Localization of Multiple Jellyfish Toxins Shows Specificity for Functionally Distinct Polyps and Nematocyst Types in a Colonial Hydrozoan
Abstract
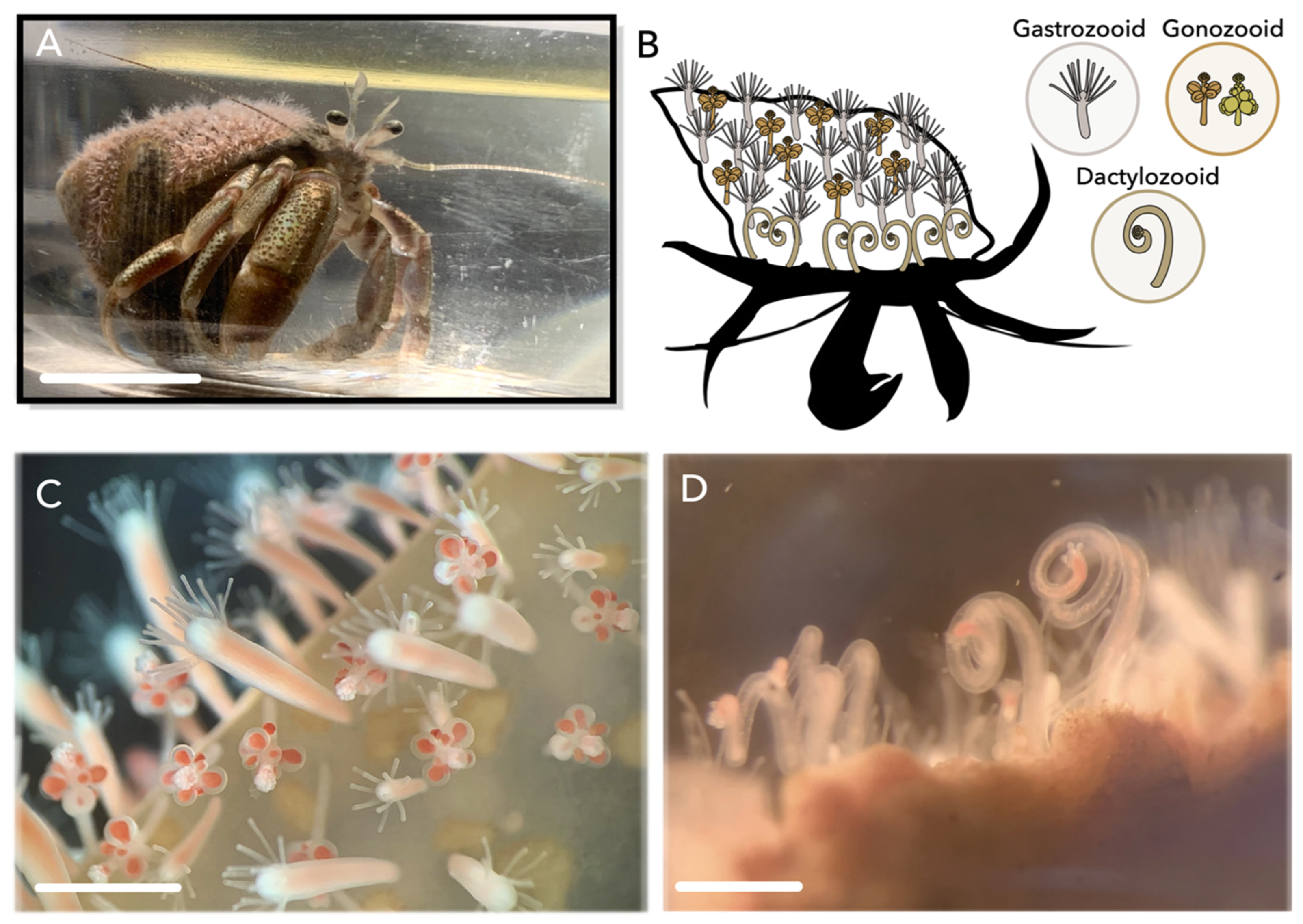
1. Introduction
2. Results
2.1. JFT Expression in Single-Cell RNA-Seq Clusters
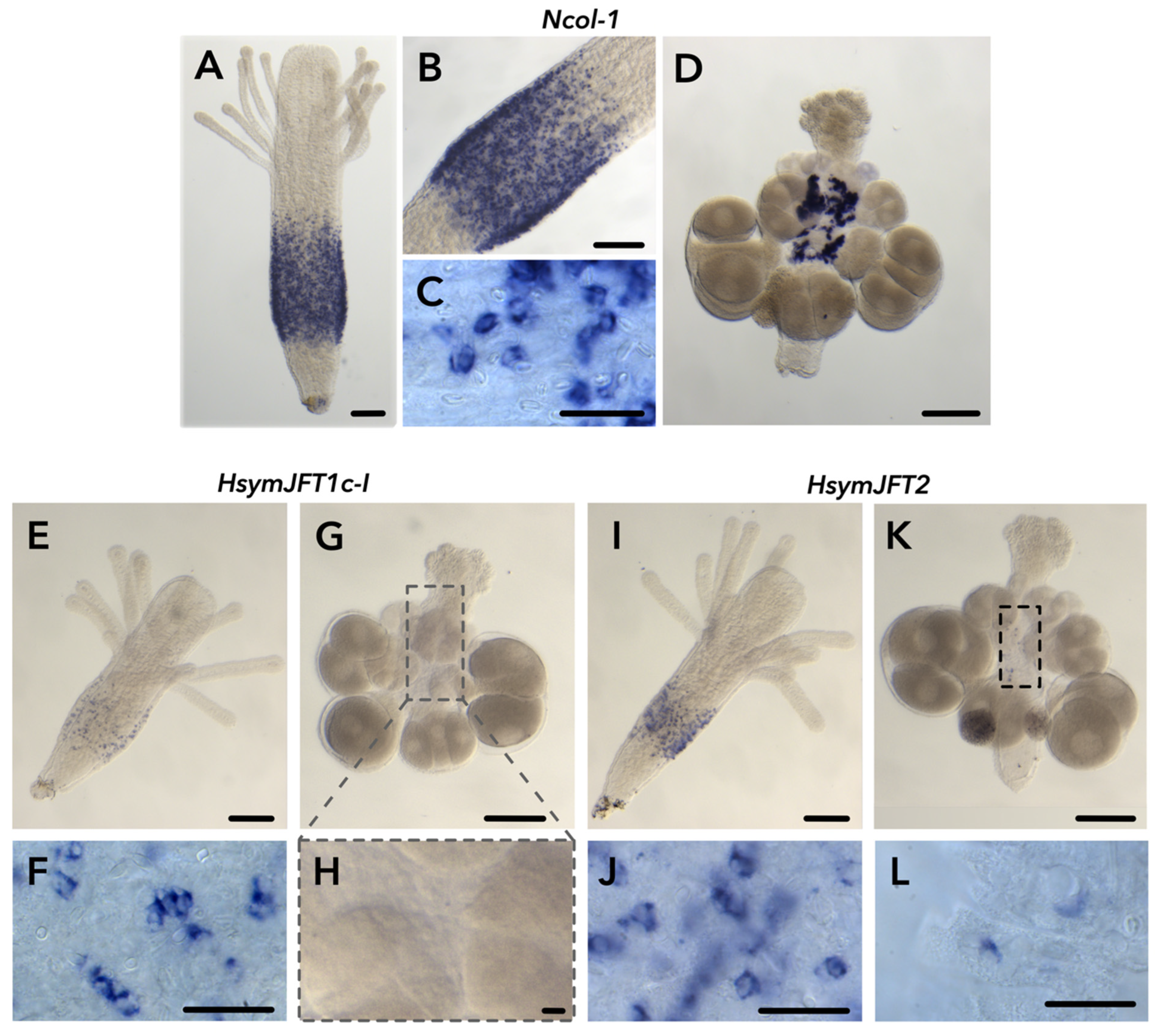
2.2. JFT Expression Is Consistent with Regions of Nematogenesis in Gastrozooids and Gonozooids
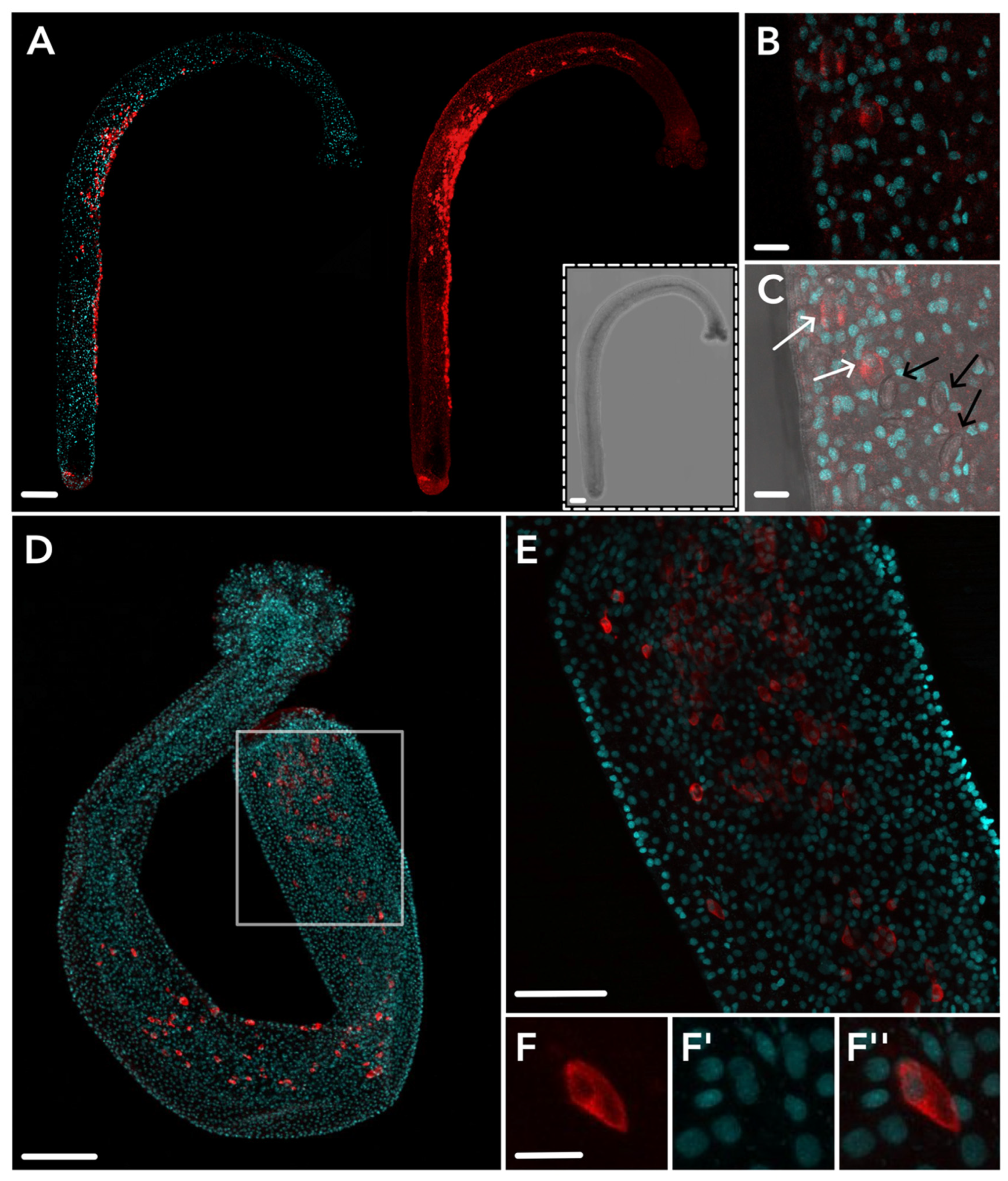
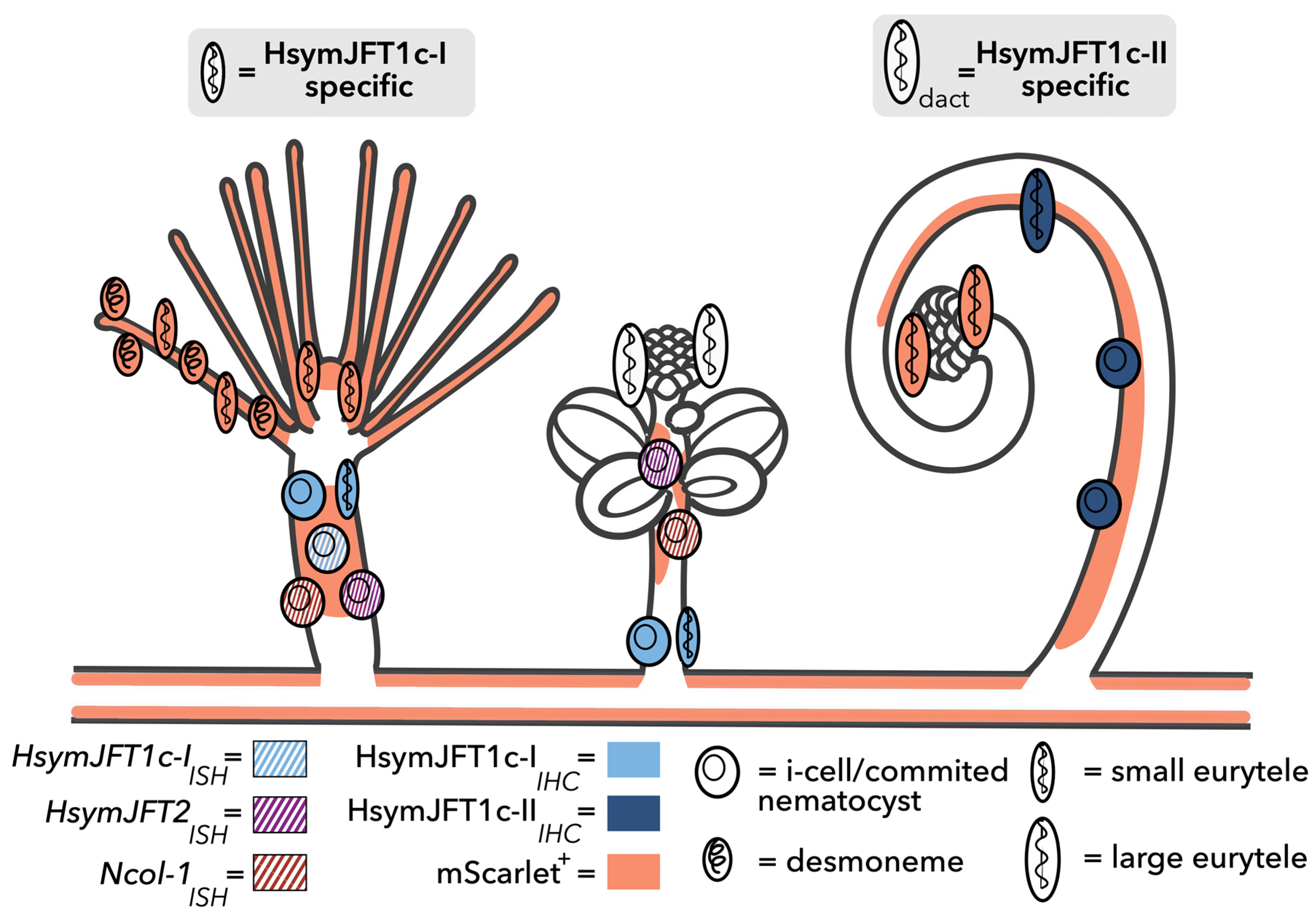
2.3. HsymJFT1c-I and HsymJFT1c-II Toxins Putatively Restricted to Specific Nematocyst Types
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Genomic JFT Identification and Evaluation of Single-Cell Expression
5.2. Animal Care
5.3. Tissue Fixation
5.4. cDNA Synthesis and in situ Hybridization (ISH)
5.5. Antibody Design, Immunohistochemistry, and Imaging
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariscal, R.N. Nematocysts. In Coelenterate Biology: Reviews and New Perspectives; Muscatine, L., Lenhoff, H.M., Eds.; Academic Press Inc.: New York, NY, USA, 1974; pp. 129–178. [Google Scholar]
- Daly, M.; Brugler, M.R.; Cartwright, P.; Collins, A.G.; Dawson, M.N.; Fautin, D.G.; France, S.C.; Mcfadden, C.S.; Opresko, D.M.; Rodriguez, E.; et al. The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. In Linnaeus Tercentenary: Progress in Invertebrate Taxonomy; Zhang, Z.-G., Shear, W.A., Eds.; Magnolia Press: Waco, TX, USA, 2007; Volume 1668, pp. 127–182. 01668p. [Google Scholar]
- Lotan, A.; Fishman, L.; Loya, Y.; Zlotkin, E. Delivery of a nematocyst toxin. Nature 1995, 375, 456. [Google Scholar] [CrossRef]
- Lotan, A.; Fishman, L.; Zlotkin, E. Toxin compartmentation and delivery in the Cnidaria: The nematocyst’s tubule as a multiheaded poisonous arrow. J. Exp. Zool. 1996, 275, 444–451. [Google Scholar] [CrossRef]
- Beckmann, A.; Özbek, S. The nematocyst: A molecular map of the cnidarian stinging organelle. Int. J. Dev. Biol. 2012, 56, 577–582. [Google Scholar] [CrossRef]
- Weill, R. Contribution a l’étude des cnidaires et de leurs nématocystes. I. Recherches sur les nématocystes. Travaux de la Station Zoologique de Wimereux. 1934, 10, 1–347.
- Weill, R. Contribution a l’étude des cnidaires et de leurs nématocystes. II. Valeur taxonomique du cnidome. Travaux de la Station Zoologique de Wimereux. 1934, 11, 348–700.
- Östman, C. A guideline to nematocyst nomenclature and classification, and some notes on the systematic value of nematocysts. Sci. Mar. 2000, 64, 31–46. [Google Scholar] [CrossRef]
- Fautin, D.G. Importance of nematocysts to actinian taxonomy. In The Biology of Nematocysts; Hessinger, D.A., Lenhoff, H.M., Eds.; Academic Press Inc.: London, UK, 1998; pp. 487–500. [Google Scholar]
- Fautin, D.G. Structural diversity, systematics, and evolution of cnidae. Toxicon 2009, 54, 1054–1064. [Google Scholar] [CrossRef]
- Hessinger, D.A. Nematocysts venoms and toxins. In The Biology of Nematocysts; Hessinger, D.A., Lenhoff, H.M., Eds.; Academic Press Inc.: London, UK, 1998; pp. 333–369. [Google Scholar]
- Doonan, L.B.; Lynham, S.; Quinlan, C.; Ibiji, S.C.; Winter, C.E.; Padilla, G.; Jaimes-Becerra, A.; Morandini, A.C.; Marques, A.C.; Long, P.F. Venom composition does not vary greatly between different nematocyst types isolated from the primary tentacles of Olindias sambaquiensis (Cnidaria: Hydrozoa). Biol. Bull. 2019, 237, 26–35. [Google Scholar] [CrossRef]
- Ashwood, L.M.; Norton, R.S.; Undheim, E.A.B.; Hurwood, D.A.; Prentis, P.J. Characterising functional venom profiles of anthozoans and medusozoans within their ecological context. Mar. Drugs 2020, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Ashwood, L.M.; Undheim, E.A.B.; Madio, B.; Hamilton, B.R.; Daly, M.; Hurwood, D.A.; King, G.K.; Prentis, P.J. Venoms for all occasions: The functional toxin profiles of different anatomical regions in sea anemones are related to their ecological function. Mol. Ecol. 2020, 31, 866–883. [Google Scholar] [CrossRef]
- Skaer, R.J. The secretion and development of nematocysts in a siphonophore. J. Cell Sci. 1973, 13, 371–393. [Google Scholar] [CrossRef]
- David, C.N.; Challoner, D. Distribution of interstitial cells and differentiating nematocytes in nests in Hydra attenuata. Am. Zool. 1974, 14, 537–542. [Google Scholar] [CrossRef]
- Holstein, T. The morphogenesis of nematocytes in Hydra and Forsklia: An ultrastructural study. J. Ultrastruct. Res. 1981, 75, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Kass-Simon, G.S.; Scappaticci, A.A., Jr. The behavioral and developmental physiology of nematocysts. Can. J. Zool. 2002, 80, 1772–1794. [Google Scholar] [CrossRef]
- Slautterback, D.B.; Fawcett, D.W. The development of the cnidoblasts of Hydra: An electron microscope study of cell differentiation. J. Cell Biol. 1959, 5, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Denker, E.; Manuel, M.; Leclère, L.; Le Guyader, H.; Rabet, N. Ordered progression of nematogenesis from stem cells through differentiation stages in the tentacle bulb of Clytia hemisphaerica (Hydrozoa, Cnidaria). Dev. Biol. 2008, 315, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Sunagar, K.; Columbus-Shenkar, Y.Y.; Fridrich, A.; Gutkovich, N.; Aharoni, R.; Moran, Y. Cell type-specific expression profiling unravels the development and evolution of stinging cells in sea anemone. BMC Biol. 2018, 16, 108. [Google Scholar] [CrossRef]
- Braverman, M.H. Differentiation and commensalism in Podocoryne carnea. Am. Midl. Nat. 1960, 63, 223–225. [Google Scholar] [CrossRef]
- Burnett, A.L.; Sindelar, W.; Diehl, N. An examination of polymorphism in the hydroid Hydractinia echinata. J. Mar. Biolog. Assoc. UK 1967, 47, 645–658. [Google Scholar] [CrossRef]
- Damiani, C.C. Reproductive costs of the symbiotic hydroid Hydractinia symbiolongicarpus (Buss and Yund) to its host hermit crab Pagurus longicarpus (Say). J. Exp. Mar. Biol. Ecol. 2003, 288, 203–222. [Google Scholar] [CrossRef]
- Schijfsma, K. Observations on Hydractinia echinata (Flem.) and Eupagurus bernhardus (L.). Arch. Néerlandaises Zool. 1935, 1, 261–314. [Google Scholar] [CrossRef]
- Klompen, A.M.L.; Sanders, S.M.; Cartwright, P. Venom system variation and the division of labor in the colonial hydrozoan Hydractinia symbiolongicarpus. Toxicon X 2022, 14, 100113. [Google Scholar] [CrossRef]
- Nagai, H. Recent progress in jellyfish toxin study. J. Health Sci. 2003, 49, 337–340. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Burnell, J.N. Biochemical and molecular characterisation of cubozoan protein toxins. Toxicon 2009, 54, 1162–1173. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Aziz, A.; Loukas, A.; Potriquet, J.; Seymour, J.; Mulvenna, J. Venom proteome of the box jellyfish Chironex fleckeri. PLoS ONE 2012, 7, e47866. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, D.L.; Konstantakopoulos, N.; McInerney, B.V.; Mulvenna, J.; Seymour, J.; Isbister, G.K.; Hodgson, W.C. Chironex fleckeri (box jellyfish) venom proteins: Expansion of a cnidarian toxin family that elicits variable cytolytic and cardiovascular effects. J. Biol. Chem. 2014, 289, 4798–4812. [Google Scholar] [CrossRef]
- Brinkman, D.L.; Jia, X.; Potriquet, J.; Kumar, D.; Dash, D.; Kvaskoff, D.; Mulvenna, J. Transcriptome and venom proteome of the box jellyfish Chironex fleckeri. BMC Genom. 2015, 16, 407. [Google Scholar] [CrossRef] [PubMed]
- Klompen, A.M.L.; Kayal, E.; Collins, A.G.; Cartwright, P. Phylogenetic and selection analysis of an expanded family of putatively pore-forming jellyfish toxins (Cnidaria: Medusozoa). Genome Biol. Evol. 2021, 13, evab081. [Google Scholar] [CrossRef]
- Hydractinia Genome Project Portal. Available online: https://research.nhgri.nih.gov/hydractinia/ (accessed on September 2022).
- Zenkert, C.; Takahashi, T.; Diesner, M.-O.; Özbek, S. Morphological and molecular analysis of the Nematostella vectensis cnidom. PLoS ONE 2011, 6, e22725. [Google Scholar] [CrossRef] [PubMed]
- Babonis, L.S.; Martindale, M.Q.; Ryan, J.F. Do novel genes drive morphological novelty? An investigation of the nematosomes in the sea anemone Nematostella vectensis. BMC Evol. Biol. 2016, 16, 114. [Google Scholar] [CrossRef]
- Kanska, J.U.; Frank, U. New roles for Nanos in neural cell fate determination revealed by studies in a cnidarian. J. Cell Sci. 2013, 126, 3192–3203. [Google Scholar] [CrossRef]
- Bradshaw, B.; Thompson, K.; Frank, U. Distinct mechanisms underlie oral vs aboral regeneration in the cnidarian Hydractinia echinata. Elife 2015, 4, e05506. [Google Scholar] [CrossRef]
- Buss, L.W.; Yund, P.O. A sibling species group of Hydractinia in the north-eastern United States. J. Mar. Biolog. Assoc. UK 1989, 69, 857–874. [Google Scholar] [CrossRef]
- Lange, R.; Plickert, G.; Müller, W.A. Histoincompatibility in a low invertebrate, Hydractinia echinata: Analysis of the mechanism of rejection. J. Exp. Zool. 1989, 249, 284–292. [Google Scholar] [CrossRef]
- Inkscape Project, Version 1.2.1. Available online: https://inkscape.org (accessed on September 2022).
- Sanders, S.M.; Shcheglovitova, M.; Cartwright, P. Differential gene expression between functionally specialized polyps of the colonial hydrozoan Hydractinia symbiolongicarpus (Phylum Cnidaria). BMC Genom. 2014, 15, 406. [Google Scholar] [CrossRef]
- Nawrocki, A.M.; Cartwright, P. A novel mode of colony formation in a hydrozoan through fusion of sexually generated individuals. Curr. Biol. 2012, 22, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, A.M.; Cartwright, P. Expression of Wnt pathway genes in polyps and medusa-like structures of Ectopleura larynx (Cnidaria: Hydrozoa). Evol. Dev. 2013, 15, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, M.; Leitz, T.; Schloßherr, J.; Plickert, G. LWamides from Cnidaria constitute a novel family of neuropeptides with morphogenetic activity. Rouxs Arch. Dev.Biol. 1996, 205, 232–242. [Google Scholar] [CrossRef]
- HeliconFocus Lite, version 8.2.0. Available online: https://www.heliconsoft.com/ (accessed on September 2022).
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological- image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J.; Johansen, A.R.; Gíslason, M.H.; Pihl, A.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]

| Toxin Name (This Study) | Gene ID | Cond. Mean, GAST | Cond. Mean, GONO | Cond. Mean, DACT | scRNA-Seq GAST Dataset ** | In Situ Hybridization | Protein Localization | ||
|---|---|---|---|---|---|---|---|---|---|
| Polyp | Location | Polyp | Location | ||||||
| HsymJFT1c-I | HyS0055.81 | 359.01+ | 33.00 | 68.83 | High relative expression; co-localized with Ncol-1, cluster 1 | GAST | Mid-body column | GAST GONO | Mid-body column Proximal region of the body column |
| HsymJFT1c-II | HyS0015.224 | 102.44 | 26.65 | 1486.59+ | Low relative expression; co-localized with Ncol-1, cluster 2 | - | - | DACT | Body column; concave region of spiral |
| HsymJFT2a | HyS0028.121 | 227.20 | 16.03 | 21.93 | Medium relative expression; co-localized with Ncol-1, cluster 2 | No signal | - | - | |
| HsymJFT2 | HyS0049.112 | 2245.62 | 522.17 | 161.37 | High relative expression; co-localized with Ncol-1, clusters 1 and 2 | GAST GONO | Mid-body column Mid-body column | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klompen, A.M.L.; Travert, M.K.; Cartwright, P. Localization of Multiple Jellyfish Toxins Shows Specificity for Functionally Distinct Polyps and Nematocyst Types in a Colonial Hydrozoan. Toxins 2023, 15, 149. https://doi.org/10.3390/toxins15020149
Klompen AML, Travert MK, Cartwright P. Localization of Multiple Jellyfish Toxins Shows Specificity for Functionally Distinct Polyps and Nematocyst Types in a Colonial Hydrozoan. Toxins. 2023; 15(2):149. https://doi.org/10.3390/toxins15020149
Chicago/Turabian StyleKlompen, Anna M. L., Matthew K. Travert, and Paulyn Cartwright. 2023. "Localization of Multiple Jellyfish Toxins Shows Specificity for Functionally Distinct Polyps and Nematocyst Types in a Colonial Hydrozoan" Toxins 15, no. 2: 149. https://doi.org/10.3390/toxins15020149
APA StyleKlompen, A. M. L., Travert, M. K., & Cartwright, P. (2023). Localization of Multiple Jellyfish Toxins Shows Specificity for Functionally Distinct Polyps and Nematocyst Types in a Colonial Hydrozoan. Toxins, 15(2), 149. https://doi.org/10.3390/toxins15020149





