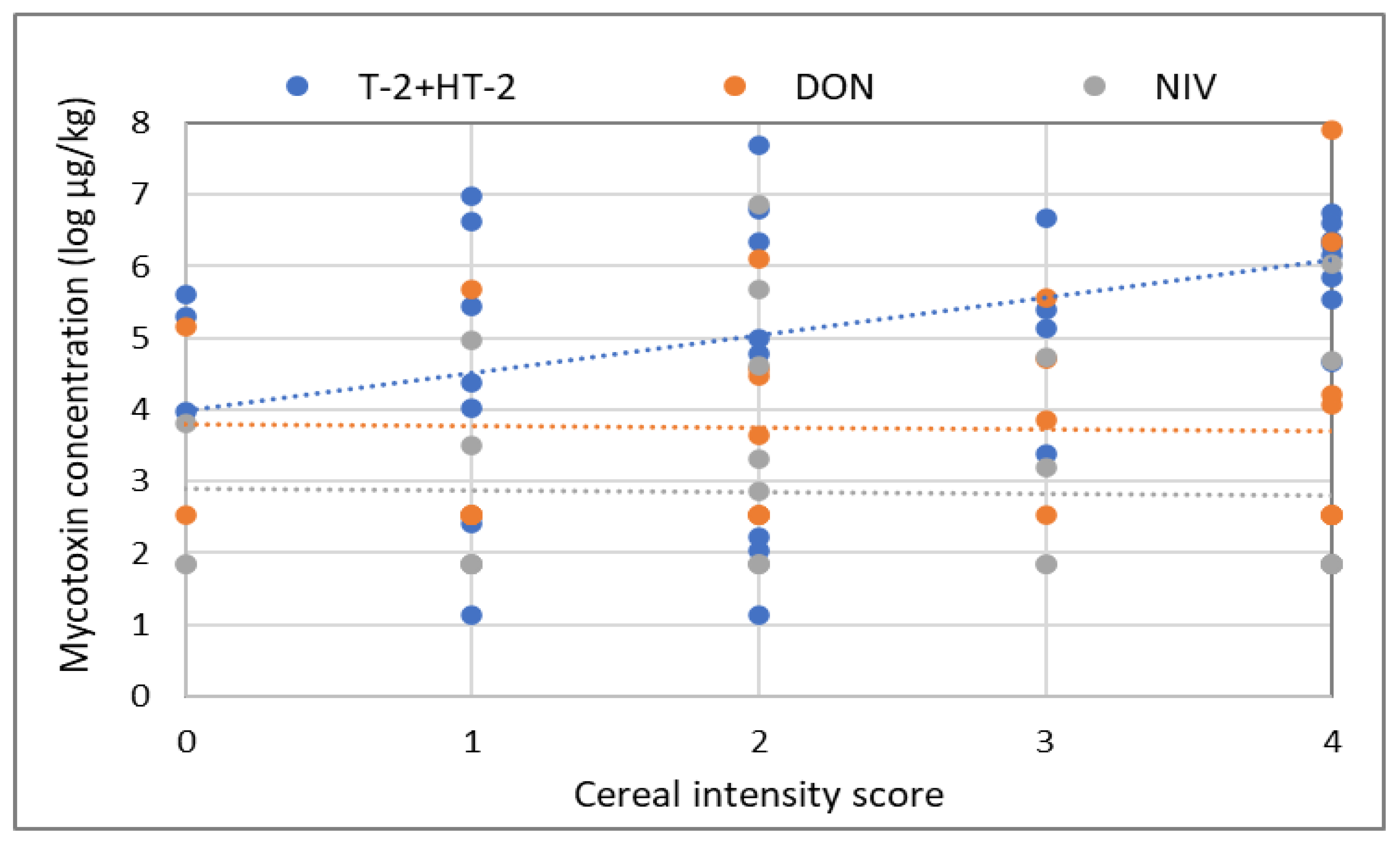
2.4. Effect of Other Agronomy Factors on Mycotoxin Concentrations in Oats
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Mycotoxin Determination in Oat Samples
4.2.1. Mycotoxin Standards
4.2.2. Extraction of Oat Samples
4.2.3. LC-MS/MS Analysis of Mycotoxins
4.3. Method Performance Validation
4.4. Data calculations and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, M.T.; Ameye, M.; Phan, L.T.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Impact of Ethnic Pre-Harvest Practices on the Occurrence of Fusarium Verticillioides and Fumonisin B1 in Maize Fields from Vietnam. Food Control 2021, 120, 107567. [Google Scholar] [CrossRef]
- Degraeve, S.; Madege, R.; Audenaert, K.; Kamala, A.; Ortiz, J.; Kimanya, M.; Tiisekwa, B.; De Meulenaer, B.; Haesaert, G. Impact of Local Pre-Harvest Management Practices in Maize on the Occurrence of Fusarium Species and Associated Mycotoxins in Two Agro-Ecosystems in Tanzania. Food Control 2016, 59, 225–233. [Google Scholar] [CrossRef]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.; Chrpova, J.; Czembor, E.; Gagkaeva, T. A European Database of Fusarium Graminearum and F. Culmorum Trichothecene Genotypes. Front. Microbiol. 2016, 7, 406. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Seehusen, T.; Riley, H.; Dill-Macky, R.; Holen, B.; Brodal, G. Fusarium and Mycotoxin Content of Harvested Grain was Not Related to Tillage Intensity in Norwegian Spring Wheat Fields. World Mycotoxin J. 2020, 13, 473–486. [Google Scholar] [CrossRef]
- Fredlund, E.; Gidlund, A.; Sulyok, M.; Börjesson, T.; Krska, R.; Olsen, M.; Lindblad, M. Deoxynivalenol and Other Selected Fusarium Toxins in Swedish oats—Occurrence and Correlation to Specific Fusarium Species. Int. J. Food Microbiol. 2013, 167, 276–283. [Google Scholar] [CrossRef]
- Polak-śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef]
- Alkadri, D.; Rubert, J.; Prodi, A.; Pisi, A.; Manes, J.; Soler, C. Natural Co-Occurrence of Mycotoxins in Wheat Grains from Italy and Syria. Food Chem. 2014, 157, 111–118. [Google Scholar] [CrossRef]
- Schollenberger, M.; Jara, H.T.; Suchy, S.; Drochner, W.; Müller, H.-M. Fusarium Toxins in Wheat Flour Collected in an Area in Southwest Germany. Int. J. Food Microbiol. 2002, 72, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.H.; Ghorbani, F.; Berg, T. Deoxynivalenol and Other Fusarium Toxins in Wheat and Rye Flours on the Danish Market. Food Addit. Contam. 2003, 20, 396–404. [Google Scholar] [CrossRef]
- Hajšlová, J.; Lancová, K.; Sehnalová, M.; Krplová, A.; Zachariášová, M.; Moravcová, H.; Nedělník, J.; Marková, J.; Ehrenbergerová, J. Occurrence of Trichothecene Mycotoxins in Cereals Harvested in the Czech Republic. Czech J. Food Sci. 2007, 25, 339–350. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium Mycotoxin Content of UK Organic and Conventional Wheat. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 496–506. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Syvahuoko, J.; Malachova, A.; Jestoi, M.; Varga, E.; Michlmayr, H.; Adam, G.; Sievilainen, E.; Berthiller, F.; Peltonen, K. Simultaneous Determination of Major Type A and B Trichothecenes, Zearalenone and Certain Modified Metabolites in Finnish Cereal Grains with a Novel Liquid Chromatography-Tandem Mass Spectrometric Method. Anal. Bioanal. Chem. 2015, 407, 4745–4755. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium Mycotoxin Content of UK Organic and Conventional Barley. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 1185–1190. [Google Scholar] [CrossRef]
- Barthel, J.; Gottschalk, C.; Rapp, M.; Berger, M.; Bauer, J.; Meyer, K. Occurrence of Type A, B and D Trichothecenes in Barley and Barley Products from the Bavarian Market. Mycotoxin Res. 2012, 28, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Drakopoulos, D.; Sulyok, M.; Krska, R.; Logrieco, A.F.; Vogelgsang, S. Raised Concerns about the Safety of Barley Grains and Straw: A Swiss Survey Reveals a High Diversity of Mycotoxins and Other Fungal Metabolites. Food Control 2021, 125, 107919. [Google Scholar] [CrossRef]
- Ivanova, L.; Sahlstrom, S.; Rud, I.; Uhlig, S.; Faeste, C.K.; Eriksen, G.S.; Divon, H.H. Effect of Primary Processing on the Distribution of Free and Modified Fusarium Mycotoxins in Naturally Contaminated Oats. World Mycotoxin J. 2017, 10, 73–88. [Google Scholar] [CrossRef]
- Edwards, S.G. Fusarium Mycotoxin Content of UK Organic and Conventional Oats. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.C.; Hennies, I.; Wessels, D.; Schwarz, K. Survey of Mycotoxins in Milling Oats Dedicated for Food Purposes between 2013 and 2019 by LC–MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 1934–1947. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jiménez, M.; Mateo, E.M. Potential Health Risk Associated with Mycotoxins in Oat Grains Consumed in Spain. Toxins 2021, 13, 421. [Google Scholar] [CrossRef]
- EC—European Commission. Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products. Off. J. Eur. Comm. L 2013, 91, 12–15. [Google Scholar]
- European Commission. Commission Regulation (EC) no 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union. 2006, 364, 5–24. [Google Scholar]
- Byrd, N.; Slaiding, I.R. Monitoring of Mycotoxins and Other Contaminants in UK Cereals used in Malting, Milling & Animal Feed. FERA 2016, PR578, 1–37. [Google Scholar]
- Monitoring of Contaminants in UK Cereals used for Processing Food and Animal Feed (2016–22); AHDB: Kenilworth, UK, 2022; pp. 1–19.
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and Validation of an LC-MS/MS Method for the Simultaneous Determination of Deoxynivalenol, Zearalenone, T-2-Toxin and some Masked Metabolites in Different Cereals and Cereal-Derived Food. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 819–835. [Google Scholar] [CrossRef]
- Rasmussen, P.H.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.C.; Jørgensen, L.N. Occurrence of Different Trichothecenes and Deoxynivalenol-3-Β-D-Glucoside in Naturally and Artificially Contaminated Danish Cereal Grains and Whole Maize Plants. Mycotoxin Res. 2012, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Bryla, M.; Ksieniewicz-Wozniak, E.; Waskiewicz, A.; Szymczyk, K.; Jedrzejczak, R. Natural Occurrence of Nivalenol, Deoxynivalenol, and Deoxynivalenol-3-Glucoside in Polish Winter Wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef]
- McCormick, S.P.; Kato, T.; Maragos, C.M.; Busman, M.; Lattanzio, V.M.T.; Galaverna, G.; Dall-Asta, C.; Crich, D.; Price, N.P.J.; Kurtzman, C.P. Anomericity of T-2 Toxin-Glucoside: Masked Mycotoxin in Cereal Crops. J. Agric. Food Chem. 2015, 63, 731–738. [Google Scholar] [CrossRef]
- Gratz, S.W.; Duncan, G.; Richardson, A.J. The Human Fecal Microbiota Metabolizes Deoxynivalenol and Deoxynivalenol-3-Glucoside and may be Responsible for Urinary Deepoxy-Deoxynivalenol. Appl. Environ. Microbiol. 2013, 79, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Dall’Erta, A.; Cirlini, M.; Dall’Asta, M.; Del Rio, D.; Galaverna, G.; Dall’Asta, C. Masked Mycotoxins are Efficiently Hydrolyzed by Human Colonic Microbiota Releasing their Aglycones. Chem. Res. Toxicol. 2013, 26, 305–312. [Google Scholar] [CrossRef]
- Gratz, S.W.; Dinesh, R.; Yoshinari, T.; Holtrop, G.; Richardson, A.J.; Duncan, G.; Macdonald, S.; Lloyd, A.; Tarbin, J. Masked Trichothecene and Zearalenone Mycotoxins Withstand Digestion and Absorption in the Upper GI Tract but are Efficiently Hydrolyzed by Human Gut Microbiota in Vitro. Mol. Nutr. Food Res. 2017, 61, 1–10. [Google Scholar] [CrossRef]
- Daud, N.; Currie, V.; Duncan, G.; Busman, M.; Gratz, S.W. Intestinal Hydrolysis and Microbial Biotransformation of Diacetoxyscirpenol-Alpha-Glucoside, HT-2-Beta-Glucoside and N-(1-Deoxy-D-Fructos-1-Yl) Fumonisin B-1 by Human Gut Microbiota in Vitro. Int. J. Food Sci. Nutr. 2020, 71, 540–548. [Google Scholar] [CrossRef]
- Daud, N.; Currie, V.; Duncan, G.; Farquharson, F.; Yoshinari, T.; Louis, P.; Gratz, S.W. Prevalent Human Gut Bacteria Hydrolyse and Metabolise Important Food-Derived Mycotoxins and Masked Mycotoxins. Toxins 2020, 12, 654. [Google Scholar] [CrossRef] [PubMed]
- Gratz, S.W.; Currie, V.; Richardson, A.J.; Duncan, G.; Holtrop, G.; Farquharson, F.; Louis, P.; Pinton, P.; Oswald, I.P. Porcine Small and Large Intestinal Microbiota Rapidly Hydrolyze the Masked Mycotoxin Deoxynivalenol-3-Glucoside and Release Deoxynivalenol in Spiked Batch Cultures in Vitro. Appl. Environ. Microbiol. 2018, 84, e02106-17. [Google Scholar] [CrossRef]
- Vidal, A.; Claeys, L.; Mengelers, M.; Vanhoorne, V.; Vervaet, C.; Huybrechts, B.; De Saeger, S.; De Boevre, M. Humans significantly Metabolize and Excrete the Mycotoxin Deoxynivalenol and its Modified Form Deoxynivalenol-3-Glucoside within 24 Hours. Sci. Rep. 2018, 8, 5255. [Google Scholar] [CrossRef]
- Edwards, S.G. Influence of Agricultural Practices on Fusarium Infection of Cereals and Subsequent Contamination of Grain by Trichothecene Mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef]
- Krupinsky, J.M.; Bailey, K.L.; McMullen, M.P.; Gossen, B.D.; Turkington, T.K. Managing Plant Disease Risk in Diversified Cropping Systems. Agron. J. 2002, 94, 198–209. [Google Scholar] [CrossRef]
- Jouany, J.P. Methods for Preventing, Decontaminating and Minimizing the Toxicity of Mycotoxins in Feeds. Anim. Feed Sci. Technol. 2007, 137, 342–362. [Google Scholar] [CrossRef]
- Blandino, M.; Reyneri, A.; Vanara, F.; Tamietti, G.; Pietri, A. Influence of Agricultural Practices on Fusarium Infection, Fumonisin and Deoxynivalenol Contamination of Maize Kernels. World Mycotoxin J. 2009, 2, 409–418. [Google Scholar] [CrossRef]
- Brodal, G.; Hofgaard, I.; Eriksen, G.; Bernhoft, A.; Sundheim, L. Mycotoxins in Organically Versus Conventionally Produced Cereal Grains and some Other Crops in Temperate Regions. World Mycotoxin J. 2016, 9, 755–770. [Google Scholar] [CrossRef]
- Edwards, S.G. Impact of Agronomic and Climatic Factors on the Mycotoxin Content of Harvested Oats in the United Kingdom. Food Addit. Contam. Part A 2017, 34, 2230–2241. [Google Scholar] [CrossRef]
- Karlsson, I.; Mellqvist, E.; Persson, P. Temporal and Spatial Dynamics of Fusarium Spp. and Mycotoxins in Swedish Cereals during 16 Years. Mycotoxin Res. 2022. ahead of print. [Google Scholar] [CrossRef]
- Monitoring of Mycotoxins and Other Contaminants in UK Cereals Used in Malting, Milling and Animal Feed (2019–2022); AHDB: Kenilworth, UK, 2022; pp. 1–22.
- Kolawole, O.; De Ruyck, K.; Greer, B.; Meneely, J.; Doohan, F.; Danaher, M.; Elliott, C. Agronomic Factors Influencing the Scale of Fusarium Mycotoxin Contamination of Oats. J. Fungi 2021, 7, 965. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Seifert, K.; Gräfenhan, T.; Keller, B.; et al. Occurrence of Fusarium Species and Mycotoxins in Swiss oats—Impact of Cropping Factors. Eur. J. Agron. 2018, 92, 123–132. [Google Scholar] [CrossRef]
- McCormick, S.P.; Price, N.P.J.; Kurtzman, C.P. Glucosylation and Other Biotransformations of T-2 Toxin by Yeasts of the Trichomonascus Clade. Appl. Environ. Microbiol. 2012, 78, 8694–8702. [Google Scholar] [CrossRef]
- Wetterhorn, K.M.; Newmister, S.A.; Caniza, R.K.; Busman, M.; McCormick, S.P.; Berthiller, F.; Adam, G.; Rayment, I. Crystal Structure of Os79 (Os04g0206600) from Oryza sativa: A UDP-Glucosyltransferase Involved in the Detoxification of Deoxynivalenol. Biochemistry 2016, 55, 6175–6186. [Google Scholar] [CrossRef]
- Yoshinari, T.; Sakuda, S.; Furihata, K.; Furusawa, H.; Ohnishi, T.; Sugita-Konish, Y.; Ishizaki, N.; Terajima, J. Structural Determination of a Nivalenol Glucoside and Development of an Analytical Method for the Simultaneous Determination of Nivalenol and Deoxynivalenol, and their Glucosides, in Wheat. J. Agric. Food Chem. 2014, 62, 1174–1180. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and Validation of a Liquid Chromatography/Tandem Mass Spectrometric Method for the Determination of 39 Mycotoxins in Wheat and Maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Varga, E.; Glauner, T.; Köppen, R.; Mayer, K.; Sulyok, M.; Schuhmacher, R.; Krska, R.; Berthiller, F. Stable Isotope Dilution Assay for the Accurate Determination of Mycotoxins in Maize by UHPLC-MS/MS. Anal. Bioanal. Chem. 2012, 402, 2675–2686. [Google Scholar] [CrossRef]
- Steiner, D.; Krska, R.; Malachová, A.; Taschl, I.; Sulyok, M. Evaluation of Matrix Effects and Extraction Efficiencies of LC-MS/MS Methods as the Essential Part for Proper Validation of Multiclass Contaminants in Complex Feed. J. Agric. Food Chem. 2020, 68, 3868–3880. [Google Scholar] [CrossRef] [PubMed]

| Mycotoxin | Oat Product | Maximum Level (µg/kg) |
|---|---|---|
| T-2 + HT-2 1 | Unprocessed oats | 1000 |
| Oat grains for direct human consumption | 200 | |
| Oat bran and flakes | 200 | |
| DON 2 | Unprocessed oats | 1750 |
| Oats intended for direct human consumption, oat flour, oat meal, oat bran, or germ | 750 | |
| Bread, pastries, biscuits, cereal snacks and breakfast cereals | 500 | |
| ZEN 2 | Unprocessed oats | 100 |
| Oats intended for direct human consumption, oat flour, oat meal, oat bran or germ | 75 | |
| Bread, pastries, biscuits, cereal snacks and breakfast cereals | 50 |
| Oat ID | T-2 µg/kg | HT-2 µg/kg | T-2-Glc µg/kg | HT-2-Glc µg/kg | T-2-Glc % | HT-2-Glc % | DON µg/kg | NIV µg/kg | DON-Glc µg/kg | NIV-Glc µg/kg | DON-Glc % | NIV-Glc % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Org1 | ND | ND | ND | 19 | --- | --- | ND | 41 | ND | ND | --- | --- |
| Org2 | 42 | 230 | ND | ND | --- | --- | 2988 | ND | 746 | ND | 25 | --- |
| Org4 | ND | 52 | ND | ND | --- | --- | ND | ND | ND | ND | --- | --- |
| Org5 | 71 | 390 | 11 | ND | 16 | --- | ND | ND | ND | ND | --- | --- |
| Org6 | ND | ND | ND | ND | --- | --- | 97 | ND | ND | ND | --- | --- |
| Org7 | 3 | 53 | ND | ND | --- | --- | ND | ND | ND | ND | --- | --- |
| Org8 | ND | 6 | ND | ND | --- | --- | 448 | 27 | ND | ND | --- | --- |
| Org9 | 9 | 138 | ND | ND | --- | --- | ND | 134 | ND | ND | --- | --- |
| Org10 | 33 | 538 | 18 | ND | 55 | --- | ND | 1253 | ND | 44 | --- | 4 |
| Org11 | 58 | 173 | ND | ND | --- | --- | 295 | ND | 84 | ND | 28 | --- |
| Org12 | 28 | 193 | ND | ND | --- | --- | ND | 156 | ND | ND | --- | --- |
| Conv1 | 266 | 1905 | ND | ND | --- | ND | 346 | ND | 20 | --- | 6 | |
| Conv2 | ND | 8 | ND | 13 | --- | 174 | 38 | ND | 50 | ND | 130 | --- |
| Conv3 | 33 | 849 | 51 | ND | 154 | --- | 88 | ND | 57 | ND | 65 | --- |
| Conv4 | 88 | 770 | 27 | ND | 31 | --- | ND | ND | ND | ND | --- | --- |
| Conv5 | 97 | 649 | 23 | ND | 24 | --- | 58 | ND | ND | ND | --- | --- |
| Conv6 | 67 | 520 | ND | ND | --- | --- | 67 | 488 | 15 | 24 | 23 | 5 |
| Conv7 | 155 | 609 | 28 | ND | 18 | --- | ND | ND | ND | ND | --- | --- |
| Conv8 | 390 | 3084 | 31 | ND | 8 | --- | ND | ND | 28 | ND | --- | --- |
| Conv9 | 836 | 2145 | 53 | ND | 6 | --- | ND | ND | ND | ND | --- | --- |
| Conv10 | 41 | 66 | ND | ND | --- | --- | 565 | ND | 101 | ND | 18 | --- |
| Conv11 | 36 | 509 | 47 | ND | 130 | --- | ND | ND | ND | ND | --- | --- |
| Conv12 | 93 | 705 | 73 | ND | 78 | --- | 48 | 33 | ND | ND | --- | --- |
| Conv13 | 32 | 137 | 44 | ND | 137 | --- | 263 | 15 | 47 | ND | 18 | --- |
| Conv14 | 126 | 343 | 14 | ND | 11 | --- | ND | ND | ND | ND | --- | --- |
| Conv15 | 48 | 298 | ND | ND | --- | --- | 2734 | 132 | 881 | 21 | 32 | 16 |
| Conv16 | 3 | 26 | ND | ND | --- | --- | 111 | ND | ND | ND | --- | --- |
| Conv17 | 44 | 157 | ND | ND | --- | --- | 173 | 64 | 89 | ND | 51 | --- |
| Conv18 | 167 | 914 | ND | 462 | --- | 51 | ND | ND | ND | ND | --- | --- |
| Conv19 | 34 | 220 | ND | 155 | --- | 70 | ND | ND | ND | ND | --- | --- |
| Conv20 | 22 | 97 | ND | 67 | --- | 70 | ND | 28 | ND | ND | --- | --- |
| Conv21 | 4 | 75 | ND | 26 | --- | 34 | ND | 145 | 29 | ND | --- | --- |
| Number of Co-Occurring Mycotoxins | Number of Combinations Found | Types of Combinations | Number (%) of Samples Organic | Number (%) of Samples Conventional |
|---|---|---|---|---|
| 4 | 1 | T-2/HT-2 + DON + NIV + ZEN | 0 (0) | 1 (4.8) |
| 3 | 2 | T-2/HT-2 + DON + NIV T-2/HT-2 + DON + ZEN | 1 (8.3) 2 (16.7) | 4 (19.0) 2 (9.5) |
| 2 | 2 | T-2/HT-2 + DON T-2/HT-2 + NIV | 0 (0) 3 (25.0) | 3 (14.3) 3 (14.3) |
| (1) | 3 | T-2/HT-2 DON NIV | 4 (33.3) 1 (8.3) 1 (8.3) | 8 (38.1) 0 (0) 0 (0) |
| Compound | RT (min) | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy | Polarity | % RA (RSD) | %SSE (RSD) | LOQ Oat |
|---|---|---|---|---|---|---|---|---|
| T-2 | 10.3 | 489.1 | 327.2 | −26.0 | +ve | 109.4 (2.2) | 103.3 (8.4) | 3.1 |
| HT-2 | 9.8 | 447.3 | 345.2 | −20.0 | +ve | 92.3 (6.8) | 91.4 (9.1) | 6.3 |
| T-2-Glc | 9.9 | 651.3 | 489.2 | −34.0 | +ve | 118.7 (3.6) | 96.5 (3.3) | 12.5 |
| HT-2-Glc | 9.4 | 609.2 | 447.1 | −34.0 | +ve | 114.1 (5.7) | 77.4 (8.8) | 12.5 |
| DAS | 8.9 | 384.2 | 307.5 | −12.0 | +ve | 95.3 (16.3) | 116.5 (5.0) | 25 |
| DAS-Glc | 8.5 | 551.2 | 389.1 | −33.0 | +ve | 112.0 (7.1) | 111.7 (1.8) | 25 |
| DON | 6.1 | 355.3 | 295.2 | 12.0 | −ve | 87.9 (7.6) | 107.3 (4.9) | 25 |
| DON-Glc | 5.9 | 517.2 | 427.2 | 23.0 | −ve | 95.1 (10.6) | 93.2 (6.4) | 12.5 |
| NIV | 5.3 | 371.2 | 281.2 | 20.0 | −ve | 101.1 (7.3) | 93.3 (7.4) | 12.5 |
| NIV-Glc | 5.1 | 533.3 | 473.2 | 14.0 | −ve | 94.7 (14.5) | 91.9 (2.8) | 12.5 |
| ZEN | 10.9 | 317.2 | 175.3 | 24.0 | −ve | 86.4 (6.4) | 104.4 (7.9) | 6.3 |
| ZEN-Glc | 9.4 | 479.4 | 317.2 | 21.0 | −ve | 70.4 (8.1) | 59.6 (5.0) | 6.3 |
| 13C22-HT-2 | 9.8 | 464.3 | 278.2 | −20.0 | +ve | |||
| 13C15-DON | 6.1 | 370.2 | 310.3 | 11.0 | −ve | |||
| 13C18-ZEN | 10.9 | 335.2 | 185.2 | 26.0 | −ve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daud, N.; Currie, V.; Duncan, G.; Filipe, J.A.N.; Yoshinari, T.; Stoddart, G.; Roberts, D.; Gratz, S.W. Free and Modified Mycotoxins in Organic and Conventional Oats (Avena sativa L.) Grown in Scotland. Toxins 2023, 15, 247. https://doi.org/10.3390/toxins15040247
Daud N, Currie V, Duncan G, Filipe JAN, Yoshinari T, Stoddart G, Roberts D, Gratz SW. Free and Modified Mycotoxins in Organic and Conventional Oats (Avena sativa L.) Grown in Scotland. Toxins. 2023; 15(4):247. https://doi.org/10.3390/toxins15040247
Chicago/Turabian StyleDaud, Noshin, Valerie Currie, Gary Duncan, Joao A. N. Filipe, Tomoya Yoshinari, Gary Stoddart, Deborah Roberts, and Silvia W. Gratz. 2023. "Free and Modified Mycotoxins in Organic and Conventional Oats (Avena sativa L.) Grown in Scotland" Toxins 15, no. 4: 247. https://doi.org/10.3390/toxins15040247
APA StyleDaud, N., Currie, V., Duncan, G., Filipe, J. A. N., Yoshinari, T., Stoddart, G., Roberts, D., & Gratz, S. W. (2023). Free and Modified Mycotoxins in Organic and Conventional Oats (Avena sativa L.) Grown in Scotland. Toxins, 15(4), 247. https://doi.org/10.3390/toxins15040247





