Simultaneous Analysis of Mycotoxins, Potentially Toxic Elements, and Pesticides in Rice: A Health Risk Assessment Study
Abstract
- Heavy metals, mycotoxins, and pesticides in rice samples in Bushehr were assessed.
- The ochratoxin A of all types was below the detection limit.
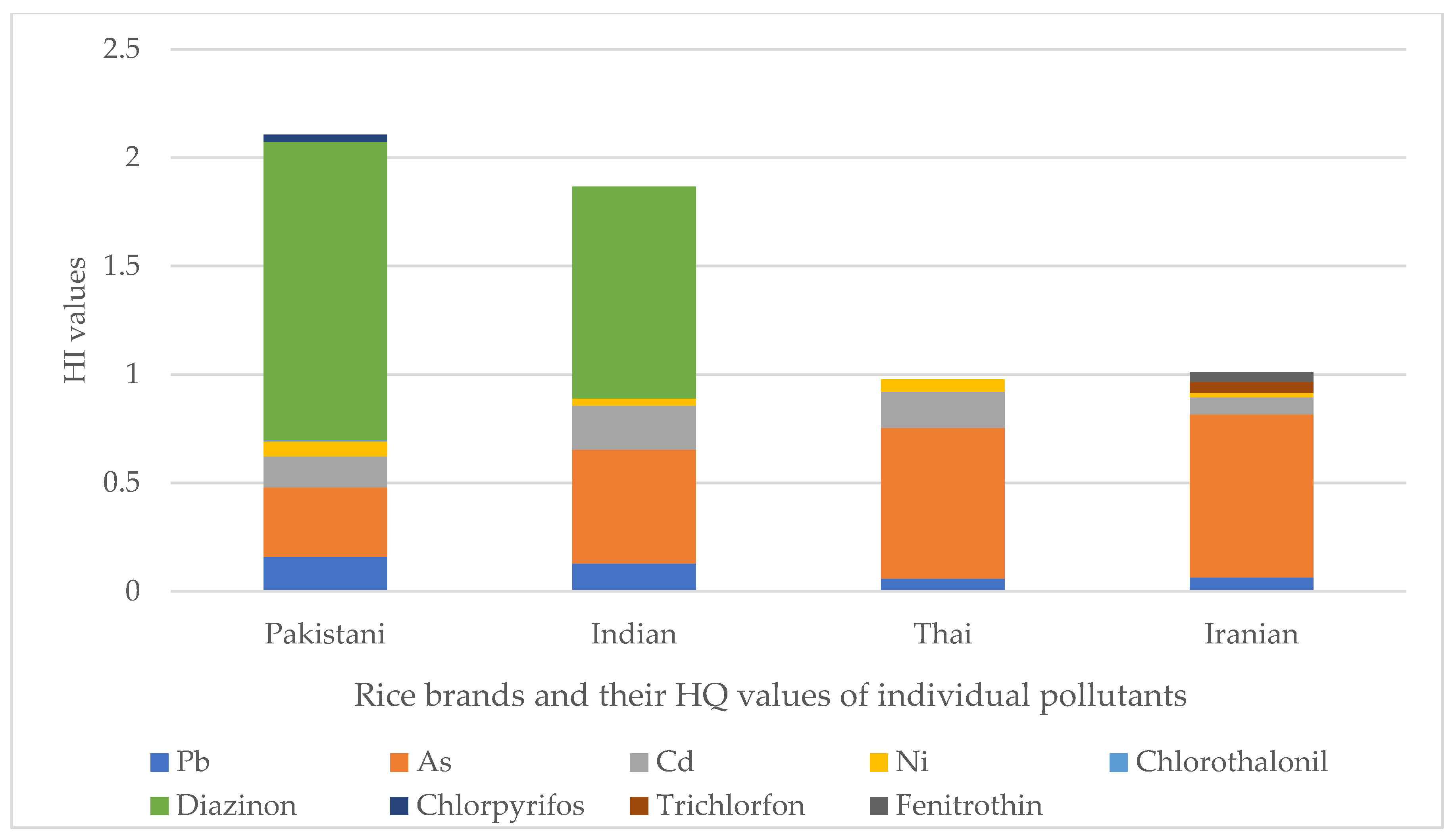
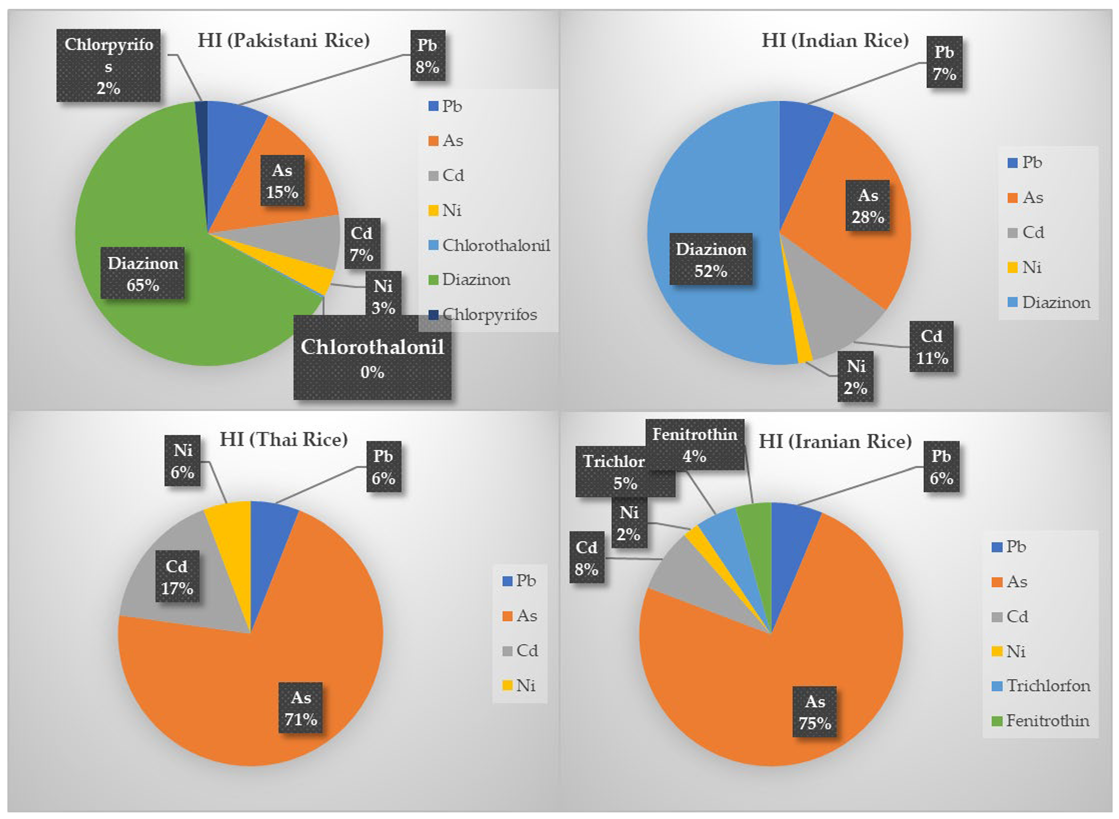
- The order of HI of rice types was HIPAK = 2.1 > HIIND = 1.86 > HIIRN = 1.01 > HITHAI = 0.98.
- The most important pollutant related to the risk index in Indian and Pakistani rice was diazinon and arsenic in Iranian and Thai rice.
- The incremental lifetime cancer risk of nickel was unacceptable in all rice types.
1. Introduction
2. Results and Discussion
2.1. Ash, Moisture, and Mold Contamination
2.2. PTEs
2.3. Mycotoxins
2.3.1. Aflatoxin B1
2.3.2. Zearalenone
2.3.3. Ochratoxin A
2.3.4. Deoxynivalenol
2.4. Pesticides
2.4.1. Chlorpyrifos
2.4.2. Trichlorfon
2.4.3. Diazinon
2.4.4. Fenitrothion
2.4.5. Chlorothalonil
2.5. Non-Carcinogenic Health Risk Assessment
2.6. Carcinogenic Health Risk Assessment
3. Conclusions
4. Materials and Methods
4.1. Chemicals, Reagents, and Apparatuses
4.2. Sample Collection and Study Design
4.3. Measurement of Moisture, Ash, and Mold Content
4.4. Preparation, Sample Digestion, and Determination of PTEs
4.5. Mycotoxins Determination
4.5.1. Aflatoxin B1
4.5.2. Ochratoxin
4.5.3. Zearalenone
4.5.4. Deoxynivalenol
4.6. Pesticides Determination
4.7. Health Risk Assessment
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sreethong, T.; Prom-u-thai, C.; Rerkasem, B.; Dell, B.; Jamjod, S. Identifying rice grains with premium nutritional quality among on-farm germplasm in the highlands of Northern Thailand. Qual. Assur. Saf. Crops Foods 2020, 12, 12–23. [Google Scholar] [CrossRef]
- He, X.; Liu, H.; Lv, C.; Wang, F.; Zhao, C.; Tao, R.; Li, J.; Liu, Z.; Du, L. Analysis of rice microbial communities under different storage conditions using culture-dependent and -independent techniques. Qual. Assur. Saf. Crops Foods 2022, 14, 1–11. [Google Scholar] [CrossRef]
- Naseri, M.; Vazirzadeh, A.; Kazemi, R.; Zaheri, F. Concentration of some heavy metals in rice types available in Shiraz market and human health risk assessment. Food Chem. 2015, 175, 243–248. [Google Scholar] [CrossRef]
- Torres-Escribano, S.; Leal, M.; Vélez, D.; Montoro, R. Total and Inorganic Arsenic Concentrations in Rice Sold in Spain, Effect of Cooking, and Risk Assessments. Environ. Sci. Technol. 2008, 42, 3867–3872. [Google Scholar] [CrossRef]
- Sharafi, K.; Yunesian, M.; Nodehi, R.N.; Mahvi, A.H.; Pirsaheb, M. A systematic literature review for some toxic metals in widely consumed rice types (domestic and imported) in Iran: Human health risk assessment, uncertainty and sensitivity analysis. Ecotoxicol. Environ. Saf. 2019, 176, 64–75. [Google Scholar] [CrossRef]
- McEvoy, J.D. Contamination of animal feedingstuffs as a cause of residues in food: A review of regulatory aspects, incidence and control. Anal. Chim. Acta 2002, 473, 3–26. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Fakhri, Y.; Gahruie, H.H.; Niakousari, M.; Sant’Ana, A.S. Mycotoxins in cereal-based products during 24 years (1983–2017): A global systematic review. Trends Food Sci. Technol. 2019, 91, 95–105. [Google Scholar] [CrossRef]
- Aranega, J.P.R.B.; Oliveira, C.A.F. Occurrence of mycotoxins in pastures: A systematic review. Qual. Assur. Saf. Crops Foods 2022, 14, 135–144. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Sharma, N.; Bhardwaj, A.; Phimolsiripol, Y. Lactic acid bacteria. Qual. Assur. Saf. Crops Foods 2022, 14, 13–31. [Google Scholar] [CrossRef]
- De Souza, C.; Mousavi Khaneghah, A.; Oliveira, C.A.F. The occurrence of aflatoxin M1 in industrial and traditional fermented milk. Ital. J. Food Sci. 2021, 33, 12–23. [Google Scholar] [CrossRef]
- Heshmati, A.; Khorshidi, M.; Mousavi Khaneghah, A. The prevalence and risk assessment of aflatoxin in sesame based products. Ital. J. Food Sci. 2021, 33, 92–102. [Google Scholar] [CrossRef]
- Jafari, K.; Ebadi Fathabad, A.; Fakhri, Y.; Shamsaei, M.; Miri, M.; Farahmandfar, R.; Mousavi Khaneghah, A. Aflatoxin M1 in traditional and industrial pasteurized milk samples from Tiran County, Isfahan Province. Ital. J. Food Sci. 2021, 33, 103–116. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.; Silva, M.J.; Alvito, P. Challenges in risk assessment of multiple mycotoxins in food. World Mycotoxin J. 2016, 9, 791–811. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Yan, Z.; Tan, Y.; Zhu, R.; Yu, D.; Yang, H.; Wu, A. Occurrence and Quantitative Risk Assessment of Twelve Mycotoxins in Eggs and Chicken Tissues in China. Toxins 2018, 10, 477. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. Part A 2008, 25, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Juraske, R.; Castells, F.; Vijay, A.; Muñoz, P.; Antón, A. Uptake and persistence of pesticides in plants: Measurements and model estimates for imidacloprid after foliar and soil application. J. Hazard. Mater. 2009, 165, 683–689. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Wei, D.; Deng, Y.-J.; Feng, W.-Z.; Gao, Q.; Shi, Y.-H.; Xiao, J.-J. Bioaccessibility and health risk of neonicotinoids in apple and pear samples as affected by in vitro digestion. Qual. Assur. Saf. Crops Foods 2021, 13, 74–81. [Google Scholar] [CrossRef]
- Arora, S.; Mukherji, I.; Kumar, A.; Tanwar, R.K. Pesticide residue analysis of soil, water, and grain of IPM basmati rice. Environ. Monit. Assess. 2014, 186, 8765–8772. [Google Scholar] [CrossRef] [PubMed]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Mansour, S.A. Pesticide exposure—Egyptian scene. Toxicology 2004, 198, 91–115. [Google Scholar] [CrossRef]
- Mansour, S.A.; Belal, M.H.; Abou-Arab, A.A.K.; Gad, M.F. Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere 2009, 75, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Meng-Jen, T.; Mousavi Khaneghah, A. Emerging techniques in food science: The resistance of chlorpyrifos pesticide pollution against arc and dielectric barrier discharge plasma. Qual. Assur. Saf. Crops Foods 2020, 12, 9–17. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Y.; Jiao, H.; Hu, D.; Lu, P. Dissipation, residues analysis and risk assessment of metconazole in grapes under field conditions using gas chromatography–tandem mass spectrometry. Qual. Assur. Saf. Crops Foods 2021, 13, 84–97. [Google Scholar] [CrossRef]
- Heshmati, A.; Mehri, F.; Karami-Momtaz, J.; Mousavi Khaneghah, A. The concentration and health risk of potentially toxic elements in black and green tea—Both bagged and loose-leaf. Qual. Assur. Saf. Crops Foods 2020, 12, 140–150. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, C.; Zhang, Q.; Li, Y.; Chen, Z.; Li, M. Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk. Food Control 2010, 21, 1757–1763. [Google Scholar] [CrossRef]
- Gao, L.; Huang, X.; Wang, P.; Chen, Z.; Hao, Q.; Bai, S.; Tang, S.; Li, C.; Qin, D. Concentrations and health risk assessment of 24 residual heavy metals in Chinese mitten crab (Eriocheir sinensis). Qual. Assur. Saf. Crops Foods 2022, 14, 82–91. [Google Scholar] [CrossRef]
- Hu, L.; Wang, X.; Zou, Y.; Wu, D.; Gao, G.; Zhong, Z.; Liu, Y.; Hu, S.; Fan, H.; Zhang, B. Effects of inorganic and organic selenium intervention on resistance of radish to arsenic stress. Ital. J. Food Sci. 2022, 34, 44–58. [Google Scholar] [CrossRef]
- Bengtsson, G. Past, present and future exposures from natural elements. In Proceedings of the Global Symposium on Soil Pollution | FAO HQ, Rome, Italy, 2–4 May 2018. [Google Scholar]
- Zdrojewicz, Z.; Popowicz, E.; Winiarski, J. Nickel-role in human organism and toxic effects. Pol. Merkur. Lek. 2016, 41, 115–118. [Google Scholar]
- Tsatsakis, A.M.; Docea, A.O.; Tsitsimpikou, C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals’ low dose exposure. Food Chem. Toxicol. 2016, 96, 174–176. [Google Scholar] [CrossRef]
- Aydin, A.; Aksu, H.; Gunsen, U. Mycotoxin levels and incidence of mould in Turkish rice. Environ. Monit. Assess. 2011, 178, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, M.; Alsaleem, T.; Jeperel, H.; Alsamti, M.; Alowaifeer, A.M. Determination of inorganic arsenic, heavy metals, pesticides and mycotoxins in Indian rice (Oryza sativa) and a probabilistic dietary risk assessment for the population of Saudi Arabia. Regul. Toxicol. Pharmacol. 2021, 125, 104986. [Google Scholar] [CrossRef] [PubMed]
- Djahed, B.; Taghavi, M.; Farzadkia, M.; Norzaee, S.; Miri, M. Stochastic exposure and health risk assessment of rice contamination to the heavy metals in the market of Iranshahr, Iran. Food Chem. Toxicol. 2018, 115, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Abduljabbar, M. Heavy metals (lead, cadmium, methylmercury, arsenic) in commonly imported rice grains (Oryza sativa) sold in Saudi Arabia and their potential health risk. Int. J. Hyg. Environ. Health 2017, 220, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Jallad, K.N. Heavy metal exposure from ingesting rice and its related potential hazardous health risks to humans. Environ. Sci. Pollut. Res. 2015, 22, 15449–15458. [Google Scholar] [CrossRef]
- Li, L.; Feng, H.; Wei, J. Toxic element (As and Hg) content and health risk assessment of commercially available rice for residents in Beijing based on their dietary consumption. Environ. Sci. Pollut. Res. 2020, 27, 13205–13214. [Google Scholar] [CrossRef]
- Zazouli, M.A.; Bandpei, A.M.; Ebrahimi, M.; Izanloo, H. Investigation of Cadmium and Lead Contents in Iranian Rice Cultivated in Babol Region. Asian J. Chem. 2010, 22, 1369–1376. [Google Scholar]
- Sharma, S.; Nagpal, A.K.; Kaur, I. Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem. 2018, 255, 15–22. [Google Scholar] [CrossRef]
- Liu, L.; Han, J.; Xu, X.; Xu, Z.; Abeysinghe, K.S.; Atapattu, A.J.; De Silva, P.M.C.S.; Lu, Q.; Qiu, G. Dietary exposure assessment of cadmium, arsenic, and lead in market rice from Sri Lanka. Environ. Sci. Pollut. Res. 2020, 27, 42704–42712. [Google Scholar] [CrossRef]
- Praveena, S.M.; Omar, N.A. Heavy metal exposure from cooked rice grain ingestion and its potential health risks to humans from total and bioavailable forms analysis. Food Chem. 2017, 235, 203–211. [Google Scholar] [CrossRef]
- Kormoker, T.; Proshad, R.; Islam, M.S.; Tusher, T.R.; Uddin, M.; Khadka, S.; Chandra, K.; Sayeed, A. Presence of toxic metals in rice with human health hazards in Tangail district of Bangladesh. Int. J. Environ. Health Res. 2022, 32, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.M. Rice is a potential dietary source of not only arsenic but also other toxic elements like lead and chromium. Arab. J. Chem. 2017, 10, S3434–S3443. [Google Scholar] [CrossRef]
- Majeed, S.; De Boevre, M.; De Saeger, S.; Rauf, W.; Tawab, A.; Fazal-e-Habib; Rahman, M.; Iqbal, M. Multiple Mycotoxins in Rice: Occurrence and Health Risk Assessment in Children and Adults of Punjab, Pakistan. Toxins 2018, 10, 77. [Google Scholar] [CrossRef]
- Eslamizad, S.; Yazdanpanah, H.; Hadian, Z.; Tsitsimpikou, C.; Goumenou, M.; Shojaee AliAbadi, M.H.; Kamalabadi, M.; Tsatsakis, A. Exposure to multiple mycotoxins in domestic and imported rice commercially traded in Tehran and possible risk to public health. Toxicol. Rep. 2021, 8, 1856–1864. [Google Scholar] [CrossRef]
- Pan, H.Y. Research on Contamination and Toxicology of Zearalenone in Paddy Rice. Master’s Thesis, Wuhan University of Technology, Wuhan, China, 2012. [Google Scholar]
- Hamid, A.; Yaqub, G.; Ayub, M.; Naeem, M. Determination of malathion, chlorpyrifos, λ-cyhalothrin and arsenic in rice. Food Sci. Technol. 2021, 41, 461–466. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moattar, F.; Monavari, S.; Arjmandi, R. Human health risk assessment of organophosphorus pesticide in rice crop from selected districts of Anzali International Wetland basin, Iran. Hum. Exp. Toxicol. 2017, 36, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Monavari, S.M.; Kiani Sadr, M.; Rahimi, R.; Mirbolooki, H. Pesticide in Soil and Rice Crop from North of Iran: Concentration and Risk Assessment. Adv. J. Chem. A 2020, 3, 211–220. [Google Scholar] [CrossRef]
- López-Dávila, E.; Houbraken, M.; De Rop, J.; Claus, G.; Wumbei, A.; Romero Romero, O.; Spanoghe, P. Pesticide traces in local crops of Sancti Spíritus, Cuba: Risk assessment study. Int. J. Food Contam. 2021, 8, 1. [Google Scholar] [CrossRef]
- Javdan, G.; Ghaffari, H.R.; Nahidi, M.; Zeraei, N.; Hoseinvandtabar, S.; Ahmadi, M.; Pourramezani, F.; Heidarinejad, Z. Health risk assessment of heavy metals (Arsenic and Cadmium) in rice (Oryza sativa L.) brands imported to Iran: Using Monte Carlo simulation. Hormozgan Med. J. 2021, 25, 104–110. [Google Scholar] [CrossRef]
- Salehipour, M.; Ghorbani, H.; Kheirabadi, H.; Afyuni, M. Health Risks from Heavy Metals via Consumption of Cereals and Vegetables in Isfahan Province, Iran. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 1920–1935. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hadei, M.; Sharafi, K. Human health risk assessment by Monte Carlo simulation method for heavy metals of commonly consumed cereals in Iran- Uncertainty and sensitivity analysis. J. Food Compos. Anal. 2021, 96, 103697. [Google Scholar] [CrossRef]
- ISIRI. Rice–Specifications and Test Methods; ISIRI: Tehran, Iran, 2021.
- AOAC. Determination of Moisture, Ash, Protein and Fat. Official Method of Analysis of the Association of Analytical Chemists; AOAC: Washington, DC, USA, 2005; pp. 141–144. [Google Scholar]
- Ho, C.-T.; Lin, J.-K.; Shahidi, F. (Eds.) Tea and Tea Products; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9780429117831. [Google Scholar]
- Miguéis, S.; Santos, C.; Saraiva, C.; Esteves, A. Evaluation of ready to eat sashimi in northern Portugal restaurants. Food Control 2015, 47, 32–36. [Google Scholar] [CrossRef]
- ISIRI. Fruits, Vegetables and Drive Products-Determination of Arsenic Content-Method Using Hydride Generation Atomic Absorption Spectrometry; ISIRI: Tehran, Iran, 2002.
- Jorhem, L.; Afthan, G.; Cumont, G.; Dypdahl, H.P.; Gadd, K.; Havre, G.N.; Julshamn, K.; Kåverud, K.; Lind, B.; Loimaranta, J.; et al. Determination of metals in foods by atomic absorption spectrometry after dry ashing: NMKL1 collaborative study. J. AOAC Int. 2000, 83, 1204–1211. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, H.; Zarghi, A.; Shafaati, A.R.; Foroutan, S.M.; Aboul-Fathi, F.; Khoddam, A.; Nazari, F.; Shaki, F. Analysis of aflatoxin b1 in Iranian foods using HPLC and a monolithic column and estimation of its dietary intake. Iran. J. Pharm. Res. IJPR 2013, 12, 83–89. [Google Scholar] [PubMed]
- ISIRI 6872; Food and Feed Stuffs-Determination of Aflatoxins B and G by HPLC Method Using Immunoaffinity Column Clean up-Test Method. ISIRI: Tehran, Iran, 2004.
- ISIRI 9238; Cereal and Cereal’s Products—Determination of Ochratoxin A by HPLC Method and Immunoaffinity Column Clean up-Test Method. ISIRI: Tehran, Iran, 2014.
- ISIRI 9239; Foodstuffs-Cereal and Cerealís Products-Determination of Zearalenon by HPLC Method and Immunoaffinity Column Clean up-Test Method. ISIRI: Tehran, Iran, 2012.
- ISIRI 10215; Cereal and Cereal Products ñ Determination of Deoxynivalenole by HPLC Method and DONSPE Column Clean up-Test Method. ISIRI: Tehran, Iran, 2012.
- United States Environmental Protection Agency. Risk Assessment Guidance for Superfund: Volume III Part A, Process for Conducting Probabilistic Risk Assessment; US Environmental Protection Agency: Washington, DC, USA, 2022.
- ISIRI. Food and Feed-Maximum Limit of Heavy Metals; ISIRI: Tehran, Iran, 2010.
- Nikooyeh, B.; Abdollahi, Z.; Salehi, F.; Nourisaeidlou, S.; Hajifaraji, M.; Zahedirad, M.; Shariatzadeh, N.; Kalayi, A.; Babaei balderlou, F.; Gholizadeh salmasi, J.; et al. Prevalence of Obesity and Overweight and Its Associated Factors in Urban Adults from West Azerbaijan, Iran: The National Food and Nutritional Surveillance Program (NFNSP). Nutr. Food Sci. Res. 2016, 3, 21–26. [Google Scholar] [CrossRef]
- Saha, N.; Zaman, M.R. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 2013, 185, 3867–3878. [Google Scholar] [CrossRef]
- Yang, S.; Lin, J.; Lin, W.; Chang, C. Cancer risk assessment for occupational exposure to chromium and nickel in welding fumes from pipeline construction, pressure container manufacturing, and shipyard building in Taiwan. J. Occup. Health 2018, 60, 515–524. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, W.; Li, M.; Huang, R.; Yang, F.; Duan, Y. Heavy Metal Contamination in Rice-Producing Soils of Hunan Province, China and Potential Health Risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. [Google Scholar] [CrossRef]
- Minnesota Department of Health MDH Health Risk Assessment Methods to Incorporate Human Equivalent Dose Calculations into Derivation of Oral Reference Doses. Available online: http://www.health.state.mn.us/divs/eh/risk/guidance/hedrefguide.pdf (accessed on 20 January 2017).
| Parameter | Pakistan | India | Thailand | Iran |
|---|---|---|---|---|
| Moisture (%) | 8.801 ± 0.288 b | 10.691 ± 0.286 a | 9.893 ± 0.458 ab | 9.273 ± 0.411 b |
| Ash (%) | 0.788 ± 0.322 a | 0.58 ± 0.025 b | 0.662 ± 0.049 b | 0.563 ± 0.036 b |
| Mold (log CFU/g) | 2.94 ± 0.34 d | 4.11 ± 0.58 a | 3.6 ± 0.48 b | 3.21 ± 0.41 c |
| Heavy Metal | Limit of National Standard (mg kg−1) | Pakistan | India | Thailand | Iran |
|---|---|---|---|---|---|
| As | 0.15 | 0.045 ± 0.019 b | 0.074 ± 0.014 ab | 0.098 ± 0.014 a | 0.106 ± 0.020 a |
| Pb | 0.15 | 0.30 ± 0.08 a | 0.24 ± 0.06 ab | 0.11 ± 0.03 b | 0.12 ± 0.04 b |
| Cd | 0.06 | 0.067 ± 0.007 b | 0.095 ± 0.011 a | 0.078 ± 0.006 ab | 0.037 ± 0.007 c |
| Ni | - | 0.650 ± 0.054 a | 0.320 ± 0.035 c | 0.53 ± 0.03 b | 0.19 ± 0.039 d |
| Mycotoxins | Pakistan | India | Thailand | Iran | Limit of National Standard (µg kg−1) |
|---|---|---|---|---|---|
| Aflatoxin B1 (AFB1) (µg kg−1) | 3.3 ± 0.04 a | <LOD | 2.4 ± 0.01 b | <LOD | 5 |
| Zearalenone (ZEN) (µg kg−1) | <LOD | 8.8 ± 0. 44 b | 10.3 ± 0.25 ab | 11.8 ± 0.32 a | 200 |
| Ochratoxin A (OTA) (µg kg−1) | <LOD | <LOD | <LOD | <LOD | 5 |
| Deoxynivalenol (DON) (µg kg−1) | 44 ± 0.88 b | 55 ± 1.7 a | 42 ± 2.54 b | 38 ± 2.33 c | 1000 |
| Pesticides | Limit of National Standard (mg kg−1) | Pakistan | India | Thailand | Iran |
|---|---|---|---|---|---|
| Chlorpyrifos | 0.1 | 0.056 ± 0.08 a | nd | nd | nd |
| Trichlorfon | 0.1 | nd | nd | nd | 0.048 ± 0.05 a |
| Diazinon | 0.1 | 0.031 ± 0.03 a | 0.022 ± 0.01 b | nd | nd |
| Fenitrothion | 0.05 | nd | nd | nd | 0.027 ± 0.006 a |
| Chlorothalonil | 0.05 | 0.044 ± 0.005 a | nd | nd | nd |
| Rice Brands and Pollutants | EDInon-carcinogenic (mg kg−1 day−1) | HQ | EDIcarcinogenic (mg kg−1 day−1) | ILCR | |
|---|---|---|---|---|---|
| Pakistani | Pb | 6.4 × 10−4 | 1.6 × 10−1 | 4.9 × 10−4 | 1.4 × 10−4left |
| As | 9.6 × 10−5 | 3.2 × 10−1 | 7.4 × 10−5 | 1.1 × 10−4 | |
| Cd | 1.4 × 10−4 | 1.4 × 10−1 | 1.1 × 10−4 | 4.2 × 10−5 | |
| Ni | 1.4 × 10−3 | 6.9 × 10−2 | 1.1 × 10−3 | 9.7 × 10−4 | |
| Chlorothalonil | 9.4 × 10−5 | 6.2 × 10−3 | 7.2 × 10−5 | 5.5 × 10−7 | |
| Diazinon | 6.6 × 10−5 | 1.4 | |||
| Chlorpyrifos | 1.2 × 10−4 | 3.3 × 10−2 | |||
| Trichlorfon | |||||
| Fenitrothion | |||||
| AFB1 | 7.03 × 10−6 | ||||
| ZEN | |||||
| OTA | |||||
| DON | 9.4 × 10−5 | ||||
| HIPakistani | 2.1 | ||||
| Indian | Pb | 5.1 × 10−4 | 1.2 × 10−1 | 3.9 × 10−4 | 1.1 × 10−4 |
| As | 1.6 × 10−4 | 5.3 × 10−1 | 1.2 × 10−4 | 1.8 × 10−4 | |
| Cd | 0.2 × 10−3 | 2.0 × 10−1 | 1.6 × 10−4 | 5.9 × 10−5 | |
| Ni | 6.8 × 10−4 | 3.0 × 10−2 | 5.3 × 10−4 | 4.8 × 10−5 | |
| Chlorothalonil | |||||
| Diazinon | 4.7 × 10−5 | 9.7 × 10−1 | |||
| Chlorpyrifos | |||||
| Trichlorfon | |||||
| Fenitrothion | |||||
| AFB1 | |||||
| ZEN | 1.9 × 10−5 | ||||
| OTA | |||||
| DON | 1.2 × 10−5 | ||||
| HIIndian | 1.86 | ||||
| Thai | Pb | 2.4 × 10−4 | 6.0 × 10−2 | 1.8 × 10−4 | 5.1 × 10−5 |
| As | 2.1 × 10−4 | 7.0 × 10−1 | 1.6 × 10−4 | 2.4 × 10−4 | |
| Cd | 1.6 × 10−4 | 1.7 × 10−1 | 1.3 × 10−4 | 4.9 × 10−5 | |
| Ni | 1.1 × 10−3 | 6.0 × 10−2 | 8.7 × 10−4 | 7.9 × 10−4 | |
| Chlorothalonil | |||||
| Diazinon | |||||
| Chlorpyrifos | |||||
| Trichlorfon | |||||
| Fenitrothion | |||||
| AFB1 | 5.1 × 10−6 | ||||
| ZEN | 2.2 × 10−5 | ||||
| OTA | |||||
| DON | 8.9 × 10−5 | ||||
| HIThai | 0.98 | ||||
| Iranian | Pb | 2.6 × 10−4 | 6.0 × 10−2 | 1.9 × 10−4 | 5.5 × 10−5 |
| As | 2.3 × 10−4 | 7.5 × 10−1 | 1.7 × 10−4 | 2.6 × 10−4 | |
| Cd | 7.9 × 10−5 | 8.0 × 10−2 | 6.1 × 10−5 | 2.3 × 10−5 | |
| Ni | 4.1 × 10−4 | 2.0 × 10−2 | 3.1 × 10−4 | 2.8 × 10−4 | |
| Chlorothalonil | |||||
| Diazinon | |||||
| Chlorpyrifos | |||||
| Trichlorfon | 1.02 × 10−4 | 5.0 × 10−2 | |||
| Fenitrothion | 5.7 × 10−5 | 4.0 × 10−2 | |||
| AFB1 | |||||
| ZEN | 2.5 × 10−5 | ||||
| OTA | |||||
| DON | 1.8 × 10−5 | ||||
| HIIranian | 1.01 | ||||
| Component | Wavelength (nm) | Sensitivity (mg/L) | LOQ | LOD | Recovery (%) | R2 |
|---|---|---|---|---|---|---|
| As | 193.7 | 0.40 | 0.032 (mg kg−1) | 0.012 (mg kg−1) | 91 | 0.93 |
| Pb | 283.3 | 0.18 | 0.055 | 0.022 | 87 | 0.98 |
| Cd | 228.8 | 0.012 | 0.01 | 0.008 | 93 | 0.97 |
| Ni | 232 | 0.08 | 0.06 | 0.015 | 89 | 0.95 |
| Chlorpyrifos | 0.2 (mg kg−1) | 0.01 (mg kg−1) | 91 | |||
| Trichlorfon | 0.2 | 0.01 | 84 | |||
| Diazinon | 0.3 | 0.02 | 88 | |||
| Fenitrothion | 0.2 | 0.01 | 87 | |||
| Chlorothalonil | 0.1 | 0.02 | 89 | |||
| AFB1 | 0.85 (µg kg−1) | 0.2 (µg kg−1) | 88.6 | 0.993 | ||
| ZEN | 13 | 8.5 | 90.3 | 0.994 | ||
| OTA | 0.65 | 0.3 | 81 | 0.992 | ||
| DON | 34.8 | 22.44 | 84 | 0.991 |
| Contaminants | Reference Dose (RfD) (mg kg−1 day−1) | Cancer Slope Factor (CSF) (mg kg−1 day−1) |
|---|---|---|
| Pb | 4 × 10−3 | 2.8 × 10−1 |
| As | 3 × 10−4 | 1.5 |
| Cd | 1 × 10−3 | 3.8 × 10−1 |
| Ni | 2 × 10−2 | 9.1 × 10−1 |
| Chlorothalonil | 1.5 × 10−2 | 7.66 × 10−3 |
| Diazinon | 4.8 × 10−5 | |
| Chlorpyrifos | 3.6 × 10−3 | |
| Trichlorfon | 2 × 10−3 | |
| Fenitrothion | 1.3 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yousefi, M.H.; Abbasi, E.; Hadidi, M.; Hashemi, S.; Ghadimi, A.H.; Yousefinejad, S.; Arfaeinia, H.; Yousefinejad, A.; Kowalczewski, P.Ł.; Tomkowiak, A.; et al. Simultaneous Analysis of Mycotoxins, Potentially Toxic Elements, and Pesticides in Rice: A Health Risk Assessment Study. Toxins 2023, 15, 102. https://doi.org/10.3390/toxins15020102
Yousefi MH, Abbasi E, Hadidi M, Hashemi S, Ghadimi AH, Yousefinejad S, Arfaeinia H, Yousefinejad A, Kowalczewski PŁ, Tomkowiak A, et al. Simultaneous Analysis of Mycotoxins, Potentially Toxic Elements, and Pesticides in Rice: A Health Risk Assessment Study. Toxins. 2023; 15(2):102. https://doi.org/10.3390/toxins15020102
Chicago/Turabian StyleYousefi, Mohammad Hashem, Esmaeel Abbasi, Milad Hadidi, Seyedenayat Hashemi, Amir Hossein Ghadimi, Saeed Yousefinejad, Hossein Arfaeinia, Abbas Yousefinejad, Przemysław Łukasz Kowalczewski, Agnieszka Tomkowiak, and et al. 2023. "Simultaneous Analysis of Mycotoxins, Potentially Toxic Elements, and Pesticides in Rice: A Health Risk Assessment Study" Toxins 15, no. 2: 102. https://doi.org/10.3390/toxins15020102
APA StyleYousefi, M. H., Abbasi, E., Hadidi, M., Hashemi, S., Ghadimi, A. H., Yousefinejad, S., Arfaeinia, H., Yousefinejad, A., Kowalczewski, P. Ł., Tomkowiak, A., Hosseinzadeh, S., & Mousavi Khaneghah, A. (2023). Simultaneous Analysis of Mycotoxins, Potentially Toxic Elements, and Pesticides in Rice: A Health Risk Assessment Study. Toxins, 15(2), 102. https://doi.org/10.3390/toxins15020102