Abstract
Nemerteans (also called Nemertines) are a phylum of predominantly marine worms that use toxins to capture prey and to defend themselves against predators. Hoplonemerteans have a proboscis armed with one or more stylets used in prey capture and are taxonomically divided into Order Monostilifera, whose members possess a single large proboscis stylet, and Order Polystilifera, whose members have multiple small stylets. Many monostiliferans contain alkaloidal toxins, including anabaseine, that stimulate and then desensitize nicotinic acetylcholine receptors that are present in all animals. These compounds also interact with pyridyl chemoreceptors in crustaceans, reducing predation and larval settlement. Anabaseine has been a lead compound in the design of alpha7 nicotinic acetylcholine receptor agonists like GTS-21 (also called DMXBA) to treat disorders of cognition such as Alzheimer’s disease and schizophrenia. These drug candidates also display anti-inflammatory activities of potential medical importance. Most polystiliferans live deep in open oceans and are relatively inaccessible. We fortunately obtained two live specimens of a large benthic polystiliferan, Paradrepanophorus crassus (Pc), from the coast of Spain. MS and NMR analyses of the Ehrlich’s reagent derivative allowed identification of anabaseine. A spectrophotometric assay for anabaseine, also based on its reaction with Ehrlich’s reagent, revealed high concentrations of anabaseine in the body and proboscis. Apparently, the biosynthetic mechanism for producing anabaseine was acquired early in the evolution of the Hoplonemertea, before the monostiliferan-polystiliferan divergence.
Keywords:
anabaseine; anabasine; ehrlich reagent; nemertean; nemertine; nicotinic acetylcholine receptor; toxin; venom Key Contribution:
The discovery of the nicotinic receptor agonist anabaseine in a polystiliferan nemertean suggests that the biosynthetic mechanism for this alkaloid originated before the poly- and monostiliferan branches of the class Hoplonemertea diverged in the course of their evolution.
1. Introduction
Based on the structure of the proboscis, it was surmised that hoplonemertean (also called hoplonemertine) worms use venom to capture their prey [1,2]. The presence of toxic substances in nemerteans was discovered in the 1930s. The Belgian pharmacologist Bacq found that crude extracts of Amphiporus lactifloreus, a relatively common hoplonemertean along north Atlantic coasts, stimulated neuromuscular and autonomic ganglionic synapses like acetylcholine, but were not inactivated by high alkaline pH [3,4]. An attempt to isolate the active compound, named “amphiporine,” by solvent extraction and picric acid crystallization methods widely used in isolating plant alkaloids was only partially successful due to the availability of only a small number of worms and to difficulties in obtaining a crystalline salt suitable for structure analysis. Nevertheless, King [5] demonstrated that “amphiporine” behaved like a weakly basic alkaloid. Thirty years later a compound with nicotinic actions on vertebrate skeletal muscle was isolated from the wandering nemertean, Paranemertes peregrina (Pp), which is widely distributed along north Pacific coastal waters of Asia and America. Anabaseine (Figure 1) was isolated as a free base using aluminum oxide thin layer, preparative layer and ultimately column chromatography [6]. A chemical assay based on Ehrlich’s reagent (p-dimethylaminobenzaldehyde, Figure 1) facilitated the initial purification and identification of anabaseine [6] and also its detection in other nemerteans [7,8]. Anabasine, a tree tobacco alkaloid, resembles anabaseine but lacks its imine bond. Spath and Mamoli, tobacco alkaloid chemists at the University of Vienna, first prepared anabaseine as an intermediate in their synthesis of anabasine [9]. Using the Ehrlich and other thin-layer chromatographic spot reagents, it was found that a number of hoplonemertean species possess anabaseine, anabasine [7,8] and/or related pyridyl compounds like 2,3′-bipyridyl [10], nemertelline [10], 3-methyl-2,3′-bipyridyl [11], and isoanatabine [12,13], suggesting that this family of natural products appeared early in monostyliferan evolution. Fortunately, the Ehrlich reagent based spectrophotometric assay for anabaseine has allowed its presence to be detected in even small nemerteans, as most species are small and difficult to collect in sufficient numbers for chemical (especially NMR) analyses.
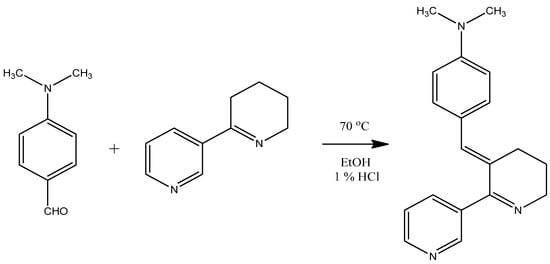
Figure 1.
Reaction of Ehrlich’s reagent (p-dimethylaminobenzaldehyde) with anabaseine. The product, DMAB-anabaseine, at 3.0 µg/mL has a high visible light absorbance of 1.00 at 490 nm in ethanol containing 1% concentrated HCl, permitting detection of very small amounts of anabaseine in tissue extracts and on thin layer chromatograms (adapted from [7]).
Current understanding of nemertean phylogeny is incomplete compared with many animal phyla, in spite of systematic investigations by a number of expert systematists. Within the hoplonemertea Order Polystilifera has been less studied because most species live deep in the open ocean and are difficult to collect. Because they are considered a monophyletic group [14,15,16,17], i.e., they evolved from a common ancestor, it is of great interest to identify their toxins to understand how the hoplonemertean proboscis and its venom-producing and -injecting system evolved over time. Currently one can only speculate that the first stylets were small and relatively simple and that this primitive weapon was largely retained by polystiliferans which, instead of developing large single stylets as found in monostiliferans, developed a basis containing many small stylets (Figure 2).
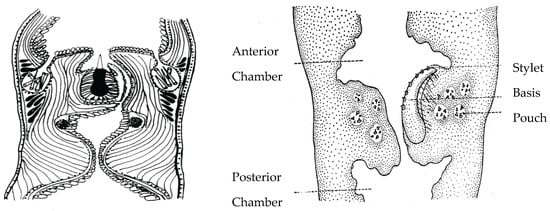
Figure 2.
Hoplonemertean stylets. Left: Stylet apparatus of a monostiliferan hoplonemertean. Right: Stylet apparatus of a pelagic polystiliferan hoplonemertean. Top of each median proboscis shows anterior proboscis chamber, bottom shows posterior proboscis chamber. The polystiliferan stylet basis containing multiple small stylets is probably used to abrade or tear the prey’s integument, facilitating venom entry. Accessory stylets are synthesized and stored in nearby mineralizing gland pouches in both probosces.
This paper is the first report of the existence of anabaseine in a species belonging to Order Polystilifera. Over 100 polystiliferan hoplonemertean species have been described by taxonomists [14,15,16,17]. Most are fragile, bathypelagic animals living at great depths in the oceans of the world and are known from just a few specimens, often mutilated during their collection with conventional nets or dredges. However, there also are a small number (~30 species) of benthic polystiliferans that live in shallow coastal waters at the lowest part of the intertidal zone or in adjacent shallow waters. Paradrepanophorus crassus (abbreviated Pc), the subject of this paper, is one of these benthic species. Pc has been reported to occur on British, Irish and Spanish Atlantic coasts as well as in the Mediterranean Sea [18,19,20]. While the presence of numerous tiny stylets in the proboscis of polystiliferans has been noted, the only previous report of a toxin in this group was Bacq’s observation that an aqueous extract of this species displayed “amphiporine” activities, stimulating skeletal muscle and autonomic synapses [3,4]. Here, we report the presence of high concentrations of anabaseine in the body and proboscis of this species (Figure 3).
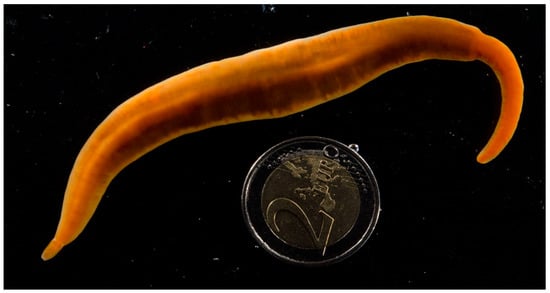
Figure 3.
Color photograph of the living Paradrepanophorus crassus specimen used in this study. The head is at the bottom left side of the photograph. While spontaneously gliding in a dish of sea water it displayed a maximum length of approximately 25 cm and a maximum width of 1.5 cm. The specimen had a regenerated tail (top right) as revealed by the sudden change in its body width. A two Euro coin (26 mm diameter) was included to provide a scale.
2. Results
2.1. Anabaseine Identification
A silica gel gradient HPLC separation of a portion of the basic methylene chloride phase is shown in Figure 4. Two major peaks eluting at 13 and 23 min were observed. The absorbance spectrum of the second peak contained two major peaks, at 230 and 260 nm with the absorbance of the 230 nm peak being ~1.5× larger than the 260 nm peak, similar to the spectrum for unionized anabaseine injected into the same column. The presumed anabaseine peak was collected, evaporated to dryness in a rotary evaporator and subjected to reverse phase C18 LC-Electrospray Ionization MS. Like synthetic anabaseine, the isolated compound produced LC-ESI-MS m/z 161 and m/z 179 ions which eluted simultaneously with the LC-UV absorbance peak, whose retention time was typical for anabaseine; the 161 peak corresponded to the cyclic iminium form of anabaseine and the 179 peak to the open-chain ammonium-ketone form of anabaseine [21,22].
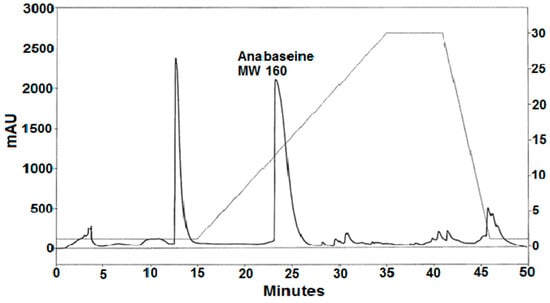
Figure 4.
Silica gel HPLC separation of a crude methylene chloride extract of alkaloids from the Pc worm. A linear gradient of an increasing concentration (2 to 30%, shown in right y-axis) of isopropanol in hexane-0.2% triethylamine was used with a Beckmann silica gel analytical column at a flow rate of 1.5 mL/min. Absorbance at 260 nm was measured with a diode array detector.
If they were present, two other known hoplonemertean alkaloids (2,3′-bipyridyl [10] and 3-methyl-2,3′-bipyridyl [11]), being less basic and polar, would have eluted earlier than anabaseine, and three other more polar nemertean alkaloids (isoanatabine [12], anabasine [8] and nemertelline [10]) would have eluted after anabaseine. However, re-HPLC of the 13 min and post-15 min peak components shown in Figure 4 failed to reveal these compounds. Smaller amounts of other pyridyl compounds might be found in future studies when more worms become available.
Most of the crude alkaline methylene chloride phase alkaloid sample was reacted with Ehrlich’s reagent under acidic conditions and subsequently purified on the same silica gel analytical HPLC column used to obtain the putative anabaseine component. The DMAB-derivative (Figure 5) eluted at a higher isopropanol concentration due to its higher polarity; synthetic DMAB-anabaseine eluted with an almost identical retention time (data not shown). A high resolution ESI-MS analysis of the DMAB-natural product resulted in an m/z 292.1811 [M+H]+ ion which was in good agreement (0.96 ppm) with the theoretical m/z 292.1808 [M+H]+ ion of the neutral elemental composition (C19H21N3), consistent with the structure of DMAB-anabaseine shown in Figure 1.
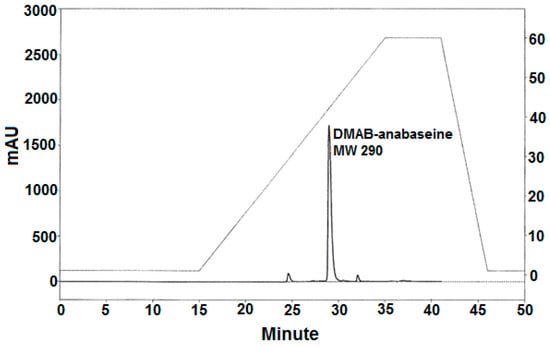
Figure 5.
Normal phase HPLC separation of the Ehrlich reaction products from the crude ethanolic Paradrepanophorus crassus body extract. The same silica gel column was used as in the normal phase HPLC separation shown in Figure 4, but a steeper gradient (%B, shown by dashed line and right y-axis dimensions) of increasing proportion of isopropanol-0.1% triethylamine in hexane-0.1% triethylamine was necessary to elute the putative 3-(4-dimethylaminobenzylidene)-anabaseine which occurred at 29 min.
The proton data (Table 1) extracted from a 300 MHz proton NMR spectrum of the HPLC-purified DMAB-derivative free base were essentially identical with the 600 MHz spectral data from the synthesized DMAB-anabaseine free base, completing the structure identification of the natural product.

Table 1.
Assignments of the hydrogen resonances in the proton NMR spectrum of the Pc natural product (NP) DMAB-derivative free base. Corresponding data for the synthetic (SYN) DMAB-anabaseine free base is included for comparison. Both spectra were recorded in CDCl3. The atoms in the heterocyclic rings are, starting with the N atom: 1’–6’ in the pyridyl ring, 1–6 in the tetrahydropyridyl ring. Numbering of the hydrogens in the dimethylaminobenzylidene moiety is as follows: hydrogen 7 is the connecting methine group, 9 and 12 are hydrogens on the two ortho-carbons and 10 and 14 are hydrogens attached to the meta-carbons.
2.2. Anabaseine Concentrations in Pc Tissues
Anabaseine concentrations within the largest Pc worm are summarized in Table 2. Previously published results [7] for Pp obtained using the same procedure are also shown to facilitate comparisons between the two species. In both species most of the anabaseine was found in the body, suggesting that it is used as a chemical defense against potential predators. Unlike the Pp proboscis, which contained 27% of the total worm anabaseine, the anterior proboscis of Pc contained only 2% of the total anabaseine, and its anabaseine tissue concentration was approximately 50× less than in the Pp anterior proboscis. It is interesting that the Pc anterior and posterior proboscis concentrations are similar. In contrast, the Pp anterior proboscis has much higher anabaseine concentrations than the body proper or the posterior proboscis [7]. In early scientific speculations, before any toxins were discovered, it was assumed that a hoplonemertean’s venom would be stored in the lumen of the posterior proboscis so that it could be ejected more readily at sites of stylet puncture of the prey organism [1,2]. The differences between the Pc and Pp anabaseine distributions, if real, might relate to different methods of prey capture. Pp wraps its anterior proboscis around its annelid prey whereas Pc may be a suctorial predator of crustaceans, like the monostiliferous chevron hoplonemertean Amphiporus angulatus [10,11,12,13]. Suctorial species probably rely on rupturing the crustacean exoskeleton to allow toxin penetration. Clearly further analyses of proboscis alkaloid concentrations in different parts of hoplonemertean probosces are needed to understand the mechanics of toxin synthesis and envenomation and how it may vary with the species.

Table 2.
Anabaseine (Ae) distribution in the large Paradrepanophoros crassus specimen. Data obtained from the single dissected Pc specimen are compared with previously published estimates [7] for Paranemertes peregrina (Pp).
Our Pc anabaseine estimates, based on a single specimen, are less reliable than the Pp estimates, which are mean values based on three separate experiments, each with ten adult worms. Additionally, the Pc anabaseine estimates may be on the low side since there was a significant period of time (one month) between the initial preservation of the Pc parts and the anabaseine determinations. Additionally, the tissues were preserved and then mailed in pure ethanol rather than a more stabilizing mixture like ethanol containing 1% glacial acetic acid where pH < 3. Anabaseine is not very stable in the intermediate (3–9) pH range [21,22,23] where both unionized and ionized iminium as well as open chain forms co-exist, due to its tendency to polymerize and possible form Schiff base adducts with other molecules [23]. As more specimens of Pc become available it will be possible to extend the current observations and search for minor pyridine alkaloid constituents that were not detected here.
3. Discussion
The body concentration of anabaseine in the large Pc specimen was one of the highest concentrations observed to date, but less than half the concentration in Pp [7,8]. A second Pc specimen of 0.4 g fresh weight was also collected. The total anabaseine concentration of this undissected worm was found to be 720 ug/g using the DMAB assay. It is possible that the lower concentrations of anabaseine in Pc relative to Pp was partly due to the lower body surface area: weight ratio of these large worms. It was previously shown that nemertean body toxins are localized in their integument [7,8,24]. The maximum length of the large Pc specimen was approximately 25 cm and its total fresh weight was 2.90 g. Assuming that the concentration of anabaseine is the same in the integumentary tissues of Pc as in Pp, and assuming a cylindrical form for both species, one calculates a surface area: fresh weight ratio of only 12 cm2/g for Pc, compared with a 36 cm2/g ratio for the Pp worms which averaged 15 cm length, 3 mm diameter and 0.4 g fresh weight. Thus, one would expect a 2–3 fold lower body anabaseine concentration in Pc relative to Pp due to its lower surface area: body weight ratio. A cross-section of the preserved worm showed that it was flattened such that the distance from the dorsal to the ventral surface was only 25–30% of its width. Since Pc is much less cylindrical than Pp, its cross-section being more like an ellipse, calculations based on a cylindrical shape are only rough approximations and are only meant to illustrate how body shape might be a key determinant of overall body anabaseine levels.
A current phylogeny [16] of nemerteans is shown in Figure 6 along with the known presence of toxins in some families. The pilidiophorans and paleonemerteans shown in the bottom half of the tree frequently contain peptide neurotoxins and cytolysins ([23,24,25,26,27]; Kem et al., in preparation). Tetrodotoxin (TTX), a bacterially synthesized guanidinium neurotoxin which blocks voltage-gated sodium channels and is found in many animals including pufferfish, the blue-ringed octopus and newts, has also been found in some paleonemertines [27,28,29].
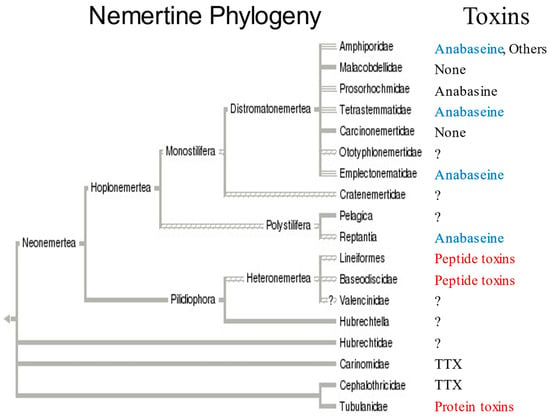
Figure 6.
Known occurrence of toxins in nemertean taxa, based on a proposed phylogenetic tree (adapted from [16]). Major families (Amphiporidae, etc.) are listed on the right side of the tree. Currently known nemertean toxins or types of toxins are listed next to the families in which they have been found. Blue color indicates presence of anabaseine; Red color indicates peptide toxins have been found in the two other major taxa. Pilidiophora were previously called Heteronemertea and the complex comprising Hubrechtidae, Carinomidae, Cephalothricidae and Tubulanidae were called Paleonemertea. One species (Amphiporus angulatus) belonging to family Amphiporidae is known to have >15 pyridine alkaloids [Kem et al., in preparation]. TTX has been found in certain species [27,28,29].
While the mineralized stylets of monostiliferan nemerteans have been studied in considerable detail by Stricker and colleagues [30,31,32], no electron microscopic or elemental analyses of polystiliferans have been reported. It would be of interest to determine if polystiliferan stylets, like monostiliferan stylets, are composed of the same mineral, amorphous calcium phosphate [32]. Perhaps such information can provide some basis for determining whether both forms of stylets evolved from a common primordial stylet or mineral deposit. We suggest that acquisition of pyridyl alkaloid toxins like anabaseine occurred first and mineralized deposits in the proboscis epithelium were elaborated later as a means of enhancing penetration of venom through the integuments of prey and potential predators.
While the prey and predators of polystiliferans are still largely unknown, available data suggest that arthropods are a major prey for at least some pelagic polystiliferans [33,34]. Thus, it is possible that compounds with insecticidal activity (besides anabaseine) may be found in these worms.
4. Concluding Remarks
The primary conclusion of this paper, that anabaseine is found in a polystiliferan hoplonemertean, suggests that the biosynthetic mechanism for producing this alkaloid was developed before the divergent evolution of the polystiliferan and monostiliferan branches of Hoplonemertea. Analyses of other polystiliferan species can reveal how generally this alkaloid is distributed within this order. It is likely that future studies of other polystiliferans as well as monostyliferan nemerteans will lead to the discovery of novel natural products of potential human use.
5. Materials and Methods
5.1. Animal Collection
Two living Pc worms were collected by a diver at 18 meters depth along the Galician (northwestern) Atlantic coast of Spain. The smallest worm (0.40 g Fr Wt) was preserved in absolute EtOH. The larger worm (2.90 g Fr. Wt.) was relaxed in 7.5% MgCl2, which is isotonic with seawater. The head was then sliced off, allowing removal of the proboscis, which was then sliced into anterior, median (stylet bearing) and posterior regions. Each part, including the body with head (minus a 0.65 g piece for mitochondrial DNA analysis), was separately preserved in absolute EtOH (approximately 10 mL/g Fr. Wt.) The two preserved specimens were then airmailed to the Kem laboratory for analysis.
5.2. Alkaloid Extraction
The ethanolic preservative was removed from each worm sample and then each worm was sliced into multiple pieces and placed in a second solution of ethanol containing 1% acetic acid to enhance extraction of the alkaloids. After shaking at 5 °C overnight, the two solvent samples of each worm were pooled, passed through a glass filter and then concentrated with a rotary evaporator at room temperature to prevent loss of volatile alkaloids like 2,3′-bipyridyl and 3-methyl-2,3′-bipyridyl. The extract for each body part was then dissolved in 2.0 mL EtOH and 0.1 mL was then used for the Ehrlich reagent spectrophotometric assay of anabaseine. The remaining body (minus proboscis) extract was used to purify anabaseine by HPLC and to obtain larger amounts of the presumed DMAB-anabaseine derivative for structural analysis.
5.3. Purification of Pc Alkaloids
Essentially the same Stas-Otto solvent extraction procedure previously used by King [5] to isolate “amphiporine” from Amphiporus lactifloreus and by Kem et al. [6] to isolate anabaseine from the wandering nemertean Paranemertes peregrina (Pp) was employed. First, methylene chloride extraction of an aqueous solution (3X with 3 Vol CH2Cl2/Vol of the crude EtOH-soluble fraction in HCl acidified water, (pH < 2) conditions was employed to remove neutral and acidic lipids, etc., followed by a similar extraction of the alkalinized (pH > 11) aqueous solution with CH2Cl2, the weakly basic alkaloids being recovered in the alkaline CH2Cl2 phase. This procedure effectively provides the alkaloids in a much purified and concentrated form suitable for chromatographic separations.
5.4. Tissue Anabaseine Determinations
The use of Ehrlich’s reagent to measure anabaseine has been described in detail in earlier publications [6,7,8]. Briefly a tissue sample is homogenized in absolute EtOH containing 1% concentrated HCl, the extract is centrifuged or filtered to remove insolubles, then a portion of this ethanolic solution is added directly to the Ehrlich reagent (1% DMAB and 1% HCl in EtOH) and incubated in a closed vial for at least 3 h at 70 °C After cooling to room temperature an absorbance spectrum over the wavelength range 300–550 nm is measured to determine the wavelength of peak absorbance and the actual absorbance at 490 nm. Dilutions in 1% HCl-EtOH may be necessary to accurately determine the maximum absorbance, optimally between absorbance range 0.2–2. A 3.0 ug/mL anabaseine (free base) solution in the reaction medium gives an absorbance A = 1.00 at 490 nm The wavelength of peak absorbance for anabaseine is 487 nm under the conditions of the assay. Since Ehrlich’s reagent also reacts with indoles and other compounds containing sites susceptible to electrophilic substitution, any presumed DMAB-anabaseine derivative should be isolated chromatographically and analyzed by MS and/or NMR, as in the present paper, to ascertain its identity, assuming that sufficient material is available for these analyses. For instance, the nemertean Amphpiporus ochraceous contains a compound related to anabaseine that also reacts with Ehrlich’s reagent, producing a derivative with slightly shorter peak absorbance wavelengths near 480 nm in 1% HCl-ethanol ([7]; Kem et al., in preparation).
5.5. MS and NMR Analyses
A reverse phase HPLC-(+)ESI-MS analysis of the presumed anabaseine peak was performed on a Thermo Fisher Scientific (Waltham, MA USA) LTQ classic quadrupole ion trap mass spectrometer operated in the positive electrospray ionization mode. ESI normal MS scans were taken and data-dependent MS/MS scans were obtained on the most abundant ion of the preceding MS scan with a 4u-isolation window, 0.3 qCID, 42.5% normalized CID and 30ms CID duration. Chromatography was performed with an Agilent (Santa Clara, CA, USA) 1100 series binary pump and a Waters (Milford, MA, USA) XTerra MS C18 (2.1 mm × 150 mm; 3.5 µm) with Phenomenex (Torrance, CA, USA) C18 Security Guard Column (2 mm × 4 mm). Mobile phase A was water plus 0.2% acetic acid and mobile phase B was methanol with 0.2% acetic acid. With a flow rate of 0.15 mL/min, the gradient was 0%B (0 min) to 30%B at 15 min then to 95%B at 60 min and held for 20 min. An Agilent (Santa Clara, CA, USA) 1100 G1314A UV/Vis detector was between the HPLC column and MS and monitored the HPLC effluent at 254 nm.
The time-of-flight high resolution mass spectrometry (TOF-HRMS) was performed on an Agilent (Santa Clara, CA, USA) 6220A time of flight HRMS interfaced to an Agilent 1100 binary HPLC. Flow injection analysis was used to introduce the sample. The TOF-HRMS was operated in the (+)ESI mode. Elemental composition of the DMAB-natural product was calculated with the Xcalibur Qual Browser Elemental Composition tool, Qual Browser, Thermo Fisher Scientific (Waltham, MA, USA) Xcalibur 2.2 SP1.48, 12 August 2011.
The proton NMR spectrum for the 300 µg natural product DMAB-derivative free base in 0.8 mL CDCl3 was acquired over a period of 80 minutes on a Varian Mercury 300 MHz spectrometer (Palo Alto, CA, USA) equipped with a 5 mm conventional probe. The proton NMR spectrum for a few mg of the synthetic DMAB-anabaseine free base in 0.6 mL CDCl3 was acquired on a Bruker Avance III-HD 600 MHz spectrometer (Billerica, Massachusetts, MA, USA) equipped with a 5 mm cryogenic probe. Chemical shift axes in both spectra were referenced to internal tetramethylsilane at 0.0 ppm.
Author Contributions
W.R.K.: conceptualization, methodology, anabaseine isolation and tissue determinations, writing and funding acquisition; J.R.R.: methodology, NMR, NMR data acquisition, curation; J.V.J.: methodology, mass spectrometry and data curation; J.J.: collection, identification, dissection and preservation of specimens. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Florida Sea Grant R/LR-MB-9 (W. Kem, P.I.). A portion of this work was performed in the McKnight Brain Institute at the National High Magnetic Field Laboratory’s Advanced Magnetic Resonance Imaging and Spectroscopy (AMRIS) Facility, which is supported by National Science Foundation Cooperative Agreement DMR-1644779* and the State of Florida. The MS data were obtained at the UF Chemistry Department Mass Spectrometry Research and Education Center funded by NIH S10 322 OD021758-01A1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Maria Cristina A. Dancel (Chemistry Dept, UF) for the TOF-HRMS analysis, Ferenc Soti (Deceased; Kem Lab) for acquiring the DMAB-natural product NMR spectrum and Eric Kem for assistance in preparing figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pawlowsky, E.N. Giftiere und Ihre Giftigkeit; Verlag von Gustav Fischer: Jena, Germany, 1927; p. 145. [Google Scholar]
- Reisinger, E. Nemertini. Schnurwurmer. Biol. Tiere Dtl. 1926, 17, 7.1–7.24. [Google Scholar]
- Bacq, Z.M. Les poisons des nemertiens. Bull. Cl. Sci. Acad. Roy. Belg. S 1936, 22, 1072–1079. [Google Scholar]
- Bacq, Z.M. L”amphiporine” et la “nemertine,” poisons des vers nemertiens. Arch. Int. Physiol. 1937, 44, 190–204. [Google Scholar] [CrossRef]
- King, H. Amphiporine, an active base from the marine worm Amphiporus lactifloreus. J. Chem. Soc. 1939, 1365, (Abstract). [Google Scholar]
- Kem, W.R.; Abbott, B.C.; Coates, R.M. Isolation and structure of a hoplonemertine toxin. Toxicon 1971, 9, 15–22. [Google Scholar] [CrossRef]
- Kem, W.R. A study of the occurrence of anabaseine in Paranemertes and other nemertines. Toxicon 1971, 9, 23–32. [Google Scholar] [CrossRef]
- Kem, W.R. Pyridine alkaloid distribution in the hoplonemertines. Hydrobiologia 1988, 156, 145–151. [Google Scholar] [CrossRef]
- Spath, E.; Mamoli, L. Eine neue synthese des D,L-anabasins. Chem. Ber. 1936, 69, 1082. [Google Scholar] [CrossRef]
- Kem, W.R.; Scott, K.N.; Duncan, J.H. Hoplonemertine worms—A new source of pyridine neurotoxins. Experientia 1976, 32, 684–686. [Google Scholar] [CrossRef]
- Kem, W.R.; Rocca, J.; Garraffo, H.M.; Spande, T.F.; Daly, J.W.; Soti, F. Synthesis and spectroscopic comparison of the eight methyl-2,3′-bipyridyls and identification of a hoplonemertine alkaloid as 3-methyl-2,3′-bipyridyl. Heterocycles 2009, 79, 1025–1041. [Google Scholar] [CrossRef]
- Xing, H.; Keshwah, S.; Rouchaud, A.; Kem, W.R. A pharmacological comparison of two isomeric nicotinic receptor agonists: The marine toxin isoanatabine and the tobacco alkaloid anatabine. Mar. Drugs 2020, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Kem, W.R.; Soti, F. Amphiporus alkaloid multiplicity implies functional diversity: Initial studies on crustacean pyridyl receptors. Hydrobiologia 2001, 456, 221–231. [Google Scholar] [CrossRef]
- Maslakova, S.A.; Norenburg, J.L. Phylogenetic study of pelagic nemerteans (Pelagica, Polystilifera.). Hydrobiologia 2001, 456, 111–132. [Google Scholar] [CrossRef]
- Kvist, S.; Laumer, C.E.; Junoy, J.; Giribet, G. New insights into the phylogeny, systematics and DNA barcoding of Nemertea. Invertebr. Syst. 2014, 28, 287–308. [Google Scholar] [CrossRef]
- Thollesson, M.; Norenburg, J.L. Ribbon worm relationships: A phylogeny of the phylum Nemertea. J. R. Soc. Lond. B 2003, 270, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Harlin, M. Tree-thinking and nemertean systematics, with a systematization of the Eureptantia. Hydrobiologia 1998, 365, 33–46. [Google Scholar] [CrossRef]
- Sheppard, E.M. On Paradrepanophorus crassus (Quatr.), a nemertean worm new to the British fauna. Ann. Mag. Nat. Hist. 1935, 15, 232–236. [Google Scholar] [CrossRef]
- Trowbridge, C.D.; Little, C.; Stirling, P.; Pilling, G.; Dlouhy-Massengale, B. Lusitanian nemertean species in Lough Hyne Marine Reserve: Paradrepanophorus crassus and Punnettia splendida. Mar. Biodiv. Rec. 2013, 6, e57. [Google Scholar] [CrossRef]
- Herrra-Bachiller, A.; Fernandez-Alvarez, F.A.; Junoy, J. A Taxonomic catalogue of the nemerteans (Phylum Nemertea) of Spain and Portugal. Zool. Sci. 2015, 32, 507–522. [Google Scholar] [CrossRef]
- Zoltewicz, J.A.; Bloom, L.B.; Kem, W.R. Quantitative determination of the ring-chain hydrolysis equilibrium constant for anabaseine and related tobacco alkaloids. J. Org. Chem. 1989, 54, 4462–4468. [Google Scholar] [CrossRef]
- Andrud, K.; Xing, H.; Gabrielsen, B.; Bloom, L.; Mahnir, V.; Lee, S.; Green, B.T.; Lindstrom, J.; Kem, W.R. Investigation of the possible pharmacologically active forms of the nicotinic acetylcholine receptor agonist anabaseine. Mar. Drugs 2019, 17, 614. [Google Scholar] [CrossRef]
- Kem, W.R. A Chemical Investigation of Nemertine Toxins. Ph.D. Dissertation, University of Illinois Urbana-Champaign, Champaign, IL, USA, 1969; pp. 1–113. [Google Scholar]
- Kem, W.R. Purification and characterization of a new family of polypeptide neurotoxins from the heteronemertine Cerebratulus lacteus (Leidy). J. Biol. Chem. 1976, 251, 4184–4192. [Google Scholar] [CrossRef] [PubMed]
- Kem, W.R.; Blumenthal, K.M. Purification and characterization of the cytolytic Cerebratulus A toxins. J. Biol. Chem. 1978, 253, 5752–5757. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, E.; Andersson, H.S.; Strand, M.; Peigneur, S.; Eriksson, C.; Loden, H.; Shariatgorji, M.; Andren, P.E.; Lebbe, E.K.M.; Rosengren, K.J.; et al. Peptide ion channel toxins from the bootlace worm, the longest animal on earth. Sci. Rep. 2018, 8, 4596. [Google Scholar] [CrossRef]
- Göransson, U.; Jacobsson, E.; Strand, M.; Andersson, H.S. The toxins of nemertean worms. Toxins 2019, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Higashiyama, M.; Ito, K.; Noguchi, T.; Arakawa, O.; Shida, Y.; Hashimoto, K. Tetrodotoxin in two species of ribbon worm (Nemertini), Lineus fuscoviridis and Tubulanus punctatus. Toxicon 1988, 26, 867–874. [Google Scholar] [CrossRef]
- Ali, A.E.; Arakawa, O.; Noguchi, T.; Miyazawa, K.; Shida, Y.; Hashimoto, K. Tetrodotoxin and related substances in a ribbon worm Cephalothrix linearis (Nemertean). Toxicon 1990, 28, 1083–1093. [Google Scholar] [CrossRef]
- Stricker, S.A.; Cloney, R.A. The stylet apparatus of the nemertean Paranemertes peregrina; Its ultrastructure and role in prey capture. Zoomorphology 1981, 87, 205–223. [Google Scholar] [CrossRef]
- Stricker, S.A.; Cloney, R.A. Stylet formation in nemerteans. Biol. Bull. 1982, 162, 387–403. [Google Scholar] [CrossRef]
- Stricker, S.A.; Weiner, S. Amorphous calcium phosphate in the stylets produced by a marine worm (Nemertea). Experientia 1985, 41, 1557–1559. [Google Scholar] [CrossRef]
- McDermott, J.J. Status of the nemertea as prey in marine ecosystems. Hydrobiologia 2001, 456, 7–20. [Google Scholar] [CrossRef]
- Feller, R.J.; Roe, P.; Norenburg, J.L. Dietary immunoassay of pelagic nemerteans by use of cross-reacting polyclonal antibodies: Preliminary findings. Hydrobiologia 1998, 365, 263–269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).