Combination Effect of Microcystins and Arsenic Exposures on CKD: A Case-Control Study in China
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Study Population
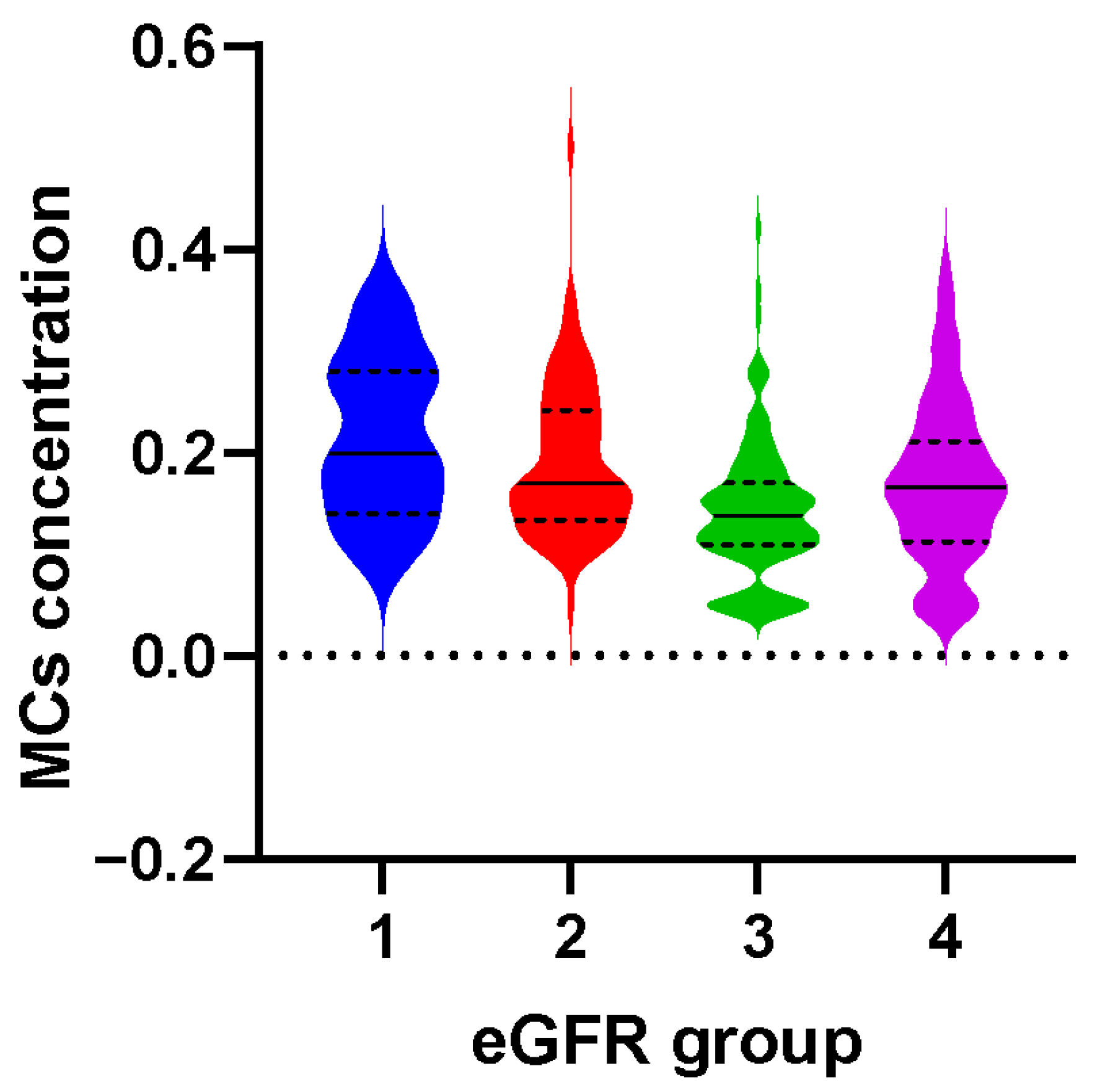
2.2. The Relationship between MCs and the Risk of CKD
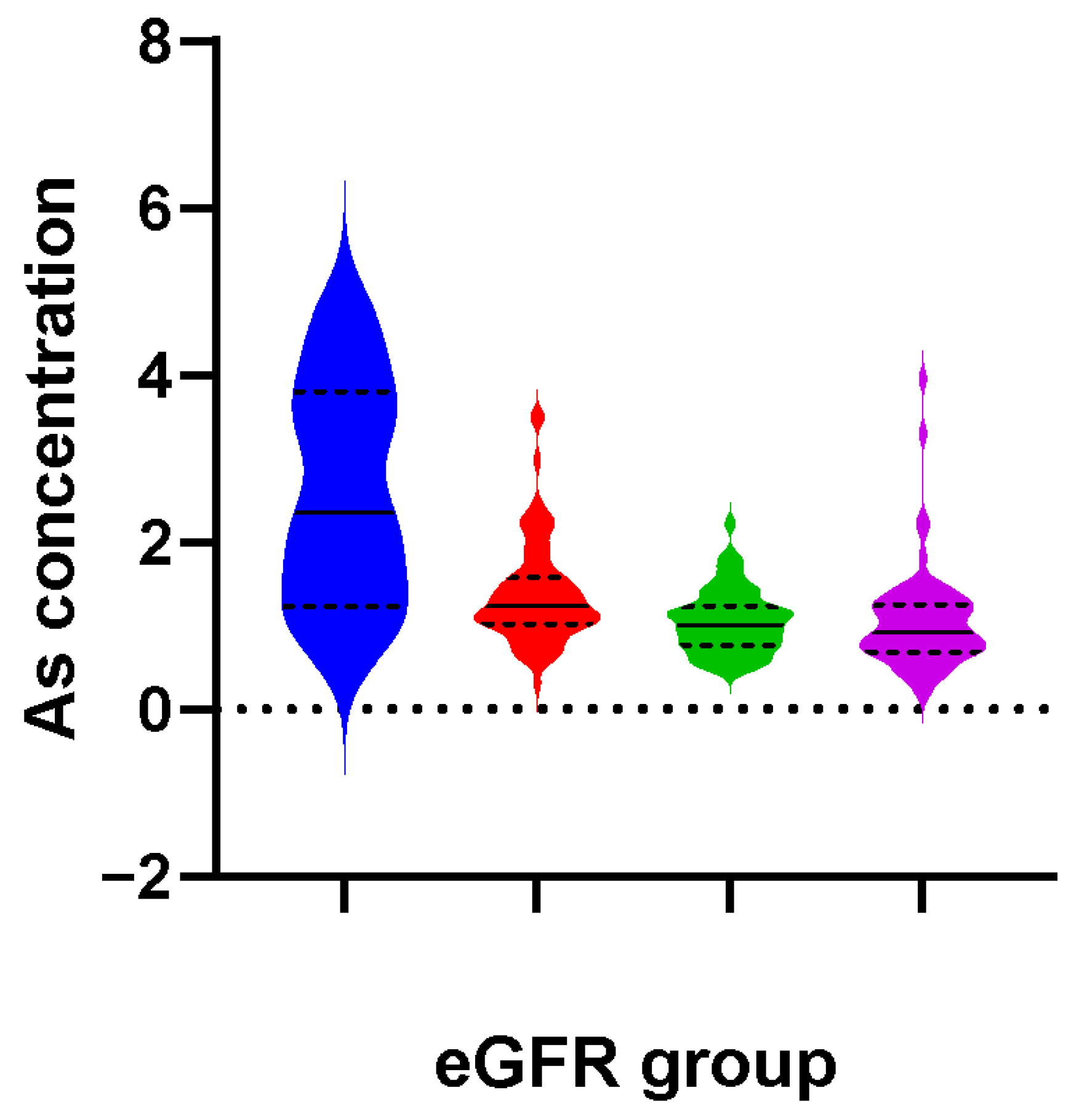
2.3. Association between As and CKD Risk
2.4. Combined Effect of MCs and As on the Risk of CKD
2.5. Combined Effects of MCs, As, and Cd on CKD
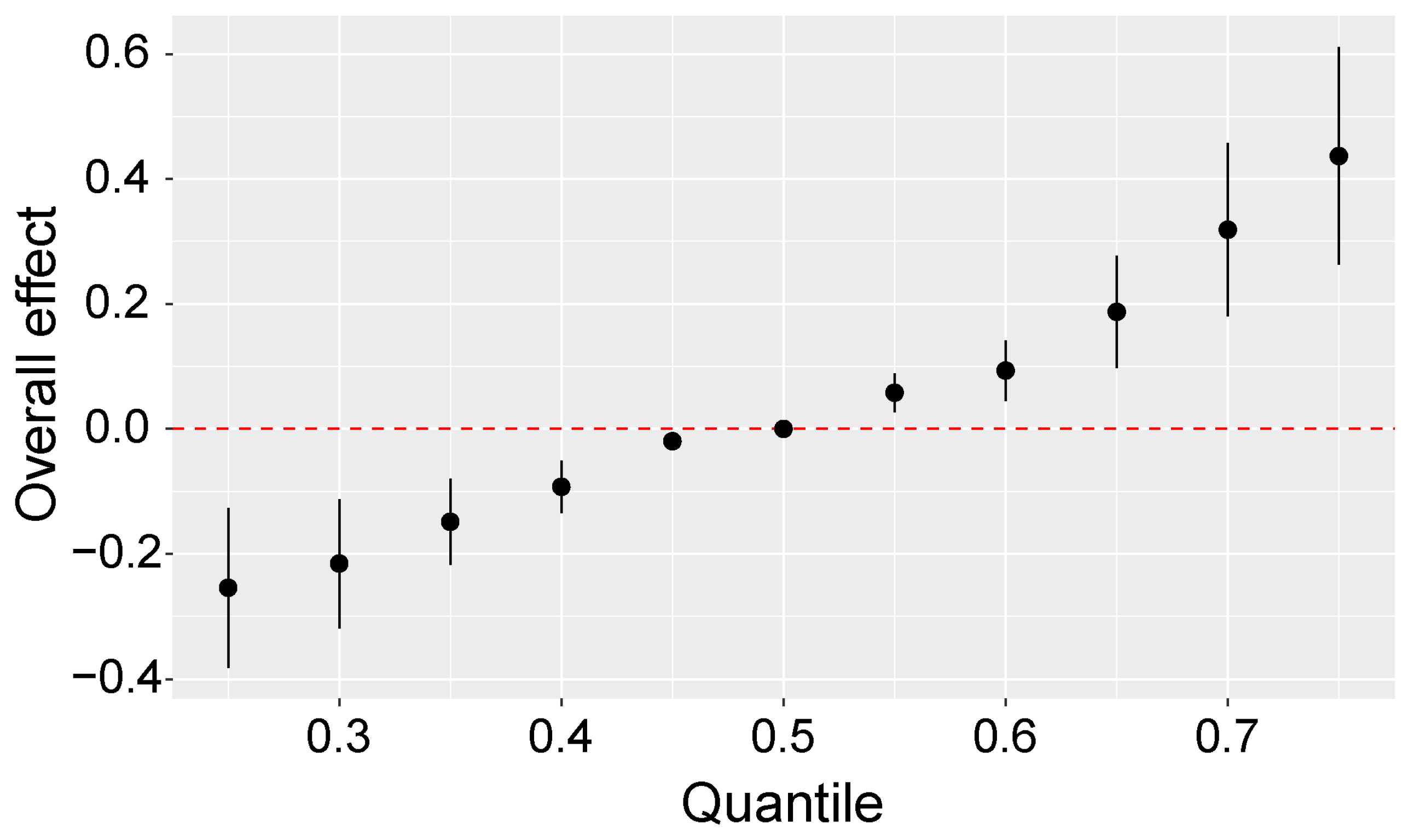
2.6. Sensitivity Analyses
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. Measurement of MCs Exposure
5.3. Measurement of As and Cd Exposures
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, J.C.; Zhang, L.X. Prevalence and Disease Burden of Chronic Kidney Disease. Adv. Exp. Med. Biol. 2019, 1165, 3–15. [Google Scholar] [PubMed]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.T.; Maddukuri, G.; Tsai, C.Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.M.; Xu, D.M.; Long, J.Y.; Shi, Y.; Zhang, L.X.; Wang, H.B.; Levin, A.; Zhao, M.H. Spectrum of chronic kidney disease in China: A national study based on hospitalized patients from 2010 to 2015. Nephrology 2019, 24, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Liang, S.; Zhu, F.L.; Liu, J.Q.; Wang, S.Y.; Chen, X.M.; Cai, G.Y.; Grp, C.-O.S. The prevalence of depression and the association between depression and kidney function and health-related quality of life in elderly patients with chronic kidney disease: A multicenter cross-sectional study. Clin. Interv. Aging 2019, 14, 905–913. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Hui, D.; Hladunewich, M.A. Chronic Kidney Disease and Pregnancy. Obs. Gynecol. 2019, 133, 1182–1194. [Google Scholar] [CrossRef]
- Lin, Y.J.; Hsiao, J.L.; Hsu, H.T. Integration of biomonitoring data and reverse dosimetry modeling to assess population risks of arsenic-induced chronic kidney disease and urinary cancer. Ecotoxicol. Environ. Saf. 2020, 206, 111212. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Ding, H.Y.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic Kidney Disease and Exposure to Nephrotoxic Metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef]
- Massey, I.Y.; Yang, F.; Ding, Z.; Yang, S.; Guo, J.; Tezi, C.; Al-Osman, M.; Kamegni, R.B.; Zeng, W.M. Exposure routes and health effects of microcystins on animals and humans: A mini-review. Toxicon 2018, 151, 156–162. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Feng, S.; Deng, S.; Tang, Y.; Liu, Y.; Yang, Y.; Xu, S.; Tang, P.; Lu, Y.; Duan, Y.; Wei, J.; et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022, 56, 15818–15827. [Google Scholar] [CrossRef]
- Falconer, I.R. An overview of problems caused by toxic blue-green algae (cyanobacteria) in drinking and recreational water. Environ. Toxicol. 1999, 14, 5–12. [Google Scholar] [CrossRef]
- Du, C.; Zheng, S.; Yang, Y.; Feng, X.; Chen, J.; Tang, Y.; Wang, H.; Yang, F. Chronic exposure to low concentration of MC-LR caused hepatic lipid metabolism disorder. Ecotoxicol. Environ. Saf. 2022, 239, 113649. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Wang, X.Y.; Chen, L.; Liu, W.Y.; Cai, D.P.; Deng, S.X.; Chu, H.Y.; Liu, Y.; Feng, X.L.; et al. Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/ Rap1b signaling pathway. J. Hazard. Mater. 2022, 440, 129793. [Google Scholar] [CrossRef]
- Veerabadhran, M.; Gnanasekaran, D.; Wei, J.; Yang, F. Anaerobic digestion of microalgal biomass for bioenergy production, removal of nutrients and microcystin: Current status. J. Appl. Microbiol. 2021, 131, 1639–1651. [Google Scholar] [CrossRef]
- Cai, D.; Wei, J.; Huang, F.; Feng, H.; Peng, T.; Luo, J.; Yang, F. The detoxification activities and mechanisms of microcystinase towards MC-LR. Ecotoxicol. Environ. Saf. 2022, 236, 113436. [Google Scholar] [CrossRef]
- Wasana, H.M.S.; Perera, G.D.R.K.; Gunawardena, P.D.; Fernando, P.S.; Bandara, J. WHO water quality standards Vs Synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci. Rep. 2017, 7, 42516. [Google Scholar] [CrossRef]
- Zheng, S.; Yang, Y.; Wen, C.; Liu, W.; Cao, L.; Feng, X.; Chen, J.; Wang, H.; Tang, Y.; Tian, L.; et al. Effects of environmental contaminants in water resources on nonalcoholic fatty liver disease. Environ. Int. 2021, 154, 106555. [Google Scholar] [CrossRef]
- Guo, J.; Wei, J.; Huang, F.; Massey, I.Y.; Luo, J.; Yang, F. Optimization of microcystin biodegradation by bacterial community YFMCD4 using response surface method. Chemosphere 2021, 274, 129897. [Google Scholar] [CrossRef]
- Lopes, T.O.M.; Passos, L.S.; Vieira, L.V.; Pinto, E.; Dorr, F.; Scherer, R.; Salustriano, N.D.; Carneiro, M.T.W.D.; Postay, L.F.; Gomes, L.C. Metals, arsenic, pesticides, and microcystins in tilapia (Oreochromis niloticus) from aquaculture parks in Brazil. Environ. Sci. Pollut. Res. 2020, 27, 20187–20200. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.G.; Yu, J.P.; Kong, C.; Li, H.R.; Yang, L.S.; Guo, Z.W.; Cui, N.; Xia, Y.J.; Wu, K.G. An investigation of the health effects caused by exposure to arsenic from drinking water and coal combustion: Arsenic exposure and metabolism. Environ. Sci. Pollut. Res. 2017, 24, 25947–25954. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Li, Y.W.; Liu, B.L.; Zhao, H.M.; Li, H.; Cai, Q.Y.; Mo, C.H.; Wong, M.H.; Li, Q.X. High ecological and human health risks from microcystins in vegetable fields in southern China. Environ. Int. 2019, 133, 105142. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Zeng, X.B.; Bai, L.Y.; Li, S.H. Soil arsenic content and its health risk assessment for agricultural products in the region surrounding Shimen arsenic sulphide mine. Ying Yong Sheng Tai Xue Bao 2010, 21, 2946–2951. [Google Scholar]
- Zhang, J.; Wei, J.; Massey, I.Y.; Peng, T.; Yang, F. Immobilization of Microbes for Biodegradation of Microcystins: A Mini Review. Toxins 2022, 14, 573. [Google Scholar] [CrossRef]
- Wang, P.F.; Yin, N.Y.; Cai, X.L.; Du, H.L.; Li, Y.; Sun, G.X.; Cui, Y.S. Comparison of bioaccessibility and relative bioavailability of arsenic in rice bran: The in vitro with PBET/SHIME and in vivo with mice model. Chemosphere 2020, 259, 127443. [Google Scholar] [CrossRef]
- Xu, S.S.; Yi, X.P.; Liu, W.Y.; Zhang, C.C.; Massey, I.Y.; Yang, F.; Tian, L. A Review of Nephrotoxicity of Microcystins. Toxins 2020, 12, 693. [Google Scholar] [CrossRef]
- Yi, X.P.; Xu, S.S.; Huang, F.Y.; Wen, C.; Zheng, S.L.; Feng, H.; Guo, J.; Chen, J.H.; Feng, X.L.; Yang, F. Effects of Chronic Exposure to Microcystin-LR on Kidney in Mice. Int. J. Environ. Res. Public Health 2019, 16, 5030. [Google Scholar] [CrossRef]
- Ishaq, A.; Gulzar, H.; Hassan, A.; Kamran, M.; Riaz, M.; Parveen, A.; Chattha, M.S.; Walayat, N.; Fatima, S.; Afzal, S.; et al. Ameliorative mechanisms of turmeric-extracted curcumin on arsenic (As)-induced biochemical alterations, oxidative damage, and impaired organ functions in rats. Environ. Sci. Pollut. Res. 2021, 28, 66313–66326. [Google Scholar] [CrossRef]
- Yang, F.; Yi, X.; Guo, J.; Xu, S.; Xiao, Y.; Huang, X.; Duan, Y.; Luo, D.; Xiao, S.; Huang, Z.; et al. Association of plasma and urine metals levels with kidney function: A population-based cross-sectional study in China. Chemosphere 2019, 226, 321–328. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Chen, F.; Chen, L.; Giesy, J.P.; Guo, Y.; Liang, G.; Deng, X.; Wang, W.; Xie, P. Health Risks of Chronic Exposure to Small Doses of Microcystins: An Integrative Metabolomic and Biochemical Study of Human Serum. Environ. Sci. Technol. 2022, 56, 6548–6559. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Li, L.; Xu, J. First Identification of the Hepatotoxic Microcystins in the Serum of a Chronically Exposed Human Population Together with Indication of Hepatocellular Damage. Toxicol. Sci. 2009, 108, 81–89. [Google Scholar] [CrossRef]
- Lin, H.; Liu, W.; Zeng, H.; Pu, C.; Zhang, R.; Qiu, Z.; Chen, J.A.; Wang, L.; Tan, Y.; Zheng, C.; et al. Determination of Environmental Exposure to Microcystin and Aflatoxin as a Risk for Renal Function Based on 5493 Rural People in Southwest China. Environ. Sci. Technol. 2016, 50, 5346–5356. [Google Scholar] [CrossRef]
- Yuan, T.H.; Ke, D.Y.; Wang, J.E.H.; Chan, C.C. Associations between renal functions and exposure of arsenic and polycyclic aromatic hydrocarbon in adults living near a petrochemical complex. Environ. Pollut. 2020, 256, 113457. [Google Scholar] [CrossRef]
- Hsu, L.I.; Hsieh, F.I.; Wang, Y.H.; Lai, T.S.; Wu, M.M.; Chen, C.J.; Chiou, H.Y.; Hsu, K.H. Arsenic Exposure From Drinking Water and the Incidence of CKD in Low to Moderate Exposed Areas of Taiwan: A 14-Year Prospective Study. Am. J. Kidney Dis. 2017, 70, 787–797. [Google Scholar] [CrossRef]
- Wang, R.X.; Long, T.F.; He, J.; Xu, Y.L.; Wei, Y.; Zhang, Y.; He, X.J.; He, M.A. Associations of multiple plasma metals with chronic kidney disease in patients with diabetes. Ecotoxicol. Environ. Saf. 2022, 244, 114048. [Google Scholar] [CrossRef]
- Palikova, M.; Papezikova, I.; Kopp, R.; Mares, J.; Markova, Z.; Navratil, S.; Adamovsky, O.; Kohoutek, J.; Navratil, L.; Blaha, L. Effect of arsenic and cyanobacterial co-exposure on pathological, haematological and immunological parameters of rainbow trout (Oncorhynchus mykiss). Neuro Endocrinol. Lett. 2015, 36 (Suppl. 1), 57–63. [Google Scholar]
- Yang, F.; Huang, F.; Feng, H.; Wei, J.; Massey, I.Y.; Liang, G.; Zhang, F.; Yin, L.; Kacew, S.; Zhang, X.; et al. A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water Res. 2020, 174, 115638. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, C.; Yi, X.; Guo, J.; Xu, S.; Huang, Z.; He, M.; Chen, X.; Luo, D.; Yang, F. Association of multi-metals exposure with intelligence quotient score of children: A prospective cohort study. Environ. Int. 2021, 155, 106692. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Ma, Y.; Chen, X.; Losiewicz, M.D.; Du, X.; Tian, Z.; Zhang, S.; Shi, L.; Zhang, H.; et al. Long-term exposure to low concentrations of MC-LR induces blood-testis barrier damage through the RhoA/ROCK pathway. Ecotoxicol. Environ. Saf. 2022, 236, 113454. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, X.; Wang, Y.; Losiewicz, M.D.; Chen, X.; Du, X.; Wang, Y.; Zhang, B.; Guo, X.; Yuan, S.; et al. Chronic Exposure to Environmentally Relevant Concentrations of Microcystin-Leucine Arginine Causes Lung Barrier Damage through PP2A Activity Inhibition and Claudin1 Ubiquitination. J. Agric. Food Chem. 2022, 70, 10907–10918. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.L.; Yang, Y.F.; Sun, B.; Yuan, J.J.; Yu, M.Z.; Stenseth, N.C.; Bullock, J.M.; Obersteiner, M. Spatial variation in biodiversity loss across China under multiple environmental stressors. Sci. Adv. 2020, 6, eabd0952. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, N.X.; Zhang, Y.Z.; Sheng, H.; Zhou, Q.; Huang, Y.X.; Yu, Z. Clay mineral composition of upland soils and its implication for pedogenesis and soil taxonomy in subtropical China. Sci. Rep. 2021, 11, 9707. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wei, J.; Yang, K.; Massey, I.Y.; Guo, J.; Zhang, C.; Yang, F. Isolation, molecular identification, and characterization of a unique toxic cyanobacterium Microcystis sp. found in Hunan Province, China. J. Toxicol. Env. Health A 2018, 81, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Clara, T.; Huang, F.; Wei, J.; Yang, F. Identification and characterization of the dominant Microcystis sp. cyanobacteria detected in Lake Dong Ting, China. J. Toxicol. Env. Health A 2019, 82, 1143–1150. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zeng, J.Y.; Ding, S.; Li, J.; Liu, X.; Guan, D.X.; Ma, L.Q. Arsenic contents, speciation and bioaccessibility in rice grains from China: Regional and variety differences. J. Hazard. Mater. 2022, 437, 129431. [Google Scholar] [CrossRef]
- Chen, H.P.; Tang, Z.; Wang, P.; Zhao, F.J. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ. Pollut. 2018, 238, 482–490. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Hudson-Edwards, K.A. Seasonal variations in arsenic mobility and bacterial diversity: The case study of Huangshui Creek, Shimen Realgar Mine, Hunan Province, China. Sci Total Environ. 2020, 749, 142353. [Google Scholar] [CrossRef]
- Kopp, R.; Mares, J.; Soukupova, Z.; Navratil, S.; Palikova, M. Influence of arsenic and cyanobacteria co-exposure on plasmatic parameters of rainbow trout (Oncorhynchus mykiss W.). Neuro Endocrinol. Lett. 2014, 3 (Suppl. 2), 57–63. [Google Scholar]
- Ji, P.Y.; Li, Z.Y.; Wang, H.; Dong, J.T.; Li, X.J.; Yi, H.L. Arsenic and sulfur dioxide co-exposure induce renal injury via activation of the NF-kappaB and caspase signaling pathway. Chemosphere 2019, 224, 280–288. [Google Scholar] [CrossRef]
- Wang, Z.; Li, G.; Wu, Q.; Liu, C.; Shen, J.; Yan, W. Microcystin-LR exposure induced nephrotoxicity by triggering apoptosis in female zebrafish. Chemosphere 2019, 214, 598–605. [Google Scholar] [CrossRef]
- Piyathilaka, M.A.; Pathmalal, M.M.; Tennekoon, K.H.; De Silva, B.G.; Samarakoon, S.R.; Chanthirika, S. Microcystin-LR-induced cytotoxicity and apoptosis in human embryonic kidney and human kidney adenocarcinoma cell lines. Microbiology 2015, 161, 819–828. [Google Scholar] [CrossRef]
- Dutta, S.; Saha, S.; Mahalanobish, S.; Sadhukhan, P.; Sil, P.C. Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. Food Chem. Toxicol. 2018, 118, 303–316. [Google Scholar] [CrossRef]
- Wan, F.; Zhong, G.L.; Wu, S.F.; Jiang, X.X.; Liao, J.Z.; Zhang, X.Y.; Zhang, H.; Mehmood, K.; Tang, Z.X.; Hu, L.M. Arsenic and antimony co-induced nephrotoxicity via autophagy and pyroptosis through ROS-mediated pathway in vivo and in vitro. Ecotoxicol. Environ. Saf. 2021, 221, 112442. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Angulo, C.; Sanchez, V.; Cuesta, A.; Cruz, A. Methylmercury, cadmium and arsenic(III)-induced toxicity, oxidative stress and apoptosis in Pacific red snapper leukocytes. Aquat. Toxicol. 2019, 213, 105223. [Google Scholar] [CrossRef]
- Huang, X.; Chen, L.; Liu, W.J.; Qiao, Q.; Wu, K.; Wen, J.; Huang, C.H.; Tang, R.; Zhang, X.Z. Involvement of oxidative stress and cytoskeletal disruption in microcystin-induced apoptosis in CIK cells. Aquat. Toxicol. 2015, 165, 41–50. [Google Scholar] [CrossRef]
- Thangapandiyan, S.; Ramesh, M.; Miltonprabu, S.; Hema, T.; Jothi, G.B.; Nandhini, V. Sulforaphane potentially attenuates arsenic-induced nephrotoxicity via the PI3K/Akt/Nrf2 pathway in albino Wistar rats. Environ. Sci. Pollut. Res. 2019, 26, 12247–12263. [Google Scholar] [CrossRef]
- Liu, P.; Xue, Y.; Zheng, B.; Liang, Y.; Zhang, J.; Shi, J.; Chu, X.; Han, X.; Chu, L. Crocetin attenuates the oxidative stress, inflammation and apoptosisin arsenic trioxide-induced nephrotoxic rats: Implication of PI3K/AKT pathway. Int. Immunopharmacol. 2020, 88, 106959. [Google Scholar] [CrossRef]
- He, L.; Liu, L.; Lin, C.; Ruan, J.; Liang, X.; Zhou, Y.; Wei, L. Effects of MC-LR on histological structure and cell apoptosis in the kidney of grass carp (Ctenopharyngodon idella). Fish. Physiol. Biochem. 2020, 46, 2005–2014. [Google Scholar] [CrossRef]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svircev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total. Environ. 2021, 764, 142319. [Google Scholar] [CrossRef]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 55–80. [Google Scholar] [CrossRef] [PubMed]
| Case/Control | OR (95% CI) | AOR (95% CI) | |
|---|---|---|---|
| MCs | |||
| Q1 (<0.10 μg/L) | 10/33 | 1.0 (reference) | 1.0 (reference) |
| Q2 (0.10 μg/L ~) | 31/34 | 3.10 (1.27, 7.58) | 3.70 (1.38, 9.93) |
| Q3 (0.14 μg/L ~) | 44/34 | 4.14 (1.72, 9.95) | 4.20 (1.62, 10.90) |
| Q4 (≥0.19 μg/L) | 50/34 | 4.05 (1.79, 9.16) | 4.81 (1.96, 11.81) |
| p for trend | 0.003 | 0.003 |
| Case/Control | OR (95% CI) | AOR (95% CI) | |
|---|---|---|---|
| As | |||
| Q1 (< 0.75 μg/L) | 20/34 | 1.0 (reference) | 1.0 (reference) |
| Q2 (0.75 μg/L ~) | 23/34 | 1.06 (0.49, 2.30) | 0.89 (0.37, 2.11) |
| Q3 (1.00 μg/L ~) | 41/34 | 2.31 (1.07, 5.02) | 2.51 (1.06, 5.96) |
| Q4 (≥ 1.31 μg/L) | 51/33 | 2.94 (1.39, 6.24) | 3.40 (1.51, 7.65) |
| p for trend | 0.003 | 0.001 |
| Variables | Case/Control | AOR (95% CI) | p | RERI (95% CI) | AP (95% CI) |
|---|---|---|---|---|---|
| MCs & As | 2.34 (0.14, 4.54) | 0.56 (0.22, 0.91) | |||
| Low & Low | 16/31 | Reference | |||
| Low & High | 25/36 | 1.40 (0.63, 3.11) | 0.41 | ||
| High & Low | 27/37 | 1.40 (0.64, 3.09) | 0.40 | ||
| High & High | 67/31 | 4.14 (1.97, 8.73) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Zhu, N.; Deng, S.; Du, C.; Tang, Y.; Tang, P.; Xu, S.; Liu, W.; Shen, M.; Xiao, X.; et al. Combination Effect of Microcystins and Arsenic Exposures on CKD: A Case-Control Study in China. Toxins 2023, 15, 144. https://doi.org/10.3390/toxins15020144
Gao H, Zhu N, Deng S, Du C, Tang Y, Tang P, Xu S, Liu W, Shen M, Xiao X, et al. Combination Effect of Microcystins and Arsenic Exposures on CKD: A Case-Control Study in China. Toxins. 2023; 15(2):144. https://doi.org/10.3390/toxins15020144
Chicago/Turabian StyleGao, Hong, Na Zhu, Shuxiang Deng, Can Du, Yan Tang, Peng Tang, Shuaishuai Xu, Wenya Liu, Minxue Shen, Xinhua Xiao, and et al. 2023. "Combination Effect of Microcystins and Arsenic Exposures on CKD: A Case-Control Study in China" Toxins 15, no. 2: 144. https://doi.org/10.3390/toxins15020144
APA StyleGao, H., Zhu, N., Deng, S., Du, C., Tang, Y., Tang, P., Xu, S., Liu, W., Shen, M., Xiao, X., & Yang, F. (2023). Combination Effect of Microcystins and Arsenic Exposures on CKD: A Case-Control Study in China. Toxins, 15(2), 144. https://doi.org/10.3390/toxins15020144






