Investigation of Best Practices for Venom Toxin Purification in Jellyfish towards Functional Characterisation
Abstract
1. Introduction
2. Results
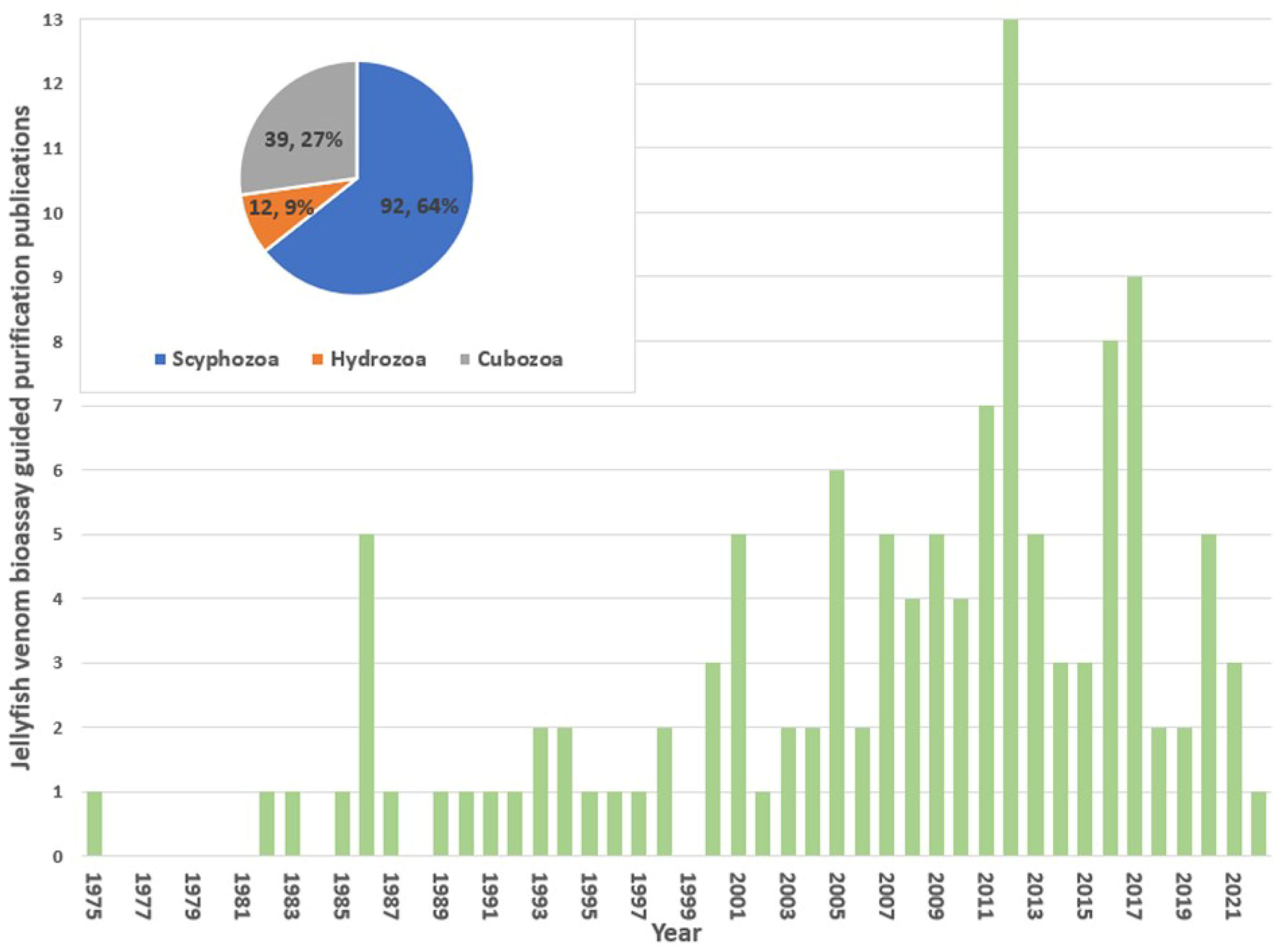
2.1. Captured Literature
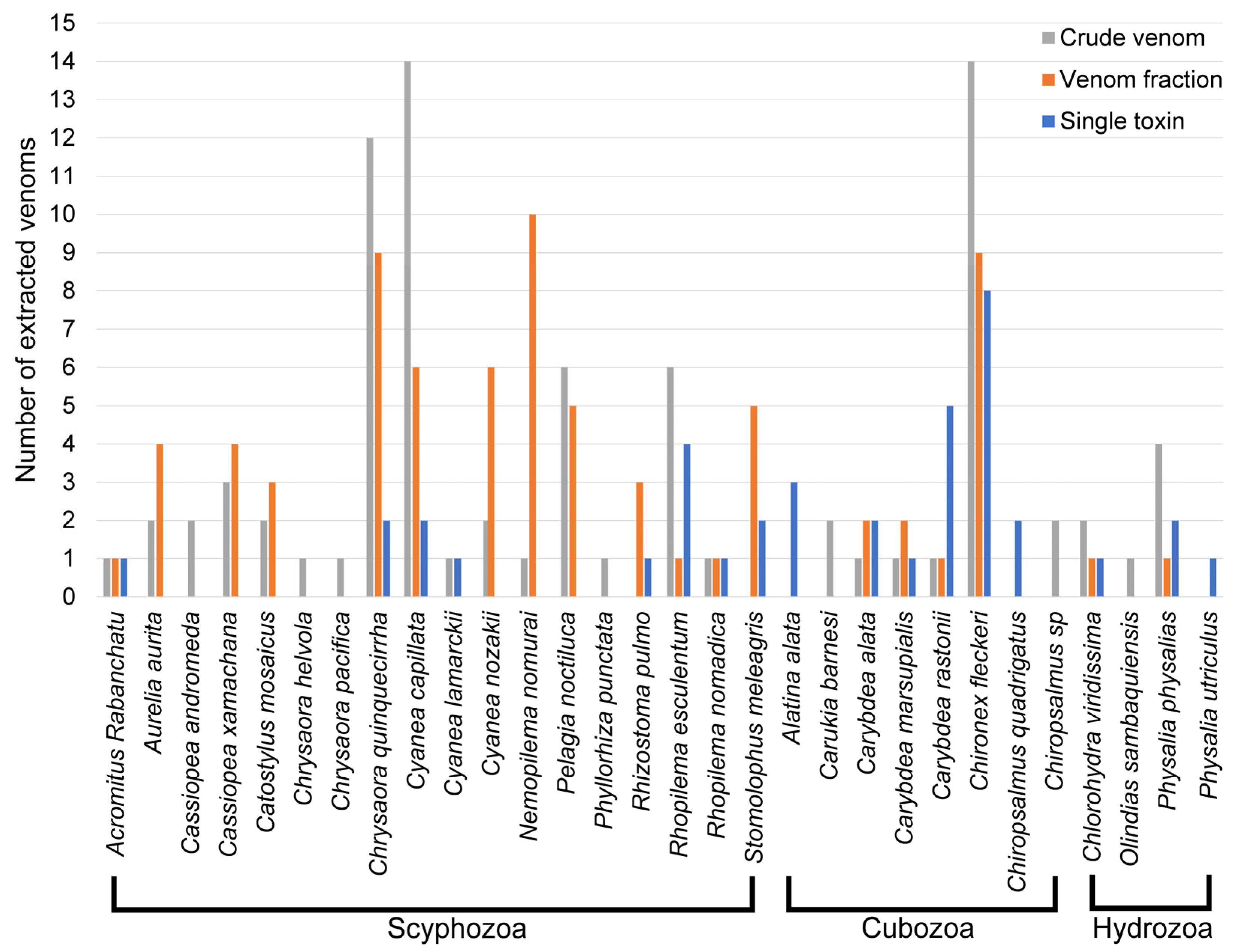
2.2. Jellyfish Venom Identification
3. Discussion
3.1. Purification Levels of Jellyfish Venom
3.2. Jellyfish Venom Extraction
3.3. Jellyfish Venom Purification
3.4. Toxicity Assays of Venom towards Proposed Function
3.5. Chironex fleckeri, a Model for Jellyfish Venom Purification
4. Conclusions
5. Method
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Castro Figueiredo Bordon, K.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Anderluh, G. Pore-forming toxins in Cnidaria. Semin. Cell Dev. Biol. 2017, 72, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Kayal, E.; Bentlage, B.; Pankey, M.S.; Ohdera, A.H.; Medina, M.; Plachetzki, D.C.; Collins, A.G.; Ryan, J.F. Phylogenomics provides a robust topology of the major cnidarian lineages and insights on the origins of key organismal traits. BMC Evol. Biol. 2018, 18, 68. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Xing, R.; Liu, S.; Li, K.; Wang, X.; Chen, X.; Li, P. Functional Elucidation of Nemopilema nomurai and Cyanea nozakii Nematocyst Venoms’ Lytic Activity Using Mass Spectrometry and Zymography. Toxins 2017, 9, 47. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, J.; Torrens, E.; Segura-Puertas, L. Partial purification and characterization of a novel neurotoxin and three cytolysins from box jellyfish (Carybdea marsupialis) nematocyst venom. Arch. Toxicol. 2005, 80, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lassen, S.; Wiebring, A.; Helmholz, H.; Ruhnau, C.; Prange, A. Isolation of a Nav channel blocking polypeptide from Cyanea capillata medusae—A neurotoxin contained in fishing tentacle isorhizas. Toxicon 2012, 59, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Endean, R. Separation of two myotoxins from nematocysts of the box jellyfish (Chironex fleckeri). Toxicon 1987, 25, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, C.; Li, R.; Xing, R.; Liu, S.; Li, P. Factors influencing hemolytic activity of venom from the jellyfish Rhopilema esculentum Kishinouye. Food Chem. Toxicol. 2007, 45, 1173–1178. [Google Scholar] [CrossRef]
- Calton, G.J.; Burnett, J.W. Partial purification of Chironex fleckeri (sea wasp) venom by immunochromatography with antivenom. Toxicon 1986, 24, 416–420. [Google Scholar] [CrossRef]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. The in vitro effects of two chirodropid (Chironex fleckeri and Chiropsalmus sp.) venoms: Efficacy of box jellyfish antivenom. Toxicon 2003, 41, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Li, A.; Yu, C.; Li, P. Refinement and Neutralization Evaluation of the F(ab’)2 Type of Antivenom against the Deadly Jellyfish Nemopilema nomurai Toxins. Int. J. Mol. Sci. 2021, 22, 12672. [Google Scholar] [CrossRef]
- D’Ambra, I.; Lauritano, C. A Review of Toxins from Cnidaria. Mar. Drugs 2020, 18, 507. [Google Scholar] [CrossRef] [PubMed]
- Nisa, S.A.; Vinu, D.; Krupakar, P.; Govindaraju, K.; Sharma, D.; Vivek, R. Jellyfish venom proteins and their pharmacological potentials: A review. Int. J. Biol. Macromol. 2021, 176, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Jouiaei, M.; Yanagihara, A.A.; Madio, B.; Nevalainen, T.J.; Alewood, P.F.; Fry, B.G. Ancient Venom Systems: A Review on Cnidaria Toxins. Toxins 2015, 7, 2251–2271. [Google Scholar] [CrossRef] [PubMed]
- Badre, S. Bioactive toxins from stinging jellyfish. Toxicon Off. J. Int. Soc. Toxinology 2014, 91, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Burnell, J.N. Biochemical and molecular characterisation of cubozoan protein toxins. Toxicon 2009, 54, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Klompen, A.M.L.; Kayal, E.; Collins, A.G.; Cartwright, P. Phylogenetic and Selection Analysis of an Expanded Family of Putatively Pore-Forming Jellyfish Toxins (Cnidaria: Medusozoa). Genome Biol. Evol. 2021, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; La Spada, G.; Crupi, R.; Esposito, E.; Marino, A. Crude Venom from Nematocysts of the Jellyfish Pelagia noctiluca as a Tool to Study Cell Physiology. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Frazão, B.; Antunes, A. Jellyfish Bioactive Compounds: Methods for Wet-Lab Work. Mar. Drugs 2016, 14, 75. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Toom, P.M.; Phillips, T.D. Effects of purified components of jellyfish toxin (Stomolophus meleagris) on active sodium transport. Toxicon 1975, 13, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.E.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (box jellyfish) venom proteins: Expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.; Burnell, J. Partial purification of cytolytic venom proteins from the box jellyfish, Chironex fleckeri. Toxicon 2008, 51, 853–863. [Google Scholar] [CrossRef]
- Saggiomo, S.L.; Seymour, J.E. Cardiotoxic effects of venom fractions from the Australian box jellyfish Chironex fleckeri on human myocardiocytes. Toxicon 2012, 60, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Endean, R.; Monks, S.A.; Cameron, A.M. Toxins from the box-jellyfish Chironex fleckeri. Toxicon 1993, 31, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.A.; Burnett, J.W.; Alderslade, P. Partial purification of box jellyfish (Chironex fleckeri) nematocyst venom isolated at the beachside. Toxicon 1998, 36, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.; Radwan, F.; Burnett, J. Toxinological and immunological studies of capillary electrophoresis fractionated Chrysaora quinquecirrha (Desor) fishing tentacle and Chironex fleckeri Southcott nematocyst venoms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 128, 75–90. [Google Scholar] [CrossRef]
- Othman, I.; Burnett, J.W. Techniques applicable for purifying Chironex fleckeri (box-jellyfish) venom. Toxicon 1990, 28, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Kintner, A.H.; Seymour, J.E.; Edwards, S.L. Variation in lethality and effects of two Australian chirodropid jellyfish venoms in fish. Toxicon 2005, 46, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Isbister, G.K.; Seymour, J.E.; Hodgson, W.C. Pharmacologically distinct cardiovascular effects of box jellyfish (Chironex fleckeri) venom and a tentacle-only extract in rats. Toxicol. Lett. 2005, 155, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ponce, D.; Brinkman, D.L.; Luna-Ramírez, K.; Wright, C.E.; Dorantes-Aranda, J.J. Comparative study of the toxic effects of Chrysaora quinquecirrha (Cnidaria: Scyphozoa) and Chironex fleckeri (Cnidaria: Cubozoa) venoms using cell-based assays. Toxicon 2015, 106, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Eldefrawi, M.; Eldefrawi, A.; Burnett, J.; Mioduszewski, R.; Menking, D.; Valdes, J. Toxicity of sea nettle toxin to human hepatocytes and the protective effects of phosphorylating and alkylating agents. Toxicon 1998, 36, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Carrette, T.; Seymour, J. A rapid and repeatable method for venom extraction from Cubozoan nematocysts. Toxicon Off. J. Int. Soc. Toxinology 2004, 44, 135–139. [Google Scholar] [CrossRef]
- Yanagihara, A.A.; Shohet, R.V. Cubozoan Venom-Induced Cardiovascular Collapse Is Caused by Hyperkalemia and Prevented by Zinc Gluconate in Mice. PLoS ONE 2012, 7, e51368. [Google Scholar] [CrossRef]
- Pereira, P.; Seymour, J.E. In vitro effects on human heart and skeletal cells of the venom from two cubozoans, Chironex fleckeri and Carukia barnesi. Toxicon 2013, 76, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Long-Rowe, K.O.; Burnett, J.W. Sea nettle (Chrysaora quinquecirrha) lethal factor: Purification by recycling on m-aminophenyl boronic acid acrylic beads. Toxicon 1994, 32, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Long, K.O.; Burnett, J.W. Isolation, characterization, and comparison of hemolytic peptides in nematocyst venoms of two species of jellyfish (Chrysaora quinquecirrha and Cyanea capillata). Comp. Biochem. Physiol. Part B Comp. Biochem. 1989, 94, 641–646. [Google Scholar] [CrossRef]
- Suganthi, K.; Bragadeeswaran, S.; Kumaran, N.S.; Thenmozhi, C.; Thangaraj, S. In vitro antioxidant activities of jelly fish Chrysaora quinquecirrha venom from southeast coast of India. Asian Pac. J. Trop. Biomed. 2012, 2, S347–S351. [Google Scholar] [CrossRef]
- Long-Rowe, K.O.; Burnett, J.W. Characteristics of hyaluronidase and hemolytic activity in fishing tentacle nematocyst venom of Chrysaora quinquecirrha. Toxicon 1994, 32, 165–174. [Google Scholar] [CrossRef]
- Olson, C.E.; Heard, M.G.; Calton, G.J.; Burnett, J.W. Interrelationships between toxins: Studies on the cross-reactivity between bacterial or animal toxins and monoclonal antibodies to two jellyfish venoms. Toxicon 1985, 23, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Calton, G.J. Sea nettle and man o’war venoms: A chemical comparison of their venoms and studies on the pathogenesis of the sting. J. Investig. Dermatol. 1974, 62, 372–377. [Google Scholar] [CrossRef]
- Russo, A.J.; Calton, G.J.; Burnett, J.W. The relationship of the possible allergic response to jellyfish envenomation and serum antibody titers. Toxicon 1983, 21, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Long, K.O.; Rubinstein, H.M. Beachside preparation of jellyfish nematocyst tentacles. Toxicon 1992, 30, 794–796. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Vucenik, I.; Shamsuddin, A.; Niculescu, F.; Burnett, J.W. Two new actions of sea nettle (Chrysaora quinquecirrha) nematocyst venom: Studies on the mechanism of actions on complement activation and on the central nervous system. Toxicon 2004, 44, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Helmholz, H. Selective toxin–lipid membrane interactions of natural, haemolytic Scyphozoan toxins analyzed by surface plasmon resonance. Biochim. Biophys. Acta (BBA) Biomembr. 2010, 1798, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Lassen, S.; Helmholz, H.; Ruhnau, C.; Prange, A. Characterisation of neurotoxic polypeptides from Cyanea capillata medusae (Scyphozoa). Hydrobiologia 2010, 645, 213–221. [Google Scholar] [CrossRef]
- Wang, B.; Liu, D.; Wang, C.; Wang, Q.; Zhang, H.; Liu, G.; Tao, X.; Zhang, L. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by tentacle extract from the jellyfish Cyanea capillata. PeerJ 2017, 5, e3172. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, L.; Zheng, J.; Wang, Q.; Wang, T.; Lu, J.; Wen, X.; Zhang, B.; Liu, G.; Zhang, W.; et al. Multiple organ dysfunction: A delayed envenomation syndrome caused by tentacle extract from the jellyfish Cyanea capillata. Toxicon 2013, 61, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xiao, L.; He, Q.; Liu, S.; Zhang, J.; Li, Y.; Zhang, Z.; Nie, F.; Guo, Y.; Zhang, L. Comparison of haemolytic activity of tentacle-only extract from jellyfish Cyanea capillata in diluted whole blood and erythrocyte suspension: Diluted whole blood is a valid test system for haemolysis study. Exp. Toxicol. Pathol. 2011, 64, 831–835. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Wang, B.; Wang, C.; Xiao, L.; Zhang, L. β adrenergic receptor/cAMP/PKA signaling contributes to the intracellular Ca2+ release by tentacle extract from the jellyfish Cyanea capillata. BMC Pharmacol. Toxicol. 2017, 18, 60. [Google Scholar] [CrossRef]
- Wang, T.; He, Q.; Xiao, L.; Wang, Q.; Zhang, B.; Wang, B.; Liu, G.; Zheng, J.; Yu, B.; Zhang, L. Mitochondrial dysfunction contributes to the cytotoxicity induced by tentacle extract from the jellyfish Cyanea capillata in rat renal tubular epithelial NRK-52E cells. Toxicon 2013, 74, 1–7. [Google Scholar] [CrossRef]
- Xiao, L.; He, Q.; Guo, Y.; Zhang, J.; Nie, F.; Li, Y.; Ye, X.; Zhang, L. Cyanea capillata tentacle-only extract as a potential alternative of nematocyst venom: Its cardiovascular toxicity and tolerance to isolation and purification procedures. Toxicon 2009, 53, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, S.; He, Q.; Wang, Q.; Ye, X.; Liu, G.; Nie, F.; Zhao, J.; Zhang, L. The Acute Toxicity and Hematological Characterization of the Effects of Tentacle-Only Extract from the Jellyfish Cyanea capillata. Mar. Drugs 2011, 9, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Beilei, W.; Ying, L.; Qianqian, W.; Sihua, L.; Yang, W.; Guoyan, L.; Jia, L.; Xuting, Y.; Liming, Z. Cardiovascular Effect Is Independent of Hemolytic Toxicity of Tentacle-Only Extract from the Jellyfish Cyanea capillata. PLoS ONE 2012, 7, e43096. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, J.; Wang, Q.-Q.; He, Q.; Liu, S.-H.; Li, Y.; Zhang, L.-M. In vitro and in vivo haemolytic studies of tentacle-only extract from jellyfish Cyanea capillata. Toxicol. Vitr. 2010, 24, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Q.Q.; Xiao, L.; Zhang, L.M. Intervention effects of five cations and their correction on hemolytic activity of tentacle extract from the jellyfish Cyanea capillata. PeerJ 2017, 5, e3338. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, B.; Wang, B.; Wang, Q.; Liu, G.; Wang, T.; He, Q.; Zhang, L. Unique Diversity of Sting-Related Toxins Based on Transcriptomic and Proteomic Analysis of the Jellyfish Cyanea capillata and Nemopilema nomurai (Cnidaria: Scyphozoa). J. Proteome Res. 2019, 18, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Helmholz, H.; Ruhnau, C.; Schütt, C.; Prange, A. Comparative study on the cell toxicity and enzymatic activity of two northern scyphozoan species Cyanea capillata (L.) and Cyanea lamarckii (Péron & Léslieur). Toxicon 2007, 50, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Lassen, S.; Helmholz, H.; Ruhnau, C.; Prange, A. A novel proteinaceous cytotoxin from the northern Scyphozoa Cyanea capillata (L.) with structural homology to cubozoan haemolysins. Toxicon 2011, 57, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Yu, H.H.; Li, T.; Li, P.C. Comprehensive Proteome Reveals the Key Lethal Toxins in the Venom of Jellyfish Nemopilema nomurai. J. Proteome Res. 2020, 19, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.K.; Lee, H.; Heo, Y.; Pyo, M.J.; Choudhary, I.; Han, C.H.; Yoon, W.D.; Kang, C.; Kim, E. In vitro characterization of jellyfish venom fibrin(ogen)olytic enzymes from Nemopilema nomurai. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Xu, J.T.; Liu, Y.L.; Zhang, X.L. Comparative study of the hemolytic and cytotoxic activities of nematocyst venoms from the jellyfish Cyanea nozakii Kishinouye and Nemopilema nomurai Kishinouye. J. Oceanol. Limnol. 2018, 36, 1255–1265. [Google Scholar] [CrossRef]
- Yu, H.; Yue, Y.; Dong, X.; Li, R.; Li, P. The Acaricidal Activity of Venom from the Jellyfish Nemopilema nomurai against the Carmine Spider Mite Tetranychus cinnabarinus. Toxins 2016, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Kim, Y.K.; Lee, H.; Cha, M.; Sohn, E.-T.; Jung, E.-S.; Song, C.; Kim, M.; Lee, H.C.; Kim, J.-S.; et al. Target organ identification of jellyfish envenomation using systemic and integrative analyses in anesthetized dogs. J. Pharmacol. Toxicol. Methods 2011, 64, 173–179. [Google Scholar] [CrossRef]
- Kang, C.; Munawir, A.; Cha, M.; Sohn, E.-T.; Lee, H.; Kim, J.-S.; Yoon, W.D.; Lim, D.; Kim, E. Cytotoxicity and hemolytic activity of jellyfish Nemopilema nomurai (Scyphozoa: Rhizostomeae) venom. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 150, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.L.M.; Hwang, D.H.; Hong, I.-H.; Chae, J.; Kang, C.; Kim, E. Danio rerio as an alternative vertebrate model for jellyfish venom study: The toxinological aspects of Nemopilema nomurai venom. Toxicol. Lett. 2020, 335, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.H.; Lee, H.; Choudhary, I.; Kang, C.; Chae, J.; Kim, E. Protective effect of epigallocatechin-3-gallate (EGCG) on toxic metalloproteinases-mediated skin damage induced by Scyphozoan jellyfish envenomation. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Xing, R.; Liu, S.; Li, K.; Wang, X.; Chen, X.; Li, P. Biochemical and kinetic evaluation of the enzymatic toxins from two stinging scyphozoans Nemopilema nomurai and Cyanea nozakii. Toxicon 2017, 125, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Bousabbeh, M.; Mabrouk, H.B.; Morjen, M.; Marrakchi, N.; Bacha, H. Impairment of the cell-to-matrix adhesion and cytotoxicity induced by the Mediterranean jellyfish Pelagia noctiluca venom and its fractions in cultured glioblastoma cells. Lipids Heal. Dis. 2012, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Dellai, A.; Ben Mansour, H.; Bacha, H.; Abid, S. Analgesic and antibutyrylcholinestrasic activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca (Forsskal, 1775). Ann. Clin. Microbiol. Antimicrob. 2012, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, J.; Lucio-Martínez, N. Isolation and prepurification of active compounds in venom from Pelagia noctiluca (Scyphozoa: Pelagiidae) from the Caribbean Sea. Cienc. Mar. 2011, 37, 369–377. [Google Scholar] [CrossRef]
- Ayed, Y.; Boussabbeh, M.; Zakhama, W.; Bouaziz, C.; Abid, S.; Bacha, H. Induction of cytotoxicity of Pelagia noctiluca venom causes reactive oxygen species generation, lipid peroxydation induction and DNA damage in human colon cancer cells. Lipids Heal. Dis. 2011, 10, 232. [Google Scholar] [CrossRef]
- Marino, A.; Morabito, R.; Pizzata, T.; La Spada, G. Effect of crude venom from nematocysts of Pelagia noctiluca (Scyphozoa) on spread discharge of acontia of Calliactis parasitica (Anthozoa). Chem. Ecol. 2008, 24, 9–17. [Google Scholar] [CrossRef]
- Marino, A.; Morabito, R.; Pizzata, T.; La Spada, G. Effect of various factors on Pelagia noctiluca (Cnidaria, Scyphozoa) crude venom-induced haemolysis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Crupi, R.; Rizzo, G.; Morabito, R.; Musci, G.; LA Spada, G. The unusual toxicity and stability properties of crude venom from isolated nematocysts of Pelagia noctiluca (Cnidaria, Scyphozoa). Cell. Mol. Biol. 2007, 53, 994–1002. [Google Scholar]
- Liu, X.; Zhang, M.S.; Zhang, C.; Liu, C.H. Angiotensin converting enzyme (ACE) inhibitory, antihypertensive and antihyperlipidaemic activities of protein hydrolysates from Rhopilema esculentum. Food Chem. 2012, 134, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Yu, H.H.; Liu, S.; Xing, R.E.; Guo, Z.Y.; Li, P.C. Factors affecting the protease activity of venom from jellyfish Rhopilema esculentum Kishinouye. Bioorganic Med. Chem. Lett. 2005, 15, 5370–5374. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yu, H.; Li, C.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Isolation and characterization of venom from nematocysts of jellyfish Rhopilema esculentum Kishinouye. Chin. J. Oceanol. Limnol. 2009, 27, 869–874. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Feng, J.; Chen, X.; Li, P. Comparative analysis of methods for concentrating venom from jellyfish Rhopilema esculentum Kishinouye. Chin. J. Oceanol. Limnol. 2009, 27, 172–176. [Google Scholar] [CrossRef]
- Yu, H.H.; Xing, R.E.; Liu, S.; Li, C.P.; Guo, Z.Y.; Li, P.C. Studies on the hemolytic activity of tentacle extracts of jellyfish Rhopilema esculentum Kishinouye: Application of orthogonal test. Int. J. Biol. Macromol. 2007, 40, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, X.; Dong, X.; Li, C.; Xing, R.; Liu, S.; Li, P. Insecticidal activity of proteinous venom from tentacle of jellyfish Rhopilema esculentum Kishinouye. Bioorganic Med. Chem. Lett. 2005, 15, 4949–4952. [Google Scholar] [CrossRef]
- Reinicke, J.; Kitatani, R.; Masoud, S.S.; Galbraith, K.K.; Yoshida, W.; Igarashi, A.; Nagasawa, K.; Berger, G.; Yanagihara, A.; Nagai, H.; et al. Isolation, Structure Determination, and Synthesis of Cyclic Tetraglutamic Acids from Box Jellyfish Species Alatina alata and Chironex yamaguchii. Molecules 2020, 25, 883. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Ito, E.; Miyake, M.; Noda, M.; Nakajima, T. Novel Proteinaceous Toxins from the Box Jellyfish (Sea Wasp) Carybdea rastoni. Biochem. Biophys. Res. Commun. 2000, 275, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Sekizaki, S.; Satoh, A.; Nakajima, T. Platelet Aggregation Caused by Carybdea Rastonii Toxins (CrTX-I, II, and III) Obtained from a Jellyfish, Carybdea rastonii. Exp. Biol. Med. 1986, 182, 34–42. [Google Scholar] [CrossRef]
- Azuma, H.; Sekizaki, S.; Satoh, A.; Nakajima, T.; Ishikawa, M. Platelet aggregation caused by a partially purified jellyfish toxin from Carybdea rastonii. Toxicon 1986, 24, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Rottini, G.; Gusmani, L.; Parovel, E.; Avian, M.; Patriarca, P. Purification and properties of a cytolytic toxin in venom of the jellyfish Carybdea marsupialis. Toxicon 1995, 33, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Isolation, identification and characterization of a novel antioxidant protein from the nematocyst of the jellyfish Stomolophus meleagris. Int. J. Biol. Macromol. 2012, 51, 274–278. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Xing, R.; Liu, S.; Qing, Y.; Li, K.; Li, B.; Meng, X.; Cui, J.; Li, P. Isolation and in vitro partial characterization of hemolytic proteins from the nematocyst venom of the jellyfish Stomolophus meleagris. Toxicol. Vitr. 2013, 27, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa, K.; Nakao, M.; Sakamoto, B.; Crow, G.L.; Nakajima, T. Isolation and Characterization of a Novel Protein Toxin from the Hawaiian Box Jellyfish (Sea Wasp) Carybdea alata. Biochem. Biophys. Res. Commun. 2000, 275, 589–594. [Google Scholar] [CrossRef]
- Chung, J.J.; Ratnapala, L.A.; Cooke, I.M.; Yanagihara, A.A. Partial purification and characterization of a hemolysin (CAH1) from Hawaiian box jellyfish (Carybdea alata) venom. Toxicon 2001, 39, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Lotan, A.; Fishman, L.; Zlotkin, E. Toxin compartmentation and delivery in the Cnidaria: The nematocyst’s tubule as a multiheaded poisonous arrow. J. Exp. Zool. 1996, 275, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Gusmani, L.; Avian, M.; Galil, B.; Patriarca, P.; Rottini, G. Biologically active polypeptides in the venom of the jellyfish Rhopilema nomadica. Toxicon 1997, 35, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.S.; Shi, Y.P.; Qiao, R.J.; Tang, W.; Sun, Z.L. Production of the angiotensin I converting enzyme inhibitory peptides and isolation of four novel peptides from jellyfish (Rhopilema esculentum) protein hydrolysate. J. Sci. Food Agric. 2016, 96, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.M.; Sultana, I.; Mahmood, S.R.; Alam, S.M. Pneumotoxic activity of crude venom and a cytolytic protein, PuTX-IVC, from a coelenterate, Physalia utriculus (Blue Bottle). Pak. J. Zool. 2006, 38, 159–165. [Google Scholar]
- Diaz-Garcia, C.; Fuentes-Silva, D.; Sanchez-Soto, C.; Pérez, D.D.; Garcia-Delgado, N.; Varela, C.; Mendoza-Hernandez, G.; Rodriguez-Romero, A.; Castaneda, O.; Hiriart, M. Toxins from Physalia physalis (Cnidaria) Raise the Intracellular Ca2+ of Beta-Cells and Promote Insulin Secretion. Curr. Med. Chem. 2012, 19, 5414–5423. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.K.; Anthony, R.L.; Calton, G.J.; Burnett, J.W. Isolation of hybridomas secreting monoclonal antibodies against Physalia physalis (Portuguese man-o’war) nematocyst venom. Toxicon 1982, 20, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.W.; Ordonez, J.V.; Calton, G.J. Differential toxicity of Physalia physalis (Portuguese man-o’war) nematocysts separated by flow cytometry. Toxicon 1986, 24, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Helmholz, H.; Naatz, S.; Lassen, S.; Prange, A. Isolation of a cytotoxic glycoprotein from the Scyphozoa Cyanea lamarckii by lectin-affinity chromatography and characterization of molecule interactions by surface plasmon resonance. J. Chromatogr. B 2008, 871, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Radwan, F.F.; Burnett, J.W.; Bloom, D.A.; Coliano, T.; Eldefrawi, M.E.; Erderly, H.; Aurelian, L.; Torres, M.; la Cotera, E.P.H.-D. A comparison of the toxinological characteristics of two Cassiopea and Aurelia species. Toxicon 2000, 39, 245–257. [Google Scholar] [CrossRef]
- Ponce, D.; López-Vera, E.; Aguilar, M.B.; Sanchez-Rodriguez, J. Preliminary Results of the in Vivo and in Vitro Characterization of a Tentacle Venom Fraction from the Jellyfish Aurelia aurita. Toxins 2013, 5, 2420–2433. [Google Scholar] [CrossRef]
- Rastogi, A.; Biswas, S.; Sarkar, A.; Chakrabarty, D. Anticoagulant activity of Moon jellyfish (Aurelia aurita) tentacle extract. Toxicon 2012, 60, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Gomes, A.; Nag Chaudhuri, A.K. Isolation of a toxin from jellyfish Acromitus rabanchatu and its effect on skeletal muscle. Toxicon 1993, 31, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Sarkar, A.; Chakrabarty, D. Partial purification and identification of a metalloproteinase with anticoagulant activity from Rhizostoma pulmo (Barrel Jellyfish). Toxicon Off. J. Int. Soc. Toxinology 2017, 132, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Ishikawa, M.; Nakajima, T.; Satoh, A.; Sekizaki, S. Calcium-dependent contractile response of arterial smooth muscle to a jellyfish toxin (pCrTX: Carybdea rastonii). Br. J. Pharmacol. 1986, 88, 549–559. [Google Scholar] [CrossRef]
- Zhang, M.; Fishman, Y.; Sher, D.; Zlotkin, E. Hydralysin, a novel animal group-selective paralytic and cytolytic protein from a noncnidocystic origin in hydra. Biochemistry 2003, 42, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Lazcano-Pérez, F.; Arellano, R.O.; Garay, E.; Arreguín-Espinosa, R.; Sánchez-Rodríguez, J. Electrophysiological activity of a neurotoxic fraction from the venom of box jellyfish Carybdea marsupialis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 191, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Radwan, F.F.Y.; Roman, L.G.; Baksi, K.; Burnett, J.W. Toxicity and mAChRs binding activity of Cassiopea xamachana venom from Puerto Rican coasts. Toxicon 2005, 45, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Radwan, F.F.; Burnett, J.W. Toxinological studies of the venom from Cassiopea xamachana nematocysts isolated by flow cytometry. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2001, 128, 65–73. [Google Scholar] [CrossRef]
- Torres, M.; Aguilar, M.B.; Falcón, A.; Sánchez, L.; Radwan, F.F.; Burnett, J.W.; la Cotera, E.P.H.-D.; Arellano, R.O. Electrophysiological and hemolytic activity elicited by the venom of the jellyfish Cassiopea xamachana. Toxicon 2001, 39, 1297–1307. [Google Scholar] [CrossRef]
- Azila, N.; Siao, F.K.; Othman, I. Haemolytic, oedema and haemorrhage inducing activities of tentacular extract of the blubber jellyfish (Catostylus mosaicus). Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991, 99, 153–156. [Google Scholar] [CrossRef]
- Wiltshire, C.J.; Sutherland, S.K.; Fenner, P.J.; Young, A.R. Optimization and preliminary characterization of venom isolated from 3 medically important jellyfish: The box (Chironex fleckeri), Irukandji (Carukia barnesi), and blubber (Catostylus mosaicus) jellyfish. Wilderness Environ. Med. 2000, 11, 241–250. [Google Scholar] [CrossRef] [PubMed]
- So, P.B.T.; Rubio, P.; Lirio, S.; Macabeo, A.P.; Huang, H.-Y.; Corpuz, M.J.-A.T.; Villaflores, O.B. In vitro angiotensin I converting enzyme inhibition by a peptide isolated from Chiropsalmus quadrigatus Haeckel (box jellyfish) venom hydrolysate. Toxicon 2016, 119, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Takuwa-Kuroda, K.; Nakao, M.; Oshiro, N.; Iwanaga, S.; Nakajima, T. A Novel Protein Toxin from the Deadly Box Jellyfish (Sea Wasp, Habu-kurage) Chiropsalmus quadrigatus. Biosci. Biotechnol. Biochem. 2002, 66, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Horiike, T.; Nagai, H.; Kitani, S. Identification of Allergens in the Box Jellyfish Chironex yamaguchii That Cause Sting Dermatitis. Int. Arch. Allergy Immunol. 2015, 167, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yu, H.; Yue, Y.; Li, P. Combined Proteome and Toxicology Approach Reveals the Lethality of Venom Toxins from Jellyfish Cyanea nozakii. J. Proteome Res. 2018, 17, 3904–3913. [Google Scholar] [CrossRef]
- Li, R.; Yu, H.; Feng, J.; Xing, R.; Liu, S.; Wang, L.; Qin, Y.; Li, K.; Li, P. Two-step purification and in vitro characterization of a hemolysin from the venom of jellyfish Cyanea nozakii Kishinouye. Int. J. Biol. Macromol. 2011, 49, 14–19. [Google Scholar] [CrossRef]
- Feng, J.; Yu, H.; Li, C.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Isolation and characterization of lethal proteins in nematocyst venom of the jellyfish Cyanea nozakii Kishinouye. Toxicon 2010, 55, 118–125. [Google Scholar] [CrossRef]
- Li, C.P.; Li, P.C.; Feng, J.H.; Li, R.F.; Yu, H.H. Cytotoxicity of the venom from the nematocysts of jellyfish Cyanea nozakii Kishinouye. Toxicol. Ind. Health 2012, 28, 186–192. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel Jellyfish (Rhizostoma pulmo) as Source of Antioxidant Peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Winkel, K.D.; Tibballs, J.; Molenaar, P.; Lambert, G.; Coles, P.; Ross-Smith, M.; Wiltshire, C.; Fenner, P.J.; Gershwin, L.-A.; Hawdon, G.M. Cardiovascular actions of the venom from the Irukandji (Carukia Barnesi) jellyfish: Effects in human, rat and guinea-pig tissues in vitro and in pigs in vitro. Clin. Exp. Pharmacol. Physiol. 2005, 32, 777–788. [Google Scholar] [CrossRef]
- Nabipour, I.; Mohebbi, G.; Vatanpour, H.; Vazirizadeh, A. Hematological parameters on the effect of the jellyfish venom Cassiopea andromeda in animal models. Data Brief 2017, 11, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, G.; Farzadinia, P.; Vatanpour, H.; Hashemi, A.; Vazirizadeh, A.; Khazaei, Z. Sub-acute toxicity of the alien Cassiopea andromeda (forsskal, 1775) jellyfish venom, in rats. Entomol. Appl. Sci. Lett. 2016, 3, 65–71. [Google Scholar]
- Qu, X.; Fan, L.; Zhong, T.; Li, G.; Xia, X.; Long, H.; Huang, D.; Shu, W. The nematocysts venom of Chrysaora helvola Brandt leads to apoptosis-like cell death accompanied by uncoupling of oxidative phosphorylation. Toxicon 2016, 110, 74–78. [Google Scholar] [CrossRef]
- Qu, X.; Xia, X.; Lai, Z.; Zhong, T.; Li, G.; Fan, L.; Shu, W. Apoptosis-like cell death induced by nematocyst venom from Chrysaora helvola Brandt jellyfish and an in vitro evaluation of commonly used antidotes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2016, 180, 31–39. [Google Scholar] [CrossRef]
- Kim, H.-J.; Noh, J.-W.; Amarsanaa, K.; Jeon, S.-C.; Yang, Y.-S.; Hwang, N.-H.; Ko, E.-A.; Kang, Y.-J.; Jung, S.-C. Peripheral Pain Modulation of Chrysaora pacifica Jellyfish Venom Requires Both Ca2+ Influx and TRPA1 Channel Activation in Rats. Neurotox. Res. 2020, 38, 900–913. [Google Scholar] [CrossRef]
- Carneiro, R.F.V.; Nascimento, N.R.F.D.; Costa, P.P.C.; Gomes, V.M.; de Souza, A.J.F.; de Oliveira, S.C.B.; Filho, E.B.D.S.D.; Zara, F.J.; Fonteles, M.C.; Toyama, D.D.O.; et al. The extract of the jellyfish Phyllorhiza punctata promotes neurotoxic effects. J. Appl. Toxicol. 2011, 31, 720–729. [Google Scholar] [CrossRef]
- Knittel, P.S.; Long, P.F.; Brammall, L.; Marques, A.C.; Almeida, M.T.; Padilla, G.; Moura-Da-Silva, A.M. Characterising the enzymatic profile of crude tentacle extracts from the South Atlantic jellyfish Olindias sambaquiensis (Cnidaria: Hydrozoa). Toxicon 2016, 119, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Amezcua, M.P.; Guerrero-Legarreta, I.; Gonzalez-Marquez, H.; Guzman-Garcia, X. In vivo analysis of effects of venom from the jellyfish Chrysaora sp. in zebrafish (Danio rerio). Toxicon 2016, 113, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Ye, R.; Ma, C.; Wang, Y.; Wang, Y.; Chen, J.; Yang, J.; Höfer, J.; Zhu, Y.; Xiao, L.; et al. Toxicity evaluation, toxin screening and its intervention of the jellyfish Phacellophora camtschatica based on a combined transcriptome-proteome analysis. Ecotoxicol. Environ. Saf. 2022, 233, 113315. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yu, H.; Li, R.; Liu, S.; Xing, R.; Li, P. Insights into individual variations in nematocyst venoms from the giant jellyfish Nemopilema nomurai in the Yellow Sea. Sci. Rep. 2019, 9, 3361. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yu, H.; Li, P. Highlights of animal venom research on the geographical variations of toxin components, toxicities and envenomation therapy. Int. J. Biol. Macromol. 2020, 165, 2994–3006. [Google Scholar] [CrossRef] [PubMed]


| Species (Class) | Toxin Name and Reference | kDa | Purification Level | LD50 µg/kg | LD50 Assay Animal | LD50 ng/mL | LD50 Cell Type | Reported Primary Activity |
|---|---|---|---|---|---|---|---|---|
| Chironex fleckeri Southcott, 1956 (Cubozoa) | CfTX-A [22] | 40 | Isolated toxin | N/A | 5 | Sheep erythrocytes | Haemolytic | |
| CfTX-B [22] | 42 | Isolated toxin | ||||||
| Toxin 1 [7] | 150 | Isolated toxin | N/A | Myotoxic | ||||
| Toxin 2 [7] | 600 | Isolated toxin | ||||||
| Major cytolysin 1 [23] | 370 | Isolated toxin | 24 | Sheep erythrocytes | Haemolytic | |||
| Major cytolysin 2 [23] | 145 | Isolated toxin | 7 | |||||
| Minor cytolysin [23] | 70 | Isolated toxin | N/A | |||||
| Fraction 2 [24] | N/A | Venom fraction | Cardiotoxic | |||||
| T4 [25] | Venom fraction | Neurotoxic | ||||||
| T5 [25] | Venom fraction | Haemolytic | ||||||
| Fraction 11–14 [26] | Venom fraction | 3.6 | Mice | Unidentified | ||||
| Cf Fraction 4 [27] | Venom fraction | N/A | Cytotoxic | |||||
| Fraction 11 [26] | Venom fraction | 6.5 | Mice | |||||
| Flow though fraction [9] | Venom fraction | 27 | 2000 | Sheep erythrocytes | Haemolytic | |||
| Specific eluate [9] | Venom fraction | 2.2 | 1100 | |||||
| Recovered Fraction [28] | Venom fraction | 28 | N/A | Cytotoxic | ||||
| Whole venom [24] | Crude venom | N/A | Cardiotoxic | |||||
| Crude venom [23] | Crude venom | 5 | Sheep erythrocytes | Haemolytic | ||||
| Cf FTNV [27] | Crude venom | N/A | Cytotoxic | |||||
| C. fleckeri venom [29] | Crude venom | 2.2 | Fish | Cardiotoxic | ||||
| Chironex fleckeri crude tentacle extract [30] | Crude venom | N/A | ||||||
| Chironex fleckeri venom [31] | Crude venom | 244 2700 | Rat cardiomyocytes Sheep erythrocytes | Haemolytic | ||||
| Nematocyst venom [26] | Crude venom | 23.4 | Mice | N/A | Cytotoxic | |||
| venom [10] | Crude venom | N/A | Neurotoxic | |||||
| Chironex venom [32] | Crude venom | 150 | Mice | Cytotoxic | ||||
| Crude tentacle venom [28] | Crude venom | 11 | 670 | Sheep erythrocytes | Haemolytic | |||
| Chironex fleckeri [33] | Crude venom | N/A | N/A | Cardiotoxic | ||||
| Chironex fleckeri venom [34] | Crude venom | N/A | 10.85 | Human erythrocytes | Haemolytic | |||
| Stock venom [9] | Crude venom | 12 | Mice | N/A | Cytotoxic | |||
| C.fleckeri venom [35] | Crude venom | N/A | Cardiotoxic | |||||
| Chrysaora Quinquecirrha Desor, 1848 (Scyphozoa) | Sea Nettle toxin [36] | 105 | Isolated toxin | 23 | Mice | Unidentified | ||
| Chrysaora hemolysin [37] | 6–10 | Isolated toxin | N/A | Haemolytic | ||||
| Cq Fraction 4 [27] | N/A | Venom fraction | Cytotoxic | |||||
| Frc-1 [38] | Venom fraction | Antioxidant | ||||||
| Frc-2 [38] | Venom fraction | |||||||
| Frc-3 [38] | Venom fraction | |||||||
| toxin fraction [37] | Venom fraction | Haemolytic | ||||||
| Partially purified Hyaluronidase [39] | Venom fraction | |||||||
| HPLC fraction [37] | Venom fraction | |||||||
| C. quinquecirrha specific fraction [40] | Venom fraction | 1000 | Chicken erythrocytes | Cardiotoxic | ||||
| Lethal proteins [41] | Venom fraction | N/A | Unidentified | |||||
| Cq FTNV [27] | Crude venom | Cytotoxic | ||||||
| Crude venom [36] | Crude venom | 58 | Mice | Unidentified | ||||
| Crude [38] | Crude venom | N/A | Antioxidant | |||||
| Crude cell free extract [37] | Crude venom | Haemolytic | ||||||
| C. quinquecirrha crude extract [40] | Crude venom | 25,000 | Chicken cardiocytes | Cardiotoxic | ||||
| Sea nettle crude toxin [42] | Crude venom | N/A | Allergen | |||||
| Chrysaora quinquecirrha [31] | Crude venom | 407 | Rat cardiocytes and cardiomyocytes | Cytotoxic | ||||
| Liquid N2 Chrysaora quinquecirrha venom [43] | Crude venom | 690 | Mice | N/A | ||||
| Chrysaora venom [32] | Crude venom | 210 | ||||||
| SN crude [44] | Crude venom | 20 | Fish | |||||
| Chrysaora quinquecirrha [33] | Crude venom | Fraction N/A | Cardiotoxic | |||||
| Cyanea capillata Linnaeus, 1758 (Scyphozoa) | CcNT [6] | 8.22 | Isolated toxin | Neurotoxic | ||||
| Major fraction [45] | N/A | Venom fraction | 13,500 8800 | Chicken erythrocytes and rabbit erythrocytes | Haemolytic | |||
| toxin fraction [37] | Venom fraction | N/A | ||||||
| HPLC fraction [37] | Venom fraction | |||||||
| C. capillata specific fraction [40] | Venom fraction | 9000 | Chicken erythrocytes | Cardiotoxic | ||||
| Fraction 3 [45] | Venom fraction | N/A | Neurotoxic | |||||
| Fraction F3B [46] | Venom fraction | |||||||
| C. capillata crude extract [40] | Crude venom | 6000 | Chicken cardiocytes | Cardiotoxic | ||||
| TE [47] | Crude venom | N/A | Phosphorylation | |||||
| TE [48] | Crude venom | Cytotoxic | ||||||
| TOE [49] | Crude venom | 156,000 | Sheep erythrocytes Erythrocytes | Haemolytic | ||||
| TE [50] | Crude venom | N/A | Cytotoxic | |||||
| TE [51] | Crude venom | |||||||
| TOE [52] | Crude venom | Cardiotoxic | ||||||
| TOE [53] | Crude venom | 4250 | Mice | Haemolytic | ||||
| C TOE [54] | Crude venom | N/A | Cardiotoxic | |||||
| TOE [55] | Crude venom | Haemolytic | ||||||
| TE [56] | Crude venom | |||||||
| CnV [57] | Crude venom | 950 | Human cancer cell line | Cytotoxic | ||||
| C. capillata venom [58] | Crude venom | N/A | ||||||
| Crude cell-free extract [37] | Crude venom | Haemolytic | ||||||
| CcTX-1 [59] | Crude venom | |||||||
| Nemopilema Nomurai Kishinouye, 1922 (Scyphozoa) | NnLF [60] | Venom fraction | ||||||
| NnV [61] | Crude venom | Proteolytic | ||||||
| Nemopilema nomurai nematocysts venom [62] | Crude venom | 63,620 | Sheep erythrocytes | Haemolytic | ||||
| NnV [57] | Crude venom | 280 | Human cancer cell line | Cytotoxic | ||||
| NnNV [4] | Crude venom | N/A | Cytolytic | |||||
| NnFV [63] | Crude venom | 29.1 | Spiders | Insecticidal | ||||
| NnV [64] | Crude venom | N/A | Cardiotoxic | |||||
| N. nomurai venom [65] | Crude venom | 2000, 1200, 151,000 | Human cancer cell line and dog erythrocytes | Cytotoxic | ||||
| NnV [66] | Crude venom | N/A | Cardiotoxic | |||||
| NnV [67] | Crude venom | Proteolytic | ||||||
| N.nomurai venom [68] | Crude venom | |||||||
| NnTXs [11] | Crude venom | Haemolytic | ||||||
| Pelagia noctiluca Forsskål, 1775 (Scyphozoa) | P. noctiluca specific fraction [40] | Venom fraction | 4000 | Chicken erythrocytes | Cardiotoxic | |||
| F1 [69] | Venom fraction | 125,000 | Human glioblastoma U87 | Cytotoxic | ||||
| F3 [69] | Venom fraction | 179,000 | Human glioblastoma U87 | |||||
| Fraction 1 [70] | Venom fraction | N/A | Analgesic | |||||
| Fraction 2 [70] | Venom fraction | |||||||
| Fraction II [71] | Venom fraction | Neurotoxic | ||||||
| P. noctiluca crude extract [40] | Crude venom | 14,000 | Chicken cardiocytes | Cardiotoxic | ||||
| Crude venom [70] | Crude venom | 20,000 | Mice | N/A | Analgesic | |||
| Crude extract [71] | Crude venom | N/A | 980 | Human erythrocytes erythrocytes | Haemolytic | |||
| Crude venom [72] | Crude venom | 300,000 | Human cancer cell line | Cytotoxic | ||||
| Pelagia Noctiluca crude venom [73] | Crude venom | N/A | Nematocysts inhibition | |||||
| Crude venom [74] | Crude venom | Haemolytic | ||||||
| Pelagia noctiluca Crude venom [75] | Crude venom | Haemolytic | ||||||
| Rhopilema Esculentum Kishinouye, 1891 (Scyphozoa) | RPH [76] | Venom fraction | ACE inhibitor | |||||
| Rhopilema esculentum Venom [77] | Crude venom | Cytotoxic | ||||||
| RNV [78] | Crude venom | 910 | Chicken erythrocytes | Haemolytic | ||||
| RFV [8] | Crude venom | 3400 | ||||||
| CT [79] | Crude venom | 12,400 | Sheep erythrocytes | |||||
| RFV [80] | Crude venom | N/A | ||||||
| R.esculentum full proteinous venom [81] | Crude venom | 123.1 | Moth | Insecticidal | ||||
| Chironex Yamaguchii Lewis and Bentlage, 2009 (Cubozoa) | 4A [82] | 0.5 | Isolated toxin | N/A | No known function | |||
| 4B [82] | 0.5 | Isolated toxin | ||||||
| 4C [82] | 0.5 | Isolated toxin | ||||||
| Carybdea rastoni (Cubozoa) Haacke, 1886 | CrTX-A [83] | 43 | Isolated toxin | 20 | Crayfish | 1.9 | Sheep erythrocytes | Haemolytic |
| CrTX-B [83] | 46 | Isolated toxin | N/A | N/A | 2.2 | |||
| CrTX-I [84] | 49 | Isolated toxin | 3.5 | Mice | N/A | |||
| CrTX-II [84] | 100 | Isolated toxin | 3.6 | |||||
| CrTX-III [84] | 51 | Isolated toxin | 3.0 | |||||
| pCrTX [84,85] | N/A | Crude venom | 127 | |||||
| Carybdea Marsupialis Linnaeus, 1758 (Cubozoa) | CARTOX [86] | 102 | Isolated toxin | N/A | 50 | Sheep erythrocytes | ||
| Stomolophus Meleagris Agassiz, 1860 (Scyphozoa) | SmP90 [87] | 90 | Isolated toxin | N/A | Antioxidant | |||
| SmTX [88] | 45–52 | Isolated toxin | 70,000 | Chicken erythrocytes | Haemolytic | |||
| Fraction A [21] | N/A | Venom fraction | >40,000 | Mice | N/A | Cardiotoxic | ||
| Fraction B [21] | Venom fraction | >40,000 | ||||||
| Fraction C [21] | Venom fraction | >40,000 | ||||||
| Fraction D [21] | Venom fraction | >40,000 | ||||||
| Fraction E [21] | Venom fraction | 32,000 | ||||||
| Carybdea alata Reynaud, 1830 (Cubozoa) | CaTX-A [89] | 43 | Isolated toxin | 25 | Crayfish | 70 | Sheep erythrocytes | Haemolytic |
| CaTX-B [89] | 45 | Isolated toxin | N/A | 80 | ||||
| First peak [90] | N/A | Venom fraction | 20 | |||||
| Second peak [90] | Venom fraction | 25 | ||||||
| Crude venom [90] | Crude venom | 290 | ||||||
| Rhopilema nomadica Spanier and Ferguson, 1990 (Scyphozoa) | PhA2 [91] | >10 | Isolated toxin | 6000 | Fish | N/A | Cytolytic | |
| Crude toxin peak 1 [92] | N/A | Venom fraction | N/A | 1250 | Human erythrocytes | Haemolytic | ||
| Crude toxin [92] | Crude venom | 1250 | ||||||
| Rhopilema esculentum Kishinouye, 1891 (Scyphozoa) | X1 [93] | 0.434 | Isolated toxin | N/A | ACE inhibitor | |||
| X2 [93] | 0.683 | Isolated toxin | ||||||
| X3 [93] | 0.754 | Isolated toxin | ||||||
| X4 [93] | 0.778 | Isolated toxin | ||||||
| Physalia utriculus Gmelin, 1788 (Hydrozoa) | PuTx-IVC [94] | N/A | Isolated toxin | 1190 | Mice | Cytolytic | ||
| Physalia physalis (Linnaeus, 1758) (Hydrozoa) | PpV9.4 [95] | 0.550 | Isolated toxin | N/A | Promote insulin secretion | |||
| PpV19.3 [95] | 4.720 | Isolated toxin | ||||||
| P. physalis specific fraction [40] | N/A | Venom fraction | 5000 | Chicken erythrocytes | Cardiotoxic | |||
| P. physalis crude extract [40] | Crude venom | 75,000 | Cardiotoxic | |||||
| Man-o-war crude toxin [42] | Crude venom | N/A | Allergen | |||||
| Portuguese Man-O-War [96] | Crude venom | Cytotoxic | ||||||
| Nematocyst fluid [97] | Crude venom | |||||||
| Cyanea lamarckii Péron and Lesueur, 1810 (Scyphozoa) | ClGp1 [98] | 25.7 | Isolated toxin | |||||
| C. lamarckii [58] | N/A | Crude venom | ||||||
| Aurelia aurita Linnaeus, 1758 (Scyphozoa) | Fraction A-B [99] | Venom fraction | 2000–3000 | Mice | Haemolytic | |||
| Fraction 4 [100] | Venom fraction | N/A | Neurotoxic | |||||
| Fraction I [101] | Venom fraction | Fibrinogenic | ||||||
| Major fraction [45] | Venom fraction | 35,300 43,100 | Chicken erythrocytes and rabbit erythrocytes | Haemolytic | ||||
| Crude venom [99] | Crude venom | 3200–4200 | Mice | N/A | Haemolytic | |||
| JFTE [101] | Crude venom | N/A | Proteolytic | |||||
| Acromitus Rabanchatu Annandale, 1915 (Scyphozoa) | T-Ar [102] | 182 | Isolated toxin | 850 | Mice | Myotoxic | ||
| Fr-II [102] | N/A | Venom fraction | 3000 7700 | |||||
| Crude venom [102] | Crude venom | |||||||
| Rhizostoma pulmo Macri, 1778 (Scyphozoa) | Rhizoprotease [103] | 95 | Isolated toxin | N/A | Proteolytic | |||
| Carybeda rastonii Haacke, 1886 (Cubozoa) | pCrTX [104] | N/A | Venom fraction | 127 | Mice | Cardiotoxic | ||
| Chlorohydra Viridissima * Pallas, 1766 (Hydrozoa) | Pure Toxin [105] | 27 | Isolated toxin | 3.6 | Flies | Cytolytic | ||
| first separation [105] | N/A | Venom fraction | 156 | |||||
| BE [105] | Crude venom | 75.2 | ||||||
| Carybdea marsupialis Linnaeus, 1758 (Cubozoa) | Fraction A [106] | Venom fraction | N/A | Neurotoxic | ||||
| CmNt [5] | Venom fraction | 15 | Crab | |||||
| Crude extract [5] | Crude venom | 1050 | ||||||
| Cassiopea xamachana Bigelow, 1892 (Scyphozoa) | Fraction III [107] | Venom fraction | 280 | Mice | Haemolytic | |||
| Fraction IV [107] | Venom fraction | 250 | ||||||
| Fraction VI [107] | Venom fraction | 120 | Cytolytic | |||||
| C. xamachana post-FACS [108] | Venom fraction | 340 | 7000 | Sheep erythrocytes | Haemolytic | |||
| CxTX [107] | Crude venom | 750 | N/A | Cytolytic | ||||
| Crude Cx Venom [109] | Crude venom | N/A | 6890 56,000 | Human erythrocytes and sheep erythrocytes | Haemolytic | |||
| C. xamachana pre-FACS [108] | Crude venom | 1600 | Mice | 110,000 | Sheep erythrocytes | |||
| Catostylus Mosaicus Quoy and Gaimard, 1824 (Scyphozoa) | Fraction bound (2) [110] | Venom fraction | N/A | N/A | ||||
| Fraction bound (3) [110] | Venom fraction | |||||||
| Fraction bound (4) [110] | Venom fraction | |||||||
| CE [110] | Crude venom | |||||||
| Blubber venom [111] | Crude venom | 2184 | Mice | |||||
| Chiropsalmus Quadrigatus Haeckel, 1880) (Cubozoa) | Fraction 3.5 peptide [112] | 0.97 | Isolated toxin | >2,000,000 | Rats | N/A | ACE inhibitor | |
| CqTX-A [113,114] | 44 | Isolated toxin | 80 | Crayfish | 160 | Sheep erythrocytes | Haemolytic | |
| Cyanea nozakii Kishinouye, 1891 (Scyphozoa) | CnLF [115] | N/A | Venom fraction | N/A | N/A | Cytolytic | ||
| CnPH [116] | Venom fraction | 5000 | Sheep erythrocytes | Haemolytic | ||||
| Nematocyst content [117] | Crude venom | 600 | Fish | N/A | N/A | Neurotoxic | ||
| CnV [115] | Crude venom | 316,000 | mice | N/A | N/A | Cardiotoxic | ||
| Cyanea nozakii nematocyst venom [62] | Crude venom | N/A | 69,690 | Sheep erythrocytes | Haemolytic | |||
| CNN [118] | Crude venom | 5.1 17.9 24.3 | Human cancer cell line | Cytotoxic | ||||
| CnNV [4] | Crude venom | N/A | Cytolytic | |||||
| C.nozakii venom [68] | Crude venom | Proteolytic | ||||||
| Rhizostoma pulmo Macri, 1778 (Scyphozoa) | SP [119] | Venom fraction | Cytotoxic | |||||
| SP > 30 [119] | Venom fraction | |||||||
| Fraction I [103] | Venom fraction | Fibrinogenic | ||||||
| Carukia barnesi Southcott, 1967 (Cubozoa) | CVE [120] | Crude venom | N/A | Cardiotoxic | ||||
| C. barnesi venom [35] | Crude venom | Cytotoxic | ||||||
| Cassiopea andromeda Forskål, 1775 (Scyphozoa) | Cassiopea andromeda venom [121] | Crude venom | 104.0 | Mice | Haemolytic | |||
| Crude tentacle-only extract [122] | Crude venom | 104.0 | Rats | |||||
| Chrysaora helvola Brandt, 1838 (Scyphozoa) | NV [123,124] | Crude venom | N/A | 3130, 220 | Human cancer cell line | Cytotoxic | ||
| Chrysaora pacifica Goette, 1886 (Scyphozoa) | CpV [125] | Crude venom | N/A | Neurotoxic | ||||
| Phyllorhiza punctata Lendenfeld, 1884(Scyphozoa) | Crude Protein Extract [126] | Crude venom | ||||||
| Olindias Sambaquiensis Müller, 1861 (Hydrozoa) | Crude venom [127] | Crude venom | Proteolytic | |||||
| Chiropsalmus sp. Agassiz, 1862 (Cubozoa) | Chiropsalmus sp. venom [29] | Crude venom | 60.370 | Fish | Cardiotoxic | |||
| Chiropsalmus sp. venom [10] | Crude venom | N/A | Neurotoxic | |||||
| Chrysaora sp. Desor, 1848 (Scyphozoa) | Chrysaora sp. venom [128] | Crude venom | Haemolytic | |||||
| Phacellophora camtschatica Brandt, 1835 (Scyphozoa) | Tentacle extract [129] | Crude venom | 3290 | Mice | 400,290 92,440 | Mouse erythrocytes and human cancer cell line | Haemolytic | |
| Species (Class) | Toxin Name | Separation Solution | Cnidocyte Separation | Venom Extraction | Purification Step 1 | Purification Step 2 |
|---|---|---|---|---|---|---|
| Chionex fleckeri (Cubozoa) | CfTX-A [22] | Seawater | 4-day autolysis (Bloom) | Sonication 20s x3 on ice | SEC | CEX |
| CfTX-B [22] | ||||||
| Alatina alata (Cubozoa) | 4A [82] | 1M NaCl | 3–6 weeks autolysis | Omotic pressure using pressure cell | RP HPLC | |
| 4B [82] | ||||||
| 4C [82] | ||||||
| Chiropsalmus quadrigatus (Cubozoa) | CqTX-A [113,114] | Seawater | 4-day autolysis (Burrnet) | Sonication in MQ | CEX | CEX |
| Carybdea rastoni (Cubozoa) | CrTX-A [83] | Whole tentacle Osmotic lysis | Sonication in 5 mM phosphate buffer | |||
| CrTX-B [83] | ||||||
| Carybdea marsupialis (Cubozoa) | CARTOX [86] | Distilled water | Osmotic pressure 5 min | Sonication in MQ x6 | ||
| Carybdea alata (Cubozoa) | CaTX-A [89] | Seawater | 4-day autolysis (Burrnet) | Sonication in MQ on ice | ||
| CaTX-B [89] | ||||||
| Stomolophus meleagris (Scyphozoa) | SmP90 [87] | 1-day autolysis | Sonication in extraction buffer | AEX | SEC | |
| Rhopilema nomadica (Scyphozoa) | PhA2 [91] | Tentacle homogenisation | Omotic pressure dialysis | CEX | ||
| Rhopilema esculentum (Scyphozoa) | X1 [93] | Omotic pressure MQ | HPLC | SEC | ||
| X2 [93] | ||||||
| X3 [93] | ||||||
| X4 [93] | ||||||
| Physalia utriculus (Hydrozoa) | PuTx-IVC [94] | Seawater | 4-day autolysis (Burrnet) | Sonication in MQ on ice | CEX | CEX |
| Physalia physalis (Hydrozoa) | PpV9.4 [95] | Tentacle homogenisation | Omotic pressure MQ | SEC | RP HPLC | |
| PpV19.3 [95] | ||||||
| Cyanea lamarckii (Scyphozoa) | ClGp1 [98] | Sonication acetate | Lectin-affinity chromatography | SEC | ||
| Chironex fleckeri (Cubozoa) | Toxin 1 [7] | Unknown | Unknown | Grinding | SEC | SECx5 |
| Toxin 2 [7] | ||||||
| Acromitus Rabanchatu (Scyphozoa) | T-Ar [102] | Tentacle homogenisation | Freeze–thaw | Acidic precipitation | AEX | |
| Cyanea capillata (Scyphozoa) | CcNT [6] | Distilled water | 10 h | Sonication in extraction buffer | SEC | RP HPLC |
| Chironex fleckeri (Cubozoa) | Major cytolysin 1 [23] | Seawater | 4-day autolysis (Bloom) | Sonication 20s x3 on ice | CEX | |
| Major cytolysin 2 [23] | ||||||
| Minor cytolysin [23] | CEX | |||||
| Chiropsalmus quadrigatus (Cubozoa) | Fraction 3.5 peptide [112] | Distilled water | 2 days autolysis | RP HPLC | ||
| Chrysaora quinquecirrha (Scyphozoa) | Sea Nettle toxin [36] | 1.5% NaCl | 4-day autolysis | Grinding | SEC | |
| Chrysaora hemolysin [37] | Seawater | 4-day autolysis (Burrnet) | Sonication in MQ on ice | N/A | ||
| Cyanea capillata (Scyphozoa) | CcTX-1 [59] | Distilled water | 10 h | Sonication in extraction buffer | CEX RP HPLC | |
| Rhizostoma pulmo (Scyphozoa) | Rhizoprotease [103] | Tentacle homogenisation | 10H Autolysis | Salting-out precipitation | SEC | |
| Stomolophus meleagris (Scyphozoa) | SmTX [88] | Distilled water | 1 day | Sonication in extraction buffer | AEX | SEC |
| Carybdea rastoni (Cubozoa) | CrTX-I [84] | Tentacle homogenisation | HPLC | |||
| CrTX-II [84] | ||||||
| CrTX-III [84] | ||||||
| Chlorohydra Viridissima (Hydrozoa) | Hydralysin [105] | Grinding | AEX | AEX | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lausen, B.; Ahang, A.; Cummins, S.; Wang, T. Investigation of Best Practices for Venom Toxin Purification in Jellyfish towards Functional Characterisation. Toxins 2023, 15, 170. https://doi.org/10.3390/toxins15030170
Lausen B, Ahang A, Cummins S, Wang T. Investigation of Best Practices for Venom Toxin Purification in Jellyfish towards Functional Characterisation. Toxins. 2023; 15(3):170. https://doi.org/10.3390/toxins15030170
Chicago/Turabian StyleLausen, Blake, Anahita Ahang, Scott Cummins, and Tianfang Wang. 2023. "Investigation of Best Practices for Venom Toxin Purification in Jellyfish towards Functional Characterisation" Toxins 15, no. 3: 170. https://doi.org/10.3390/toxins15030170
APA StyleLausen, B., Ahang, A., Cummins, S., & Wang, T. (2023). Investigation of Best Practices for Venom Toxin Purification in Jellyfish towards Functional Characterisation. Toxins, 15(3), 170. https://doi.org/10.3390/toxins15030170





