A Review on Genotoxic and Genoprotective Effects of Biologically Active Compounds of Animal Origin
Abstract
1. Introduction
2. Results of Literature Search
2.1. Coelenterata
2.2. Sponges (Poryphera)
2.3. Annelida
2.4. Mollusca
2.5. Arachnida
2.5.1. Scorpions
2.5.2. Araneae
2.6. Insecta
2.7. Echinodermata
2.8. Ascidacea
2.9. Pisces
2.10. Amphibia
2.11. Reptiles (Serpentes)
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McDermott, A. News Feature: Venom Back in Vogue as a Wellspring for Drug Candidates. Proc. Natl. Acad. Sci. USA 2020, 117, 10100–10104. [Google Scholar] [CrossRef]
- Holford, M.; Daly, M.; King, G.F.; Norton, R.S. Venoms to the Rescue. Science 2018, 361, 842–844. [Google Scholar] [CrossRef]
- King, G.F. Venoms as a Platform for Human Drugs: Translating Toxins into Therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern Venomics-Current Insights, Novel Methods, and Future Perspectives in Biological and Applied Animal Venom Research. Gigascience 2022, 11, giac048. [Google Scholar] [CrossRef]
- Abd El-Aziz, T.M.; Garcia Soares, A.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic Application of Anti-Arthritis, Pain-Releasing, and Anti-Cancer Effects of Bee Venom and Its Constituent Compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Kalita, B.; Saviola, A.J.; Mukherjee, A.K. From Venom to Drugs: A Review and Critical Analysis of Indian Snake Venom Toxins Envisaged as Anticancer Drug Prototypes. Drug Discov. Today 2021, 26, 993–1005. [Google Scholar] [CrossRef]
- Qi, J.; Zulfiker, A.H.M.; Li, C.; Good, D.; Wei, M.Q. The Development of Toad Toxins as Potential Therapeutic Agents. Toxins 2018, 10, 336. [Google Scholar] [CrossRef]
- Saez, N.J.; Senff, S.; Jensen, J.E.; Er, S.Y.; Herzig, V.; Rash, L.D.; King, G.F. Spider-Venom Peptides as Therapeutics. Toxins 2010, 2, 2851–2871. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Harvey, A. From Demons to Darlings: Drugs from Venoms. Drug Discov. Today 1998, 3, 531–532. [Google Scholar] [CrossRef]
- Gajski, G.; Čimbora-Zovko, T.; Osmak, M.; Garaj-Vrhovac, V. Bee Venom and Melittin Are Cytotoxic against Different Types of Tumor and Non-Tumor Cell Lines In Vitro. In Advancements in Cancer Research; Viktorsson, K., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 163–178. ISBN 978-1-61470-252-8. [Google Scholar]
- Herzig, V.; King, G.F. The Cystine Knot Is Responsible for the Exceptional Stability of the Insecticidal Spider Toxin ω-Hexatoxin-Hv1a. Toxins 2015, 7, 4366–4380. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A Lytic Peptide with Anticancer Properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Chatterjee, B. Animal Venoms Have Potential to Treat Cancer. Curr. Top. Med. Chem. 2018, 18, 2555–2566. [Google Scholar] [CrossRef]
- Lewis, R.J.; Garcia, M.L. Therapeutic Potential of Venom Peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Greener, M. The next Generation of Venom-based Drugs. Prescriber 2020, 31, 28–32. [Google Scholar] [CrossRef]
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Rožman, M.; Osmak, M.; Garaj-Vrhovac, V. Combined Antitumor Effects of Bee Venom and Cisplatin on Human Cervical and Laryngeal Carcinoma Cells and Their Drug Resistant Sublines. J. Appl. Toxicol. 2014, 34, 1332–1341. [Google Scholar] [CrossRef]
- Gajski, G.; Čimbora-Zovko, T.; Rak, S.; Osmak, M.; Garaj-Vrhovac, V. Antitumour Action on Human Glioblastoma A1235 Cells through Cooperation of Bee Venom and Cisplatin. Cytotechnology 2016, 68, 1197–1205. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Venom-Derived Bioactive Compounds as Potential Anticancer Agents: A Review. Int. J. Pept. Res. Ther. 2021, 27, 129–147. [Google Scholar] [CrossRef]
- Chaisakul, J.; Hodgson, W.C.; Kuruppu, S.; Prasongsook, N. Effects of Animal Venoms and Toxins on Hallmarks of Cancer. J. Cancer 2016, 7, 1571–1578. [Google Scholar] [CrossRef]
- Viegas, S.; Ladeira, C.; Costa-Veiga, A.; Perelman, J.; Gajski, G. Forgotten Public Health Impacts of Cancer—An Overview. Arch. Ind. Hyg. Toxicol. 2017, 68, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, P.; Mirmohammadi, M.; Houshdar Tehrani, M.H. Anticancer Peptides Mechanisms, Simple and Complex. Chem. Biol. Interact. 2022, 368, 110194. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Bee Venom in Cancer Therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Domijan, A.-M.; Žegura, B.; Štern, A.; Gerić, M.; Novak Jovanović, I.; Vrhovac, I.; Madunić, J.; Breljak, D.; Filipič, M.; et al. Melittin Induced Cytogenetic Damage, Oxidative Stress and Changes in Gene Expression in Human Peripheral Blood Lymphocytes. Toxicon 2016, 110, 56–67. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Bee Venom Induced Cytogenetic Damage and Decreased Cell Viability in Human White Blood Cells after Treatment in Vitro: A Multi-Biomarker Approach. Environ. Toxicol. Pharmacol. 2011, 32, 201–211. [Google Scholar] [CrossRef]
- Garaj-Vrhovac, V.; Gajski, G. Evaluation of the Cytogenetic Status of Human Lymphocytes after Exposure to a High Concentration of Bee Venom in Vitro. Arch. Hig. Rada Toksikol. 2009, 60, 27–34. [Google Scholar] [CrossRef]
- Sjakste, N.; Djelić, N.; Dzintare, M.; Živković, L. DNA-BINDING and DNA-Protecting Activities of Small Natural Organic Molecules and Food Extracts. Chem. Biol. Interact. 2020, 323, 109030. [Google Scholar] [CrossRef]
- Muhamedejevs, R.; Živković, L.; Dzintare, M.; Sjakste, N. DNA-Binding Activities of Compounds Acting as Enzyme Inhibitors, Ion Channel Blockers and Receptor Binders. Chem. Biol. Interact. 2021, 348, 109638. [Google Scholar] [CrossRef]
- Sladic, D.; Gasic, M. Reactivity and Biological Activity of the Marine Sesquiterpene Hydroquinone Avarol and Related Compounds from Sponges of the Order Dictyoceratida. Molecules 2006, 11, 1–33. [Google Scholar] [CrossRef]
- Pejin, B.; Iodice, C.; Kojic, V.; Jakimov, D.; Lazovic, M.; Tommonaro, G. In Vitro Evaluation of Cytotoxic and Mutagenic Activity of Avarol. Nat. Prod. Res. 2016, 30, 1293–1296. [Google Scholar] [CrossRef]
- Su, J.-H.; Chang, W.-B.; Chen, H.-M.; El-Shazly, M.; Du, Y.-C.; Kung, T.-H.; Chen, Y.-C.; Sung, P.-J.; Ho, Y.-S.; Kuo, F.-W.; et al. 10-Acetylirciformonin B, A Sponge Furanoterpenoid, Induces DNA Damage and Apoptosis in Leukemia Cells. Molecules 2012, 17, 11839–11848. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, B.C.; Sombra, C.M.L.; de Oliveira, J.H.H.L.; de Berlinck, R.G.S.; de Moraes, M.O.; Pessoa, C. Cytotoxicity and Genotoxicity of Ingenamine G Isolated from the Brazilian Marine Sponge Pachychalina Alcaloidifera. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2008, 147, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Canals, A.; Arribas-Bosacoma, R.; Albericio, F.; Álvarez, M.; Aymamí, J.; Coll, M. Intercalative DNA Binding of the Marine Anticancer Drug Variolin B. Sci. Rep. 2017, 7, 39680. [Google Scholar] [CrossRef] [PubMed]
- Dave, J.R.; Williams, A.J.; Moffett, J.R.; Koenig, M.L.; Tortella, F.C. Studies on Neuronal Apoptosis in Primary Forebrain Cultures: Neuroprotective/Anti-Apoptotic Action of NR2B NMDA Antagonists. Neurotox. Res. 2003, 5, 255–264. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.H.; da Rosa, L.G.; Uliano, M.R.; da Silva Prado, L.; Ferraz, A.G.; Conter, L.U.; Grivicich, I.; Dallegrave, E.; Gomez, M.V.; Picada, J.N. Evaluation of DNA Damage in Spinal Cord and Mutagenic Effect of a Phα1β Recombinant Toxin with Analgesic Properties from the Phoneutria Nigriventer Spider. Basic Clin. Pharmacol. Toxicol. 2019, 124, 615–620. [Google Scholar] [CrossRef]
- de Santana, C.J.C.; Pires Júnior, O.R.; Fontes, W.; Palma, M.S.; Castro, M.S. Mastoparans: A Group of Multifunctional α-Helical Peptides With Promising Therapeutic Properties. Front. Mol. Biosci. 2022, 9, 331. [Google Scholar] [CrossRef]
- Liang, X.; Yan, J.; Lu, Y.; Liu, S.; Chai, X. The Antimicrobial Peptide Melectin Shows Both Antimicrobial and Antitumor Activity via Membrane Interference and DNA Binding. Drug Des. Devel. Ther. 2021, 15, 1261–1273. [Google Scholar] [CrossRef]
- Gajski, G.; Domijan, A.-M.; Garaj-Vrhovac, V. Alterations of GSH and MDA Levels and Their Association with Bee Venom-Induced DNA Damage in Human Peripheral Blood Leukocytes. Environ. Mol. Mutagen. 2012, 53, 469–477. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Genotoxic Potential of Bee Venom (Apis Mellifera) on Human Peripheral Blood Lymphocytes in Vitro Using Single Cell Gel Electrophoresis Assay. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2008, 43, 1279–1287. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Increased Frequency of Sister Chromatid Exchanges and Decrease in Cell Viability and Proliferation Kinetics in Human Peripheral Blood Lymphocytes after In Vitro Exposure to Whole Bee Venom. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2010, 45, 1654–1659. [Google Scholar] [CrossRef]
- Malyarenko, O.S.; Malyarenko, T.V.; Kicha, A.A.; Ivanchina, N.V.; Ermakova, S.P. Effects of Polar Steroids from the Starfish Patiria (=Asterina) Pectinifera in Combination with X-Ray Radiation on Colony Formation and Apoptosis Induction of Human Colorectal Carcinoma Cells. Molecules 2019, 24, 3154. [Google Scholar] [CrossRef]
- Guirouilh-Barbat, J.; Redon, C.; Pommier, Y. Transcription-Coupled DNA Double-Strand Breaks Are Mediated via the Nucleotide Excision Repair and the Mre11-Rad50-Nbs1 Complex. Mol. Biol. Cell 2008, 19, 3969–3981. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Wang, Y.-M.; Pan, X.; Chi, C.-F.; Wang, B. Antioxidant Mechanisms of the Oligopeptides (FWKVV and FMPLH) from Muscle Hydrolysate of Miiuy Croaker against Oxidative Damage of HUVECs. Oxid. Med. Cell. Longev. 2021, 2021, 9987844. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Giri, B.; Gomes, A. Apoptogenic Activity and Toxicity Studies of a Cytotoxic Protein (BMP1) from the Aqueous Extract of Common Indian Toad (Bufo Melanostictus Schneider) Skin. Toxicon 2011, 57, 225–236. [Google Scholar] [CrossRef] [PubMed]
- da Machado, K.C.; de Sousa, L.Q.; Lima, D.J.B.; Soares, B.M.; Cavalcanti, B.C.; Maranhão, S.S.; Noronha, J.; de Jesus Rodrigues, D.; Militão, G.C.G.; Chaves, M.H.; et al. Marinobufagin, a Molecule from Poisonous Frogs, Causes Biochemical, Morphological and Cell Cycle Changes in Human Neoplasms and Vegetal Cells. Toxicol. Lett. 2018, 285, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Domínguez, I.; Mateos, S.; Cortés, F. A Comparative Study of Genotoxic Effects of Anti-Topoisomerase II Drugs ICRF-193 and Bufalin in Chinese Hamster Ovary Cells. Mutat. Res. 2002, 515, 171–180. [Google Scholar] [CrossRef]
- Doumanov, J.; Mladenova, K.; Topouzova-Hristova, T.; Stoitsova, S.; Petrova, S. Effects of Vipoxin and Its Components on HepG2 Cells. Toxicon 2015, 94, 36–44. [Google Scholar] [CrossRef]
- Machado, A.R.T.; Aissa, A.F.; Ribeiro, D.L.; Costa, T.R.; Ferreira Jr., R.S.; Sampaio, S.V.; Antunes, L.M.G. Cytotoxic, Genotoxic, and Oxidative Stress-Inducing Effect of an l-Amino Acid Oxidase Isolated from Bothrops Jararacussu Venom in a Co-Culture Model of HepG2 and HUVEC Cells. Int. J. Biol. Macromol. 2019, 127, 425–432. [Google Scholar] [CrossRef]
- Machado, A.R.T.; Aissa, A.F.; Ribeiro, D.L.; Hernandes, L.C.; Machado, C.S.; Bianchi, M.L.P.; Sampaio, S.V.; Antunes, L.M.G. The Toxin BjussuLAAO-II Induces Oxidative Stress and DNA Damage, Upregulates the Inflammatory Cytokine Genes TNF and IL6, and Downregulates the Apoptotic-Related Genes BAX, BCL2 and RELA in Human Caco-2 Cells. Int. J. Biol. Macromol. 2018, 109, 212–219. [Google Scholar] [CrossRef]
- de Moura Leão, M.F.; Duarte, J.A.; Sauzen, P.D.; da Piccoli, J.C.E.; de Oliveira, L.F.S.; Machado, M.M. Cytotoxic and Genotoxic Effects of Antihypertensives Distributed in Brazil by Social Programs: Are They Safe? Environ. Toxicol. Pharmacol. 2018, 63, 1–5. [Google Scholar] [CrossRef]
- Marcussi, S.; Santos, P.R.S.; Menaldo, D.L.; Silveira, L.B.; Santos-Filho, N.A.; Mazzi, M.V.; da Silva, S.L.; Stábeli, R.G.; Antunes, L.M.G.; Soares, A.M. Evaluation of the Genotoxicity of Crotalus Durissus Terrificus Snake Venom and Its Isolated Toxins on Human Lymphocytes. Mutat. Res. 2011, 724, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Boussabbeh, M.; Zakhama, W.; Bouaziz, C.; Abid, S.; Bacha, H. Induction of Cytotoxicity of Pelagia Noctiluca Venom Causes Reactive Oxygen Species Generation, Lipid Peroxydation Induction and DNA Damage in Human Colon Cancer Cells. Lipids Health Dis. 2011, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Bouaziz, C.; Brahmi, D.; Zaid, C.; Abid, S.; Bacha, H. Cell Death in Relation to DNA Damage after Exposure to the Jellyfish Pelagia Noctiluca Nematocysts. Environ. Toxicol. 2014, 29, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Abbas, S.Q.; Shah, S.A.A.; Akhter, N.; Batool, S.; Hassan, S.S.U. Marine Sponges as a Drug Treasure. Biomol. Ther. 2016, 24, 347–362. [Google Scholar] [CrossRef]
- Varijakzhan, D.; Loh, J.-Y.; Yap, W.-S.; Yusoff, K.; Seboussi, R.; Lim, S.-H.E.; Lai, K.-S.; Chong, C.-M. Bioactive Compounds from Marine Sponges: Fundamentals and Applications. Mar. Drugs 2021, 19, 246. [Google Scholar] [CrossRef]
- Talevska, A.; Pejin, B.; Kojic, V.; Beric, T.; Stankovic, S. A Contribution to Pharmaceutical Biology of Freshwater Sponges. Nat. Prod. Res. 2018, 32, 568–571. [Google Scholar] [CrossRef]
- Ferretti, C.; Marengo, B.; De Ciucis, C.; Nitti, M.; Pronzato, M.; Marinari, U.; Pronzato, R.; Manconi, R.; Domenicotti, C. Effects of Agelas Oroides and Petrosia Ficiformis Crude Extracts on Human Neuroblastoma Cell Survival. Int. J. Oncol. 2007, 30, 161–169. [Google Scholar] [CrossRef]
- Aiub, C.; Giannerini, A.; Ferreira, F.; Mazzei, J.; Stankevicins, L.; Lobo-Hajdu, G.; Guimarães, P.; Hajdu, E.; Felzenszwalb, I. Genotoxic Evaluation of Extracts from Aplysina Fulva, a Brazilian Marine Sponge. Mutat. Res. 2006, 611, 34–41. [Google Scholar] [CrossRef]
- Vujčić, M.T.; Tufegdžić, S.; Novaković, I.; Djikanović, D.; Gašić, M.J.; Sladić, D. Studies on the Interactions of Bioactive Quinone Avarone and Its Methylamino Derivatives with Calf Thymus DNA. Int. J. Biol. Macromol. 2013, 62, 405–410. [Google Scholar] [CrossRef]
- Patiño Cano, L.P.; Bartolotta, S.A.; Casanova, N.A.; Siless, G.E.; Portmann, E.; Schejter, L.; Palermo, J.A.; Carballo, M.A. Isolation of Acetylated Bile Acids from the Sponge Siphonochalina Fortis and DNA Damage Evaluation by the Comet Assay. Steroids 2013, 78, 982–986. [Google Scholar] [CrossRef]
- D’Ambrosio, M.; Ramos, Í.; Martins, C.; Costa, P.M. An Investigation into the Toxicity of Tissue Extracts from Two Distinct Marine Polychaeta. Toxicon X 2022, 14, 100116. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.-H.; Muttenthaler, M.; Dutertre, S.; Himaya, S.W.A.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Conotoxins: Chemistry and Biology. Chem. Rev. 2019, 119, 11510–11549. [Google Scholar] [CrossRef] [PubMed]
- Escoubas, P.; Diochot, S.; Corzo, G. Structure and Pharmacology of Spider Venom Neurotoxins. Biochimie 2000, 82, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Souza, T.G.; Melo, M.E.G.; Silva, J.M.; Lima, J.R.; Lira, A.F.A.; de Aguiar-Júnior, F.C.A.; Martins, R.D.; Jorge, R.J.B.; Chagas, C.A.; et al. Tityus Stigmurus Venom Causes Genetic Damage in Blood and Testicular Cells and Affects the Number and Morphology of Gametogenic Lineage Cells in Mice. Toxicon 2020, 185, 114–119. [Google Scholar] [CrossRef]
- Galvani, N.C.; Vilela, T.C.; Domingos, A.C.; Fagundes, M.Í.; Bosa, L.M.; Della Vechia, I.C.; Scussel, R.; Pereira, M.; Steiner, B.T.; Damiani, A.P.; et al. Genotoxicity Evaluation Induced by Tityus Serrulatus Scorpion Venom in Mice. Toxicon 2017, 140, 132–138. [Google Scholar] [CrossRef]
- Das Gupta, S.; Debnath, A.; Saha, A.; Giri, B.; Tripathi, G.; Vedasiromoni, J.R.; Gomes, A.; Gomes, A. Indian Black Scorpion (Heterometrus Bengalensis Koch) Venom Induced Antiproliferative and Apoptogenic Activity against Human Leukemic Cell Lines U937 and K562. Leuk. Res. 2007, 31, 817–825. [Google Scholar] [CrossRef]
- da Silva, M.S.; Lopes, P.H.; Elias, M.C.; Tambourgi, D. V Cytotoxic and Genotoxic Effects on Human Keratinocytes Triggered by Sphingomyelinase D from Loxosceles Venom. Arch. Toxicol. 2020, 94, 3563–3577. [Google Scholar] [CrossRef]
- Luo, L.; Kamau, P.M.; Lai, R. Bioactive Peptides and Proteins from Wasp Venoms. Biomolecules 2022, 12, 527. [Google Scholar] [CrossRef]
- Niidome, T.; Urakawa, M.; Takaji, K.; Matsuo, Y.; Ohmori, N.; Wada, A.; Hirayama, T.; Aoyagi, H. Influence of Lipophilic Groups in Cationic Alpha-Helical Peptides on Their Abilities to Bind with DNA and Deliver Genes into Cells. J. Pept. Res. 1999, 54, 361–367. [Google Scholar] [CrossRef]
- Wang, J.; Yan, Z.; Xiao, S.; Wang, B.; Fang, Q.; Schlenke, T.; Ye, G. Characterization of a Cell Death-Inducing Endonuclease-like Venom Protein from the Parasitoid Wasp Pteromalus Puparum (Hymenoptera: Pteromalidae). Pest Manag. Sci. 2021, 77, 224–233. [Google Scholar] [CrossRef]
- Khalil, S.R.; Abd-Elhakim, Y.M.; Selim, M.E.; Al-Ayadhi, L.Y. Apitoxin Protects Rat Pups Brain from Propionic Acid-Induced Oxidative Stress: The Expression Pattern of Bcl-2 and Caspase-3 Apoptotic Genes. Neurotoxicology 2015, 49, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Garaj-Vrhovac, V. Radioprotective Effects of Honeybee Venom (Apis Mellifera) Against 915-MHz Microwave Radiation-Induced DNA Damage in Wistar Rat Lymphocytes: In Vitro Study. Int. J. Toxicol. 2009, 28, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, M.M.; Marin-Morales, M.A. Anti-Genotoxicity and Anti-Mutagenicity of Apis Mellifera Venom. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 762, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hoshina, M.M.; Santos, L.D.; Palma, M.S.; Marin-Morales, M.A. Cytotoxic, Genotoxic/Antigenotoxic and Mutagenic/Antimutagenic Effects of the Venom of the Wasp Polybia Paulista. Toxicon 2013, 72, 64–70. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Zeng, Z.; Lin, C.; Lin, Y.; Cao, D.; Ma, W.; Xu, W.; Xiang, Q.; Luo, L.; et al. Trabectedin in Cancers: Mechanisms and Clinical Applications. Curr. Pharm. Des. 2022, 28, 1949–1965. [Google Scholar] [CrossRef]
- D’Incalci, M.; Galmarini, C.M. A Review of Trabectedin (ET-743): A Unique Mechanism of Action. Mol. Cancer Ther. 2010, 9, 2157–2163. [Google Scholar] [CrossRef]
- Feuerhahn, S.; Giraudon, C.; Martínez-Díez, M.; Bueren-Calabuig, J.A.; Galmarini, C.M.; Gago, F.; Egly, J.-M. XPF-Dependent DNA Breaks and RNA Polymerase II Arrest Induced by Antitumor DNA Interstrand Crosslinking-Mimetic Alkaloids. Chem. Biol. 2011, 18, 988–999. [Google Scholar] [CrossRef]
- Bueren-Calabuig, J.A.; Giraudon, C.; Galmarini, C.M.; Egly, J.M.; Gago, F. Temperature-Induced Melting of Double-Stranded DNA in the Absence and Presence of Covalently Bonded Antitumour Drugs: Insight from Molecular Dynamics Simulations. Nucleic Acids Res. 2011, 39, 8248–8257. [Google Scholar] [CrossRef]
- Mills, A.; Gago, F. Insight into the Sequence-Specific Elements Leading to Increased DNA Bending and Ligase-Mediated Circularization Propensity by Antitumor Trabectedin. J. Comput. Aided. Mol. Des. 2021, 35, 707–719. [Google Scholar] [CrossRef]
- Pellegrino, F.J.; Risso, A.; Corrada, Y.; Gambaro, R.C.; Seoane, A.I. Influence of Dietary Fish Oil Supplementation on DNA Damage in Peripheral Blood Lymphocytes of Nine Healthy Dogs. Vet. Rec. Open 2021, 8, e12. [Google Scholar] [CrossRef]
- Giri, B.; Gomes, A.; Debnath, A.; Saha, A.; Biswas, A.K.; Dasgupta, S.C.; Gomes, A. Antiproliferative, Cytotoxic and Apoptogenic Activity of Indian Toad (Bufo Melanostictus, Schneider) Skin Extract on U937 and K562 Cells. Toxicon 2006, 48, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019, 7, 218. [Google Scholar] [CrossRef]
- Moridikia, A.; Zargan, J.; Sobati, H.; Goodarzi, H.R.; Hajinourmohamadi, A. Anticancer and Antibacterial Effects of Iranian Viper (Vipera Latifii) Venom; an In-Vitro Study. J. Cell. Physiol. 2018, 233, 6790–6797. [Google Scholar] [CrossRef]
- Novak Zobiole, N.; Caon, T.; Wildgrube Bertol, J.; de Pereira, C.A.S.; Okubo, B.M.; Moreno, S.E.; Tramontini Gomes de Sousa Cardozo, F. In Vitro and in Vivo Genotoxic Evaluation of Bothrops Moojeni Snake Venom. Pharm. Biol. 2015, 53, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Marcussi, S.; Stábeli, R.G.; Santos-Filho, N.A.; Menaldo, D.L.; Silva Pereira, L.L.; Zuliani, J.P.; Calderon, L.A.; da Silva, S.L.; Antunes, L.M.G.; Soares, A.M. Genotoxic Effect of Bothrops Snake Venoms and Isolated Toxins on Human Lymphocyte DNA. Toxicon 2013, 65, 9–14. [Google Scholar] [CrossRef]
- Naves, M.P.C.; de Morais, C.R.; de Freitas, V.; Ribeiro, D.L.; Lopes, D.S.; Antunes, L.M.G.; de Melo Rodrigues, V.; de Rezende, A.A.A.; Spanó, M.A. Mutagenic and Genotoxic Activities of Phospholipase A2 Bothropstoxin-I from Bothrops Jararacussu in Drosophila Melanogaster and Human Cell Lines. Int. J. Biol. Macromol. 2021, 182, 1602–1610. [Google Scholar] [CrossRef]
- Cardoso Trento, M.V.; de Faria Eleutério, M.W.; Silva Abreu, T.; Andrade Machado, G.H.; Cesar, P.H.S.; Assaid Simão, A.; Marcussi, S. The Protective Effect Exerted by Ascorbic Acid on DNA Fragmentation of Human Leukocytes Induced by Lachesis Muta Muta Venom. J. Cell Biochem 2019, 120, 3520–3528. [Google Scholar] [CrossRef]
- Modahl, C.M.; Saviola, A.J.; Mackessy, S.P. Integration of Transcriptomic and Proteomic Approaches for Snake Venom Profiling. Expert Rev. Proteom. 2021, 18, 827–834. [Google Scholar] [CrossRef]
- Costa, M.T.; da Silva Goulart, A.; Salgueiro, A.C.F.; da Rosa, H.S.; Perazzo, G.X.; Folmer, V. Cytotoxicity and Inflammation Induced by Philodryas Patagoniensis Venom. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 257, 109356. [Google Scholar] [CrossRef]
- Abreu, T.S.; Braga, M.A.; Simão, A.A.; Trento, M.V.C.; de Eleutério, M.W.F.; Silva Pereira, L.L.; Cunha, E.F.F.; Marcussi, S. Mitochondriotropic Action and DNA Protection: Interactions between Phenolic Acids and Enzymes. J. Biochem. Mol. Toxicol. 2020, 34, e22417. [Google Scholar] [CrossRef]
- Pan, H.; Soman, N.R.; Schlesinger, P.H.; Lanza, G.M.; Wickline, S.A. Cytolytic Peptide Nanoparticles (‘NanoBees’) for Cancer Therapy. WIREs Nanomed. Nanobiotechnol. 2011, 3, 318–327. [Google Scholar] [CrossRef]
- Dabbagh Moghaddam, F.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Khaledi, L.; Reihani, A.H.; Javidfar, M.; Mortazavi, P. Delivery of Melittin-Loaded Niosomes for Breast Cancer Treatment: An in Vitro and in Vivo Evaluation of Anti-Cancer Effect. Cancer Nanotechnol. 2021, 12, 14. [Google Scholar] [CrossRef]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K.; Ho, D.; Johnstone, E.; Pfleger, K.; Redfern, A.; et al. Honeybee Venom and Melittin Suppress Growth Factor Receptor Activation in HER2-Enriched and Triple-Negative Breast Cancer. NPJ Precis. Oncol. 2020, 4, 24. [Google Scholar] [CrossRef]
- Choi, K.; Hwang, C.; Gu, S.; Park, M.; Kim, J.; Park, J.; Ahn, Y.; Kim, J.; Song, M.; Song, H.; et al. Cancer Cell Growth Inhibitory Effect of Bee Venom via Increase of Death Receptor 3 Expression and Inactivation of NF-Kappa B in NSCLC Cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef]
- de Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Robles-Loaiza, A.A.; Pinos-Tamayo, E.A.; Mendes, B.; Ortega-Pila, J.A.; Proaño-Bolaños, C.; Plisson, F.; Teixeira, C.; Gomes, P.; Almeida, J.R. Traditional and Computational Screening of Non-Toxic Peptides and Approaches to Improving Selectivity. Pharmaceuticals 2022, 15, 323. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Orozco, R.Q.; Rezende, S.B.; Rodrigues, G.; Oshiro, K.G.N.; Cândido, E.S.; Franco, O.L. Computer-Aided Design of Antimicrobial Peptides: Are We Generating Effective Drug Candidates? Front. Microbiol. 2020, 10, 3097. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.C.R.; Maasch, J.R.M.A.; de la Fuente-Nunez, C. Accelerating Antibiotic Discovery through Artificial Intelligence. Commun. Biol. 2021, 4, 1050. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Cao, J.; Franco, O.L.; Lu, T.K.; de la Fuente-Nunez, C. Synthetic Biology and Computer-Based Frameworks for Antimicrobial Peptide Discovery. ACS Nano 2021, 15, 2143–2164. [Google Scholar] [CrossRef] [PubMed]
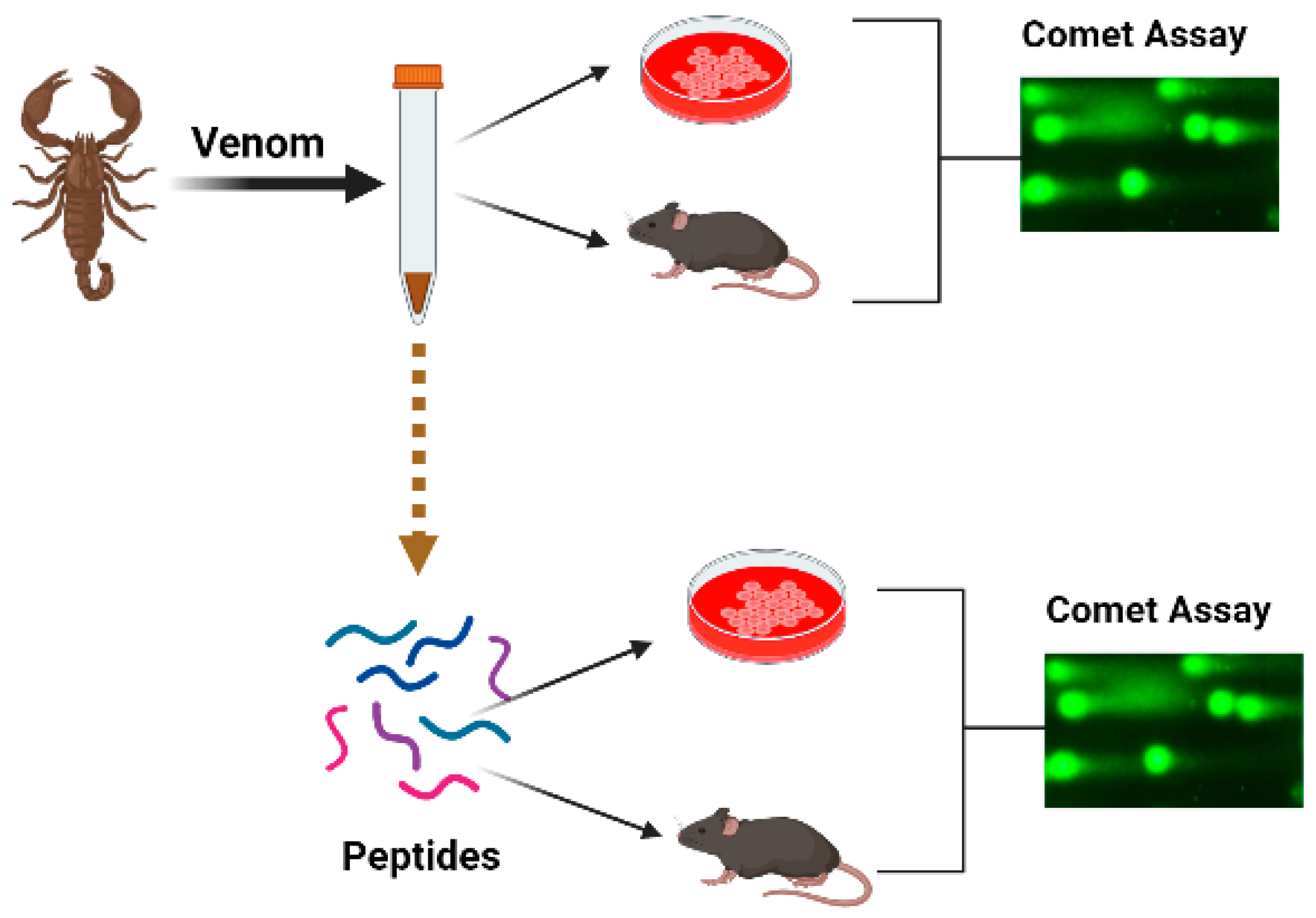
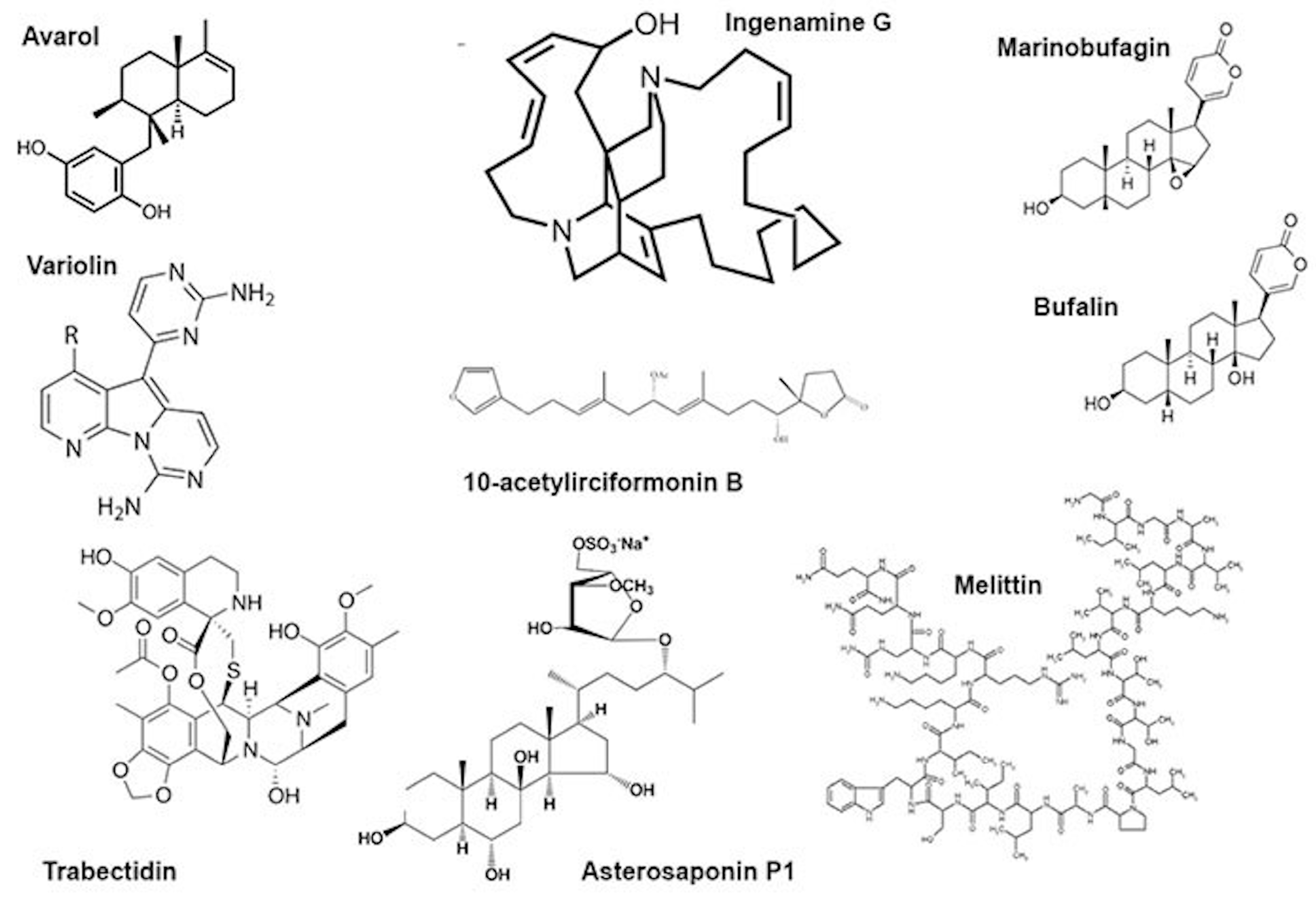

| Compound | Source | Genotoxic/Genoprotective Effect | Cell Types | Pharmacological Action | References |
|---|---|---|---|---|---|
| Avarol | Dysidea avara | Genotoxic | Human cancer (HT29) cell line and Friend leukaemia cells | Antitumour, antibacterial, antiviral | [30,31] |
| 10-Acetylirciformonin B | Ircinia sp. | Genotoxic | Human cancer (HT29) cell line | Antitumour | [32] |
| Ingenamine G | Pachychalina alcaloidifera | Genotoxic | Human lymphocytes | Antitumour | [33] |
| Variolin B | Kirckpatrickia variolosa | Genotoxic | Human cancer cells | Antitumour | [34] |
| ω-Conotoxin | Conus magus | Genoprotective | Primary cultures of rat forebrain neurons | Analgesic | [35] |
| Phα1β peptide | Phoneutria nigriventer | Genotoxic | Rat spinal cord cells | Analgesic | [36] |
| Mastoparans | Polibia paulista, Vespa sp. | Genotoxic | Cancer cell lines | Antitumour, antimicrobial, antiviral | [37] |
| Melectin | Melecta albifrons | Genotoxic | Cancer cell lines | Antimicrobial, antitumour | [38] |
| Melittin | Apis mellifera | Genotoxic | Human peripheral blood cells | Antitumour | [25,26,27,39,40,41] |
| Asterosaponin P1 | Patiria pectinifera | Genotoxic | Colorectal carcinoma cells HT-29 | Radiomimetic | [42] |
| Trabectidin | Ecteinascidia turbinata | Genotoxic | Human cancer cells | Antitumour | [43] |
| FWKVV and FMPLH | Miichthys miiuy | Genoprotectiuve | HUVEC cells | Antimutagenic | [44] |
| BMP1 | Bufo melanostictus | Genotoxic | Ehrlich ascites cells | Antitumour | [45] |
| Marinobufagin | Bufo rubescens Bufo marinus | Non-genotoxic | Human cancer cells | Antitumour | [46] |
| Bufalin | Bufo gargarizans | Non-genotoxic | Human cancer cells | Antitumour | [47] |
| Vipoxin | Vipera ammodytes meridionalis | Genotoxic | Human hepatocellular carcinoma (HepG2) | Neurotoxic | [48] |
| BjussuLAAO-II | Bothrops jararacussu | Genotoxic | HepG2 HUVEC Caco-2 cells | Antitumour | [49,50] |
| Captopril | Bothrops jararaca | Genotoxic | Human lymphocytes Human macrophages | Antihypertensive | [51] |
| Crotoxin | Crotalus durissus terrificus | Genotoxic | Human lymphocytes | Immunomodulatory, anti-inflammatory, anti-microbial, antitumour and analgesic | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sjakste, N.; Gajski, G. A Review on Genotoxic and Genoprotective Effects of Biologically Active Compounds of Animal Origin. Toxins 2023, 15, 165. https://doi.org/10.3390/toxins15020165
Sjakste N, Gajski G. A Review on Genotoxic and Genoprotective Effects of Biologically Active Compounds of Animal Origin. Toxins. 2023; 15(2):165. https://doi.org/10.3390/toxins15020165
Chicago/Turabian StyleSjakste, Nikolajs, and Goran Gajski. 2023. "A Review on Genotoxic and Genoprotective Effects of Biologically Active Compounds of Animal Origin" Toxins 15, no. 2: 165. https://doi.org/10.3390/toxins15020165
APA StyleSjakste, N., & Gajski, G. (2023). A Review on Genotoxic and Genoprotective Effects of Biologically Active Compounds of Animal Origin. Toxins, 15(2), 165. https://doi.org/10.3390/toxins15020165






