Hemolysin Co-Regulatory Protein 1 Enhances the Virulence of Clinically Isolated Escherichia coli in KM Mice by Increasing Inflammation and Inducing Pyroptosis
Abstract
1. Introduction
2. Results
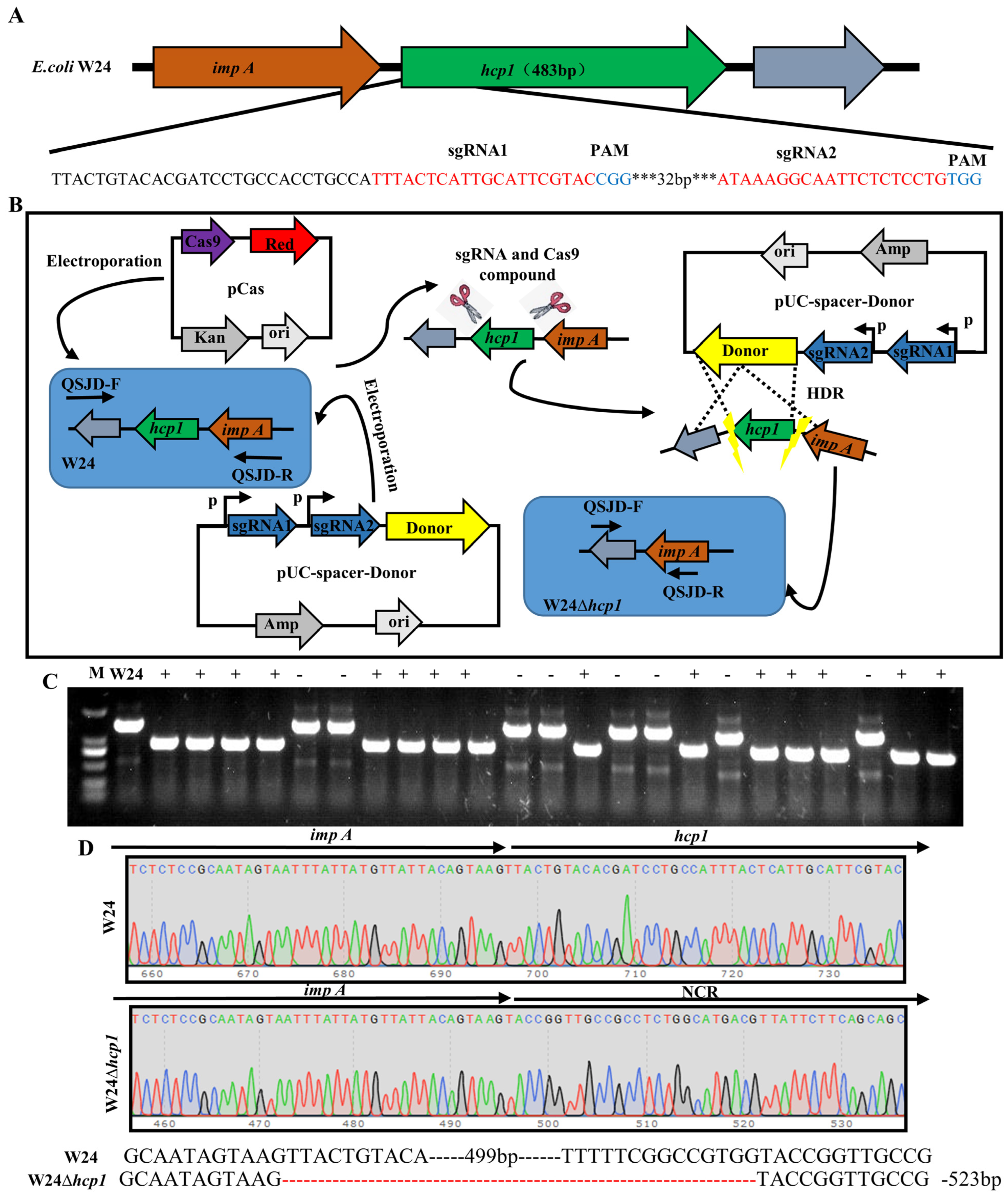
2.1. Deletion of E. coli Toxin Gene Hcp1
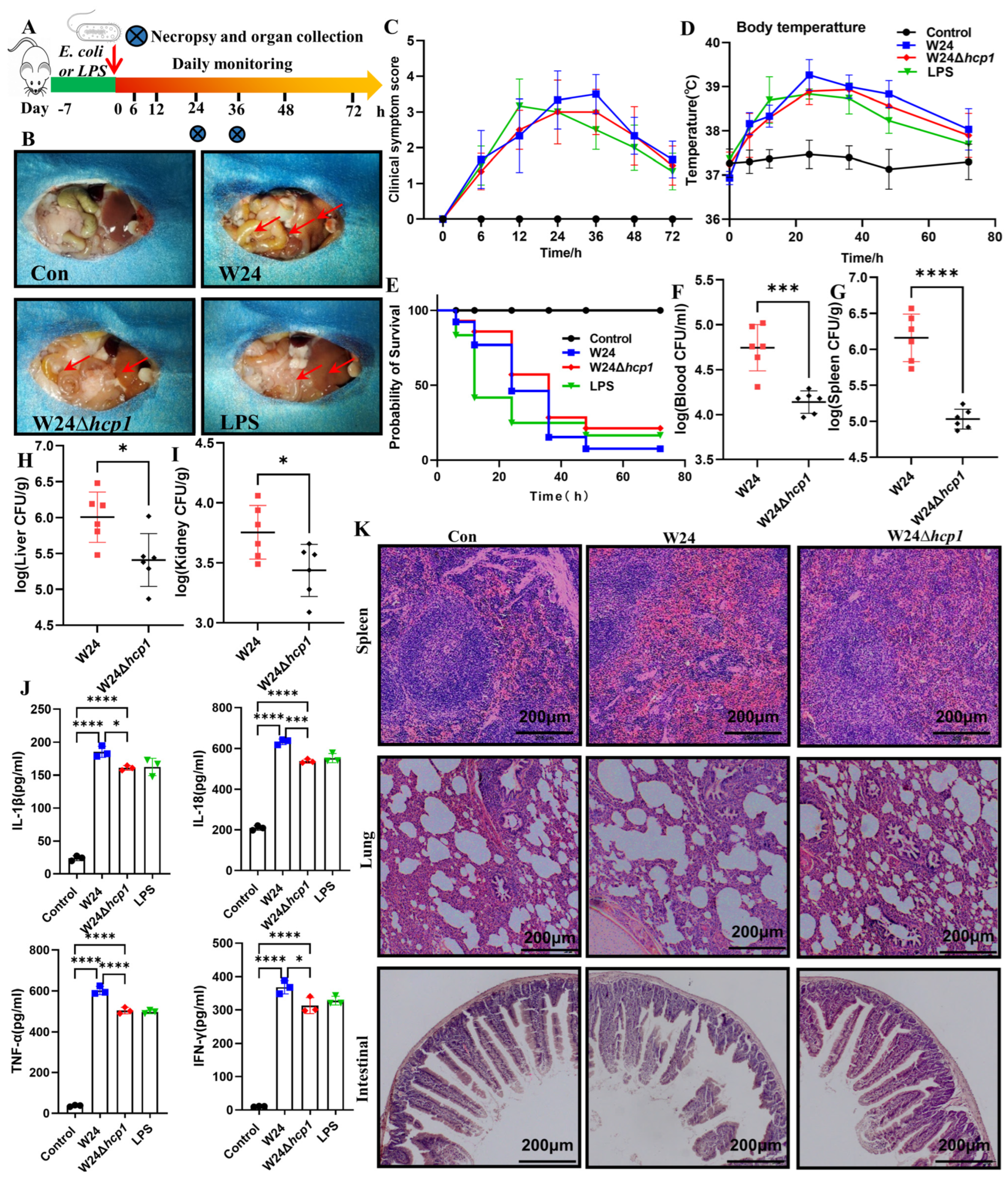
2.2. Hcp1 Enhances E. coli Virulence against KM Mice
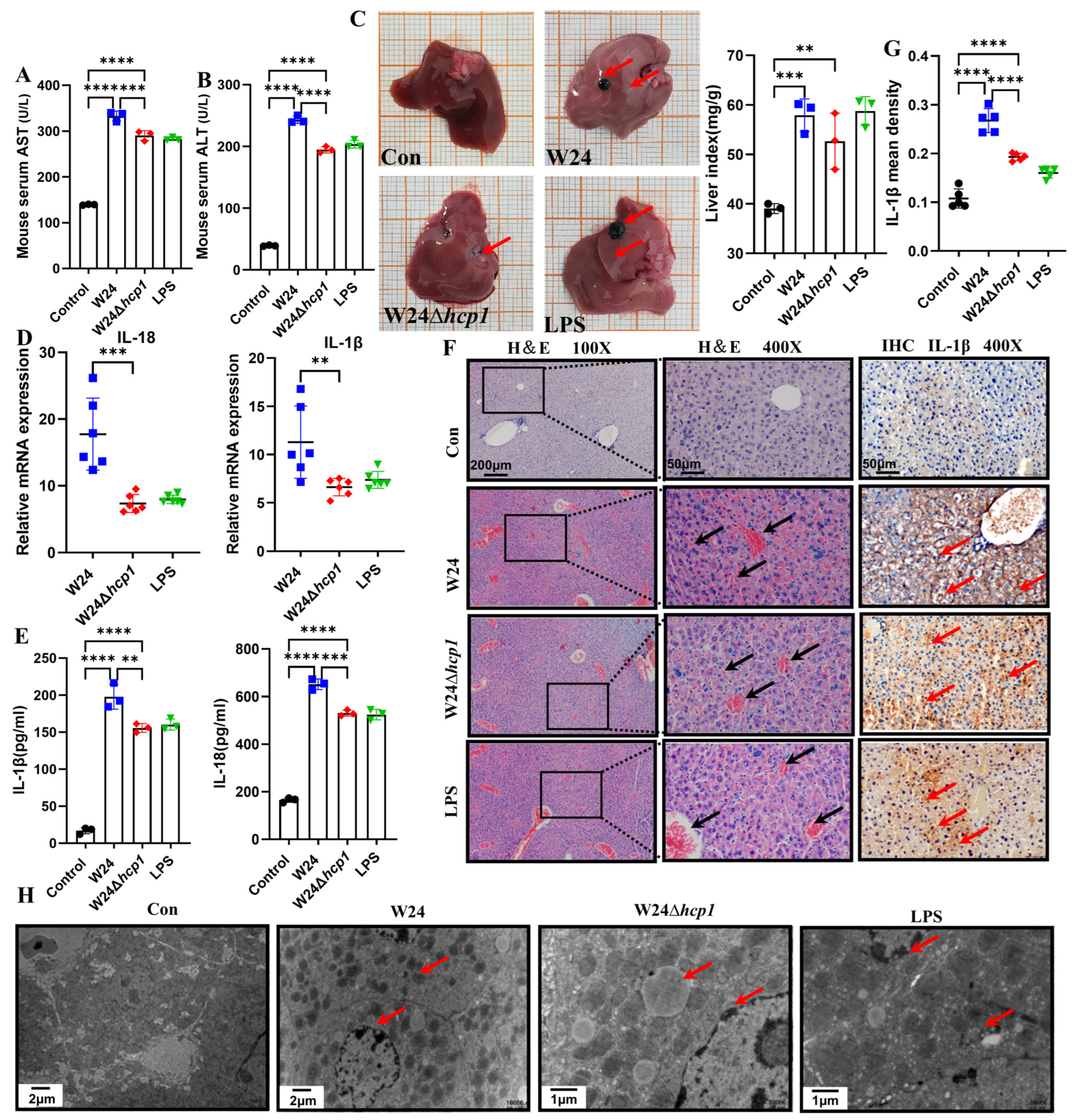
2.3. Hcp1 Promotes E. coli-Induced Acute Liver Injury in Mice
2.4. Hcp1 Promotes E. coli-Induced Acute Kidney Injury in Mice
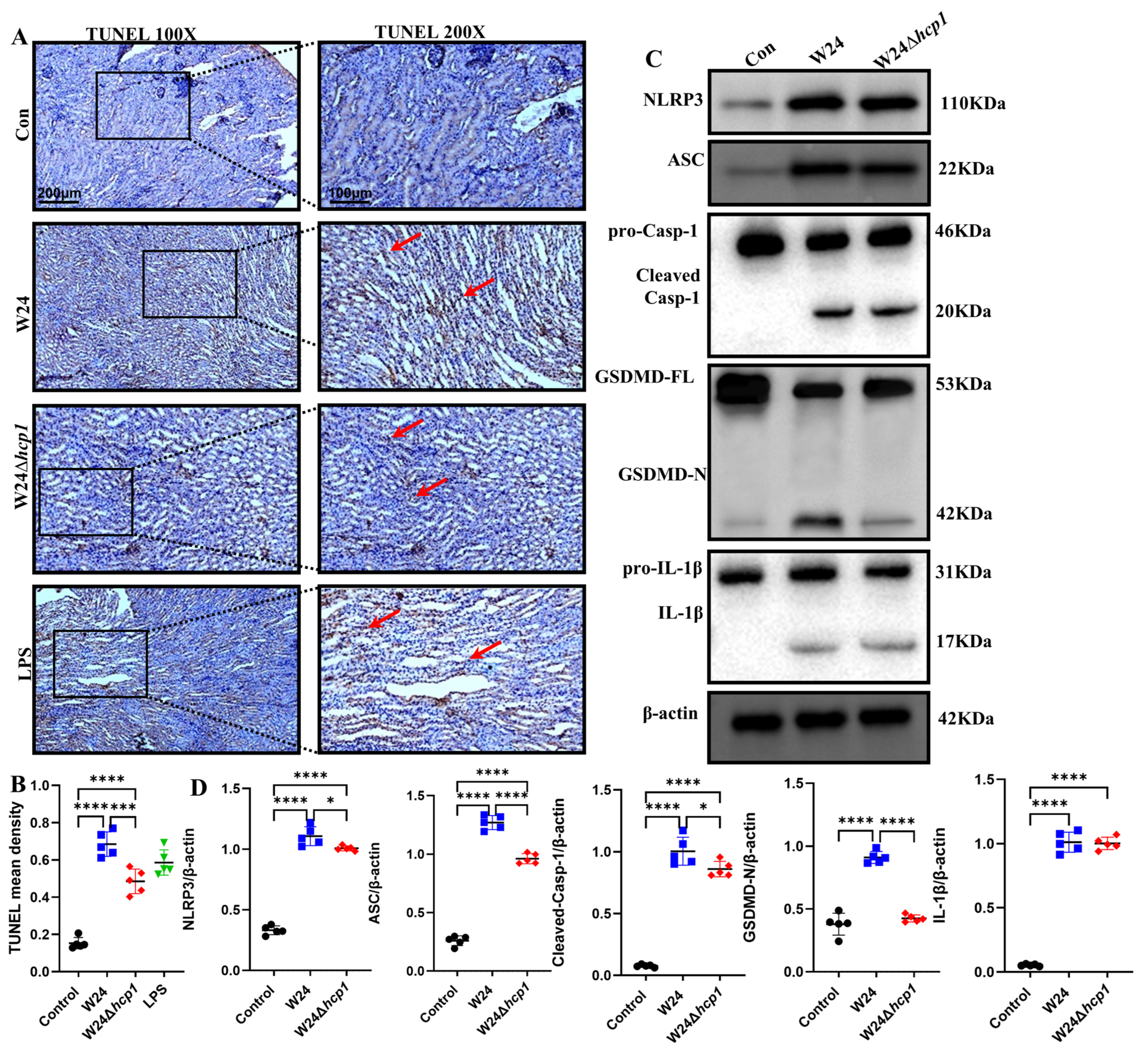
2.5. Hcp1 Induces Pyroptosis in the Kidney
2.6. Hcp1 Binds to NLRP3 Protein
3. Discussion
4. Materials and Methods
4.1. Strains and Plasmids
4.2. Deletion of E. coli Hcp1
4.3. KM Mice Infection Model
4.4. Clinical Signs and Assigned Clinical Scores
4.5. Biochemical Analysis and ELISA Assay
4.6. Liver and Kidney Organ Index
4.7. Histopathology
4.8. Immunohistochemistry
4.9. Transmission Electron Microscopy
4.10. RNA Extraction and Real-Time PCR
4.11. TUNEL Staining
4.12. Western Blotting Analysis
4.13. Protein Docking
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mougous, J.D.; Cuff, M.E.; Raunser, S.; Shen, A.; Zhou, M.; Gifford, C.A.; Goodman, A.L.; Joachimiak, G.; Ordonez, C.L.; Lory, S.; et al. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 2006, 312, 1526–1530. [Google Scholar] [CrossRef]
- Anderson, M.C.; Vonaesch, P.; Saffarian, A.; Marteyn, B.S.; Sansonetti, P.J. Shigella sonnei Encodes a Functional T6SS Used for Interbacterial Competition and Niche Occupancy. Cell Host Microbe 2017, 21, 769–776.e763. [Google Scholar] [CrossRef]
- Hood, R.D.; Singh, P.; Hsu, F.; Guvener, T.; Carl, M.A.; Trinidad, R.R.; Silverman, J.M.; Ohlson, B.B.; Hicks, K.G.; Plemel, R.L.; et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 2010, 7, 25–37. [Google Scholar] [CrossRef]
- Nolan, L.M.; Cain, A.K.; Clamens, T.; Furniss, R.C.D.; Manoli, E.; Sainz-Polo, M.A.; Dougan, G.; Albesa-Jove, D.; Parkhill, J.; Mavridou, D.A.I.; et al. Identification of Tse8 as a Type VI secretion system toxin from Pseudomonas aeruginosa that targets the bacterial transamidosome to inhibit protein synthesis in prey cells. Nat. Microbiol. 2021, 6, 1199–1210. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; Wang, B.; Chen, L.; Zhao, Z.; Waterfield, N.R.; Yang, G.; Jin, Q. The Pseudomonas aeruginosa Type VI Secretion PGAP1-like Effector Induces Host Autophagy by Activating Endoplasmic Reticulum Stress. Cell Rep. 2016, 16, 1502–1509. [Google Scholar] [CrossRef]
- Chen, H.; Yang, D.; Han, F.; Tan, J.; Zhang, L.; Xiao, J.; Zhang, Y.; Liu, Q. The Bacterial T6SS Effector EvpP Prevents NLRP3 Inflammasome Activation by Inhibiting the Ca(2+)-Dependent MAPK-Jnk Pathway. Cell Host Microbe 2017, 21, 47–58. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, X.; Shou, J.; Zong, B.; Zhang, Y.; Tan, J.; Chen, J.; Hu, L.; Zhu, Y.; Chen, H.; et al. Roles of Hcp family proteins in the pathogenesis of the porcine extraintestinal pathogenic Escherichia coli type VI secretion system. Sci. Rep. 2016, 6, 26816. [Google Scholar] [CrossRef]
- Ma, J.; Sun, M.; Pan, Z.; Song, W.; Lu, C.; Yao, H. Three Hcp homologs with divergent extended loop regions exhibit different functions in avian pathogenic Escherichia coli. Emerg. Microbes Infect. 2018, 7, 49. [Google Scholar] [CrossRef]
- Kalindamar, S.; Abdelhamed, H.; Kordon, A.O.; Pinchuk, L.M.; Karsi, A. Hemolysin Co-regulated Family Proteins Hcp1 and Hcp2 Contribute to Edwardsiella ictaluri Pathogenesis. Front. Vet. Sci. 2021, 8, 681609. [Google Scholar] [CrossRef]
- Sengyee, S.; Yarasai, A.; Janon, R.; Morakot, C.; Ottiwet, O.; Schmidt, L.K.; West, T.E.; Burtnick, M.N.; Chantratita, N.; Brett, P.J. Melioidosis Patient Survival Correlates With Strong IFN-gamma Secreting T Cell Responses Against Hcp1 and TssM. Front. Immunol. 2021, 12, 698303. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, J.; Yu, H.; Ni, J.; Zeng, L.; Teng, Q.; Kim, K.S.; Zhao, G.P.; Guo, X.; Yao, Y. Hcp family proteins secreted via the type VI secretion system coordinately regulate Escherichia coli K1 interaction with human brain microvascular endothelial cells. Infect. Immun. 2012, 80, 1243–1251. [Google Scholar] [CrossRef]
- Zhao, M.; Zhang, L.; Lv, S.; Zhang, C.; Wang, L.; Chen, H.; Zhou, Y.; Lou, J. IQGAP1 Mediates Hcp1-Promoted Escherichia coli Meningitis by Stimulating the MAPK Pathway. Front. Cell. Infect. Microbiol. 2017, 7, 132. [Google Scholar] [CrossRef]
- Broz, P.; Pelegrin, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.C.; Shao, F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef]
- Ghayur, T.; Banerjee, S.; Hugunin, M.; Butler, D.; Herzog, L.; Carter, A.; Quintal, L.; Sekut, L.; Talanian, R.; Paskind, M.; et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature 1997, 386, 619–623. [Google Scholar] [CrossRef]
- Miao, E.A.; Leaf, I.A.; Treuting, P.M.; Mao, D.P.; Dors, M.; Sarkar, A.; Warren, S.E.; Wewers, M.D.; Aderem, A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 2010, 11, 1136–1142. [Google Scholar] [CrossRef]
- Wang, H.; Miao, F.; Ning, D.; Shan, C. Ellagic acid alleviates hepatic ischemia-reperfusion injury in C57 mice via the Caspase-1-GSDMD pathway. BMC Vet. Res. 2022, 18, 229. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Deng, W.; Bai, Y.; Deng, F.; Pan, Y.; Mei, S.; Zheng, Z.; Min, R.; Wu, Z.; Li, W.; Miao, R.; et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 2022, 602, 496–502. [Google Scholar] [CrossRef]
- Liu, L.; Song, L.; Deng, R.; Lan, R.; Jin, W.; Tran Van Nhieu, G.; Cao, H.; Liu, Q.; Xiao, Y.; Li, X.; et al. Citrobacter freundii Activation of NLRP3 Inflammasome via the Type VI Secretion System. J. Infect. Dis. 2021, 223, 2174–2185. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Z.; Magupalli, V.G.; Pablo, J.L.; Dong, Y.; Vora, S.M.; Wang, L.; Fu, T.M.; Jacobson, M.P.; Greka, A.; et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 2021, 593, 607–611. [Google Scholar] [CrossRef]
- He, W.-T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.; Abrams, J.; Adam, D.; Agostinis, P.; Alnemri, E.; Altucci, L.; Amelio, I.; Andrews, D.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death. Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Q.; Zhong, X.; Zeng, M.; Zeng, H.; Shi, X.; Li, Z.; Wang, Y.; Zhao, Q.; Shao, F.; et al. Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis. Cell 2020, 180, 941–955.e920. [Google Scholar] [CrossRef]
- Chen, X.; He, W.-T.; Hu, L.; Li, J.; Fang, Y.; Wang, X.; Xu, X.; Wang, Z.; Huang, K.; Han, J. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016, 26, 1007–1020. [Google Scholar] [CrossRef]
- Wang, P.; Dong, J.F.; Li, R.Q.; Li, L.; Zou, Q.H. Roles of the Hcp family proteins in the pathogenicity of Salmonella typhimurium 14028s. Virulence 2020, 11, 1716–1726. [Google Scholar] [CrossRef]
- Noreen, Z.; Jobichen, C.; Abbasi, R.; Seetharaman, J.; Sivaraman, J.; Bokhari, H. Structural basis for the pathogenesis of Campylobacter jejuni Hcp1, a structural and effector protein of the Type VI Secretion System. FEBS J. 2018, 285, 4060–4070. [Google Scholar] [CrossRef]
- Kong, P.; Cui, Z.; Huang, X.; Zhang, D.; Guo, R.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Dong, R.; Huang, K.; Wang, C.; Gu, J.; Luo, H.; Liu, K.; Wu, J.; Sun, H.; et al. Luteolin ameliorates LPS-induced acute liver injury by inhibiting TXNIP-NLRP3 inflammasome in mice. Phytomed. Int. J. Phytother. Phytopharm. 2021, 87, 153586. [Google Scholar] [CrossRef]
- Hong, M.-Y.; Tseng, C.-C.; Chuang, C.-C.; Chen, C.-L.; Lin, Y.-H.; Hsieh, C.-Y.; Chang, Y.-T.; Hsing, C.-H.; Chang, K.-Y.; Lin, C.-F. Uropathogenic Escherichia coli causes cortical tubular necrotic cell death and the release of macrophage migration inhibitory factor. Cytokine 2013, 61, 945–952. [Google Scholar] [CrossRef]
- Bock, F.; Tait, S. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Valderrama, A.; Riestra, A.; Gao, N.; LaRock, C.; Gupta, N.; Ali, S.R.; Hoffman, H.; Ghosh, P.; Nizet, V. Group A Streptococcal M protein activates the NLRP3 inflammasome. Nat. Microbiol. 2017, 2, 1425–1434. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Xu, M.-M.; Sun, Y.; Ding, Z.-X.; Wei, Y.-Y.; Zhang, D.-W.; Wang, Y.-G.; Shen, J.-L.; Wu, H.-M.; Fei, G.-H. Melatonin attenuates LPS-induced pyroptosis in acute lung injury by inhibiting NLRP3-GSDMD pathway via activating Nrf2/HO-1 signaling axis. Int. Immunopharmacol. 2022, 109, 108782. [Google Scholar] [CrossRef]
- Tonnus, W.; Maremonti, F.; Belavgeni, A.; Latk, M.; Kusunoki, Y.; Brucker, A.; von Mässenhausen, A.; Meyer, C.; Locke, S.; Gembardt, F.; et al. Gasdermin D-deficient mice are hypersensitive to acute kidney injury. Cell Death Dis. 2022, 13, 792. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, B.; Duan, C.; Sun, B.; Yang, J.; Yang, S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 2015, 81, 2506–2514. [Google Scholar] [CrossRef]
- Lin, X.; Yang, F.; Zhou, Y.; Zhu, Z.; Jin, G.; Zhang, S.; Zhao, Z. Highly-efficient colony PCR method for red yeasts and its application to identify mutations within two leucine auxotroph mutants. Yeast (Chichester Engl.) 2012, 29, 467–474. [Google Scholar] [CrossRef]
- Huang, C.; Ding, T.; Wang, J.; Wang, X.; Guo, L.; Wang, J.; Zhu, L.; Bi, C.; Zhang, X.; Ma, X.; et al. CRISPR-Cas9-assisted native end-joining editing offers a simple strategy for efficient genetic engineering in Escherichia coli. Appl. Microbiol. Biotechnol. 2019, 103, 8497–8509. [Google Scholar] [CrossRef]
- Domínguez-Punaro, M.; Segura, M.; Plante, M.-M.; Lacouture, S.; Rivest, S.; Gottschalk, M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J. Immunol. (Baltim. Md. 1950) 2007, 179, 1842–1854. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Zhang, Y.; Xu, F.; Chen, J.; Duan, L.; Zhang, T.; Wang, J.; Zhang, F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox. Biol. 2020, 32, 101500. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Lv, L.-B.; Chen, L.-P.; Xiao, J.-L.; Shen, J.; Gao, B.; Zhao, J.-G.; Han, D.-M.; Chen, B.-X.; Wang, S.; et al. Hemolysin Co-Regulatory Protein 1 Enhances the Virulence of Clinically Isolated Escherichia coli in KM Mice by Increasing Inflammation and Inducing Pyroptosis. Toxins 2023, 15, 171. https://doi.org/10.3390/toxins15030171
Wang H, Lv L-B, Chen L-P, Xiao J-L, Shen J, Gao B, Zhao J-G, Han D-M, Chen B-X, Wang S, et al. Hemolysin Co-Regulatory Protein 1 Enhances the Virulence of Clinically Isolated Escherichia coli in KM Mice by Increasing Inflammation and Inducing Pyroptosis. Toxins. 2023; 15(3):171. https://doi.org/10.3390/toxins15030171
Chicago/Turabian StyleWang, Hao, Long-Bao Lv, Li-Ping Chen, Jin-Long Xiao, Jue Shen, Bin Gao, Jin-Gang Zhao, Dong-Mei Han, Bin-Xun Chen, Shuai Wang, and et al. 2023. "Hemolysin Co-Regulatory Protein 1 Enhances the Virulence of Clinically Isolated Escherichia coli in KM Mice by Increasing Inflammation and Inducing Pyroptosis" Toxins 15, no. 3: 171. https://doi.org/10.3390/toxins15030171
APA StyleWang, H., Lv, L.-B., Chen, L.-P., Xiao, J.-L., Shen, J., Gao, B., Zhao, J.-G., Han, D.-M., Chen, B.-X., Wang, S., Liu, G., Xin, A.-G., Xiao, P., & Gao, H. (2023). Hemolysin Co-Regulatory Protein 1 Enhances the Virulence of Clinically Isolated Escherichia coli in KM Mice by Increasing Inflammation and Inducing Pyroptosis. Toxins, 15(3), 171. https://doi.org/10.3390/toxins15030171






