Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine
Abstract
:1. Introduction
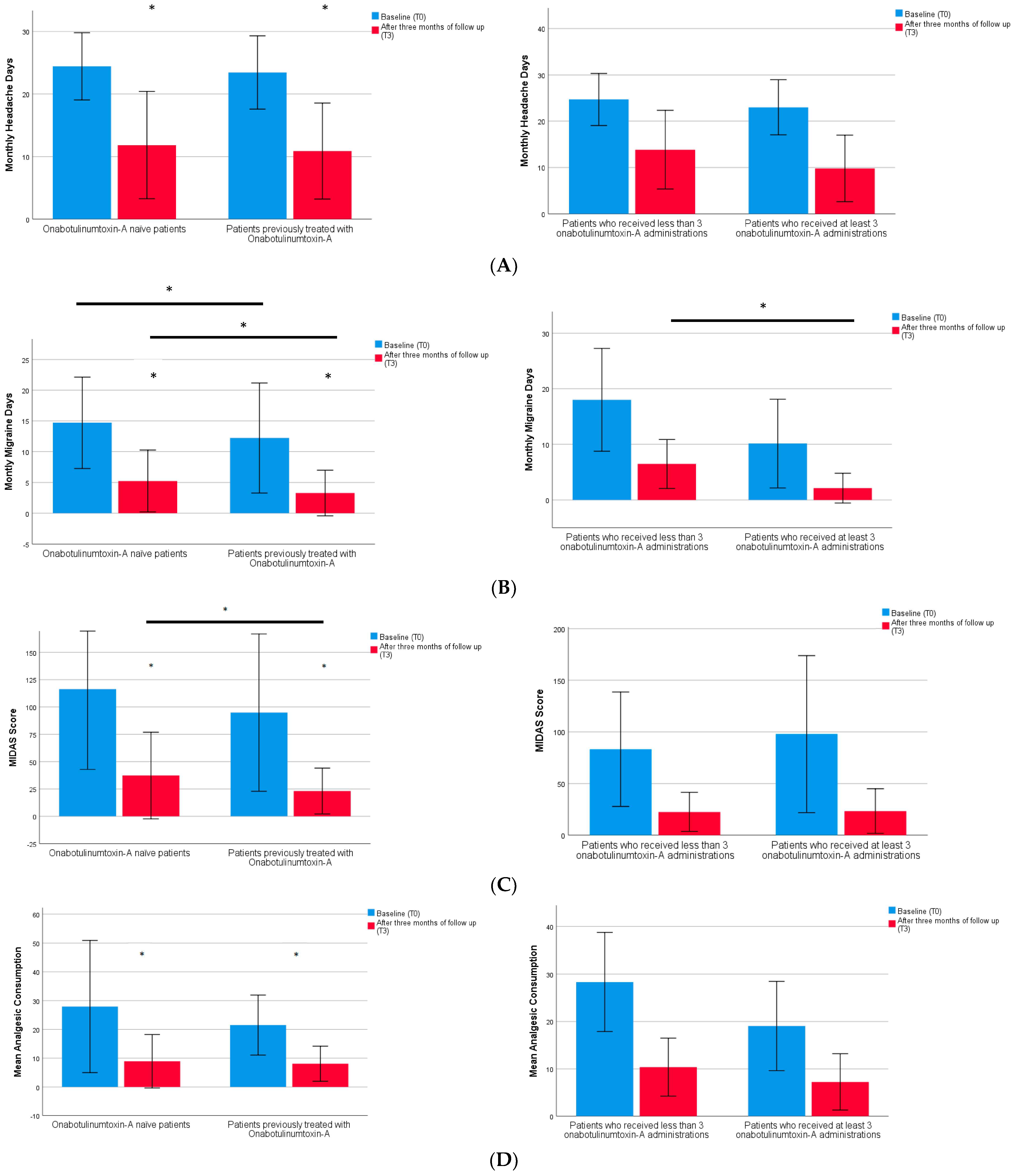
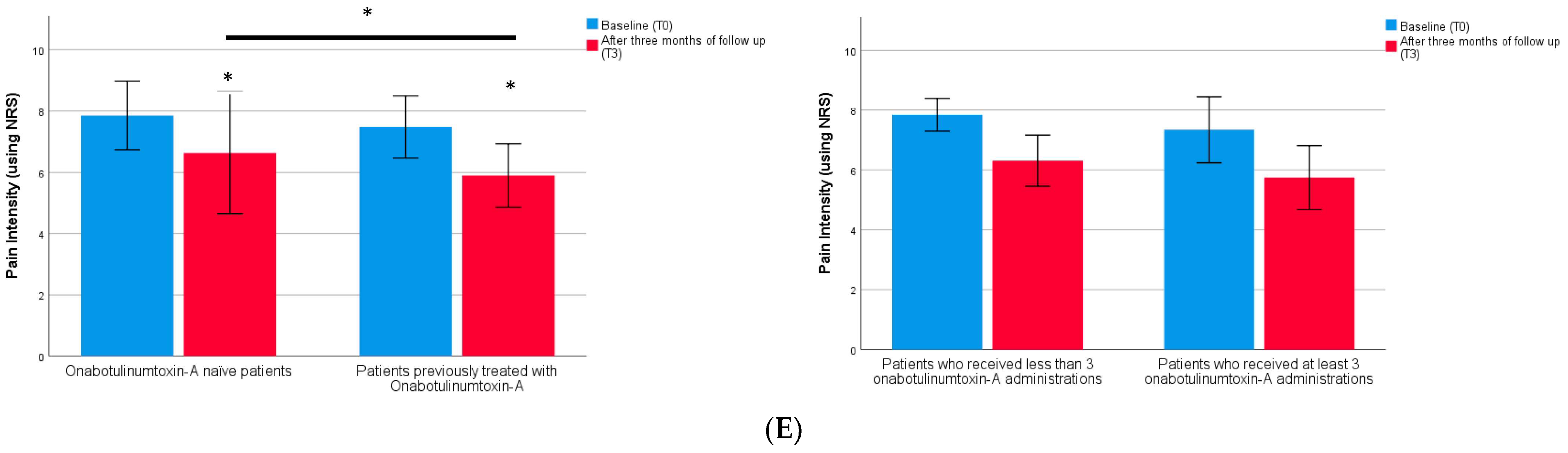
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Standard Protocol Approvals and Patient Consents
5.2. Study Design and Participants
5.3. Outcome Measures
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.; Dodick, D.W. Migraine. Nat. Rev. Dis. Primers 2022, 8, 2. [Google Scholar] [CrossRef]
- Mungoven, T.J.; Henderson, L.A.; Meylakh, N. Chronic Migraine Pathophysiology and Treatment: A Review of Current Perspectives. Front. Pain Res. 2021, 2, 705276. [Google Scholar] [CrossRef]
- Burstein, R.; Zhang, X.; Levy, D.; Aoki, K.R.; Brin, M.F. Selective inhibition of meningeal nociceptors by botulinum neurotoxin type A: Therapeutic implications for migraine and other pains. Cephalalgia 2014, 34, 853–869. [Google Scholar] [CrossRef]
- Bendtsen, L.; Sacco, S.; Ashina, M.; Mitsikostas, D.; Ahmed, F.; Pozo-Rosich, P.; Martelletti, P. Guideline on the use of onabotulinumtoxinA in chronic migraine: A consensus statement from the European Headache Federation. J. Headache Pain 2018, 19, 91. [Google Scholar] [CrossRef]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Manack Adams, A.; Brin, M.F. Mechanism of Action of OnabotulinumtoxinA in Chronic Migraine: A Narrative Review. Headache 2020, 60, 1259–1272. [Google Scholar] [CrossRef]
- Durham, P.L.; Cady, R.; Cady, R. Regulation of calcitonin gene-related peptide secretion from trigeminal nerve cells by botulinum toxin type A: Implications for migraine therapy. Headache 2004, 44, 35–42. [Google Scholar] [CrossRef]
- Shimizu, T.; Shibata, M.; Toriumi, H.; Iwashita, T.; Funakubo, M.; Sato, H.; Kuroi, T.; Ebine, T.; Koizumi, K.; Suzuki, N. Reduction of TRPV1 expression in the trigeminal system by botulinum neurotoxin type-A. Neurobiol. Dis. 2012, 48, 367–378. [Google Scholar] [CrossRef]
- Melo-Carrillo, A.; Strassman, A.M.; Schain, A.J.; Noseda, R.; Ashina, S.; Adams, A.; Brin, M.F.; Burstein, R. Exploring the effects of extracranial injections of botulinum toxin type A on prolonged intracranial meningeal nociceptors responses to cortical spreading depression in female rats. Cephalalgia 2019, 39, 1358–1365. [Google Scholar] [CrossRef]
- Sacco, S.; Amin, F.M.; Ashina, M.; Bendtsen, L.; Deligianni, C.I.; Gil-Gouveia, R.; Katsarava, Z.; MaassenVanDenBrink, A.; Martelletti, P.; Mitsikostas, D.D.; et al. European Headache Federation guideline on the use of monoclonal antibodies targeting the calcitonin gene related peptide pathway for migraine prevention—2022 update. J. Headache Pain 2022, 23, 67. [Google Scholar] [CrossRef]
- Croop, R.; Lipton, R.B.; Kudrow, D.; Stock, D.A.; Kamen, L.; Conway, C.M.; Stock, E.G.; Coric, V.; Goadsby, P.J. Oral rimegepant for preventive treatment of migraine: A phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet 2021, 397, 51–60. [Google Scholar] [CrossRef]
- Pozo-Rosich, P.; Ailani, J.; Ashina, M.; Goadsby, P.J.; Lipton, R.B.; Reuter, U.; Guo, H.; Schwefel, B.; Lu, K.; Boinpally, R.; et al. Atogepant for the preventive treatment of chronic migraine (PROGRESS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 402, 775–785. [Google Scholar] [CrossRef]
- Melo-Carrillo, A.; Strassman, A.M.; Nir, R.R.; Schain, A.J.; Noseda, R.; Stratton, J.; Burstein, R. Fremanezumab-A Humanized Monoclonal Anti-CGRP Antibody-Inhibits Thinly Myelinated (Aδ) But Not Unmyelinated (C) Meningeal Nociceptors. J. Neurosci. 2017, 37, 10587–10596. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef]
- Tepper, S.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D.; Lenz, R. Safety and efficacy of erenumab for preventive treatment of chronic migraine: A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017, 16, 425–434. [Google Scholar] [CrossRef]
- Dodick, D.W.; Lipton, R.B.; Silberstein, S.; Goadsby, P.J.; Biondi, D.; Hirman, J.; Cady, R.; Smith, J. Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia 2019, 39, 1075–1085. [Google Scholar] [CrossRef]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in chronic migraine: The randomized, double-blind, placebo-controlled REGAIN study. Neurology 2018, 91, e2211–e2221. [Google Scholar] [CrossRef]
- Barbanti, P.; Egeo, G.; Aurilia, C.; d’Onofrio, F.; Albanese, M.; Cetta, I.; Di Fiore, P.; Zucco, M.; Filippi, M.; Bono, F.; et al. Fremanezumab in the prevention of high-frequency episodic and chronic migraine: A 12-week, multicenter, real-life, cohort study (the FRIEND study). J. Headache Pain 2022, 23, 46. [Google Scholar] [CrossRef]
- Lipton, R.B.; Goadsby, P.J.; Smith, J.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Cady, R. Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. Neurology 2020, 94, e1365–e1377. [Google Scholar] [CrossRef]
- Schiano di Cola, F.; Caratozzolo, S.; Bolchini, M.; Ceccardi, G.; Cortinovis, M.; Liberini, P.; Padovani, A.; Rao, R. CGRP-monoclonal antibodies in difficult-to-treat chronic migraine patients. Neurol. Sci. 2022, 43, 5763–5764. [Google Scholar] [CrossRef]
- Scuteri, D.; Tonin, P.; Nicotera, P.; Vulnera, M.; Altieri, G.C.; Tarsitano, A.; Bagetta, G.; Corasaniti, M.T. Pooled Analysis of Real-World Evidence Supports Anti-CGRP mAbs and OnabotulinumtoxinA Combined Trial in Chronic Migraine. Toxins 2022, 14, 529. [Google Scholar] [CrossRef]
- Vernieri, F.; Brunelli, N.; Marcosano, M.; Aurilia, C.; Egeo, G.; Lovati, C.; Favoni, V.; Perrotta, A.; Maestrini, I.; Rao, R.; et al. Maintenance of response and predictive factors of 1-year GalcanezumAb treatment in real-life migraine patients in Italy: The multicenter prospective cohort GARLIT study. Eur. J. Neurol. 2023, 30, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Aicua-Rapun, I.; Martínez-Velasco, E.; Rojo, A.; Hernando, A.; Ruiz, M.; Carreres, A.; Porqueres, E.; Herrero, S.; Iglesias, F.; Guerrero, A.L. Real-life data in 115 chronic migraine patients treated with Onabotulinumtoxin A during more than one year. J. Headache Pain 2016, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Corbelli, I.; Verzina, A.; Leone De Magistris, I.; De Vanna, G.; Eusebi, P.; Mataluni, G.; Pisani, A.; Prudenzano, A.M.P.; Trojano, M.; Delussi, M.; et al. Sustained Efficacy, Safety and High Adherence Rate of Onabotulinum Toxin Type A in Chronic Migraine Patients: A Multicentric Prospective Real-Life Study. Toxins 2022, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Guerzoni, S.; Baraldi, C.; Pani, L. The association between onabotulinumtoxinA and anti-CGRP monoclonal antibodies: A reliable option for the optimal treatment of chronic migraine. Neurol. Sci. 2022, 43, 5687–5695. [Google Scholar] [CrossRef] [PubMed]
- Silvestro, M.; Tessitore, A.; Scotto di Clemente, F.; Battista, G.; Tedeschi, G.; Russo, A. Additive Interaction Between Onabotulinumtoxin-A and Erenumab in Patients With Refractory Migraine. Front. Neurol. 2021, 12, 656294. [Google Scholar] [CrossRef] [PubMed]
- Cohen, F.; Armand, C.; Lipton, R.B.; Vollbracht, S. Efficacy and Tolerability of Calcitonin Gene-Related Peptide-Targeted Monoclonal Antibody Medications as Add-on Therapy to OnabotulinumtoxinA in Patients with Chronic Migraine. Pain Med. 2021, 22, 1857–1863. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Dermitzakis, E.V.; Xiromerisiou, G.; Vikelis, M. OnabotulinumtoxinA Add-On to Monoclonal Anti-CGRP Antibodies in Treatment-Refractory Chronic Migraine. Toxins 2022, 14, 847. [Google Scholar] [CrossRef]
- Pellesi, L.; Do, T.P.; Ashina, H.; Ashina, M.; Burstein, R. Dual Therapy with Anti-CGRP Monoclonal Antibodies and Botulinum Toxin for Migraine Prevention: Is There a Rationale? Headache 2020, 60, 1056–1065. [Google Scholar] [CrossRef]
- Armanious, M.; Khalil, N.; Lu, Y.; Jimenez-Sanders, R. Erenumab and OnabotulinumtoxinA Combination Therapy for the Prevention of Intractable Chronic Migraine without Aura: A Retrospective Analysis. J. Pain Palliat. Care Pharmacother. 2021, 35, 1–6. [Google Scholar] [CrossRef]
- Blumenfeld, A.M.; Frishberg, B.M.; Schim, J.D.; Iannone, A.; Schneider, G.; Yedigarova, L.; Manack Adams, A. Real-World Evidence for Control of Chronic Migraine Patients Receiving CGRP Monoclonal Antibody Therapy Added to OnabotulinumtoxinA: A Retrospective Chart Review. Pain Ther. 2021, 10, 809–826. [Google Scholar] [CrossRef]
- Mechtler, L.; Saikali, N.; McVige, J.; Hughes, O.; Traut, A.; Adams, A.M. Real-World Evidence for the Safety and Efficacy of CGRP Monoclonal Antibody Therapy Added to OnabotulinumtoxinA Treatment for Migraine Prevention in Adult Patients With Chronic Migraine. Front. Neurol. 2021, 12, 788159. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [CrossRef]
| Demographic and Clinical Characteristics | Entire Sample (n 128) | Patients Previously Treated with Onabotulinumtoxin-A (n 51) | Onabotulinumtoxin-A Naïve Patients (n 77) | p Value |
|---|---|---|---|---|
| Gender (female) | 108 (84.4) | 43 (84.3) | 65 (84.4) | 0.988 |
| Age | 44.9 (10.9) | 46.3 (10.9) | 44.0 (10.9) | 0.866 |
| Body mass index | 22.6 (3.9) | 22.4 (3.4) | 22.7 (4.2) | 0.168 |
| Years of migraine | 27.1 (10.8) | 27.70 (10.9) | 26.8 (10.7) | 0.649 |
| Years of chronic migraine | 9.2 (6.7) | 9.4 (5.8) | 9.1 (7.4) | 0.102 |
| Number of analgesic overusers | 98 (76.6) | 38 (74.5) | 60 (77.9) | 0.559 |
| Number of triptan responders | 84 (65.6) | 38 (74.5) | 46 (59.7) | 0.147 |
| Presence of allodynia | 66 (51.6) | 32 (62.7) | 34 (44.2) | 0.026 * |
| Presence of associated symptoms | 104 (81.3) | 38 (74.5) | 66 (85.7) | 0.030 * |
| Anti-CGRP monoclonal antibody | 0.085 | |||
| Erenumab | 55 (43.0) | 16 (31.4) | 39 (50.6) | |
| Fremanezumab | 23 (18.0) | 10 (19.6) | 13 (16.9) | |
| Galcanezumab | 50 (39.0) | 25 (49.0) | 25 (32.5) | |
| Number of previous prophylactic treatments | 3 (0.9) | 3.6 (0.9) | 2.8 (0.9) | 0.470 |
| Monthly headache days | 24.0 (5.6) | 23.3 (6.1) | 23.9 (5.5) | 0.162 |
| Monthly migraine days | 13.6 (8.2) | 12.6 (9.1) | 14.8 (7.2) | 0.023 * |
| Analgesic consumption | 25.0 (18.6) | 22.7 (14.1) | 26.3 (21.4) | 0.089 |
| Pain intensity (using NRS) | 7.7 (1.1) | 7.5 (1.0) | 7.9 (1.1) | 0.793 |
| MIDAS score | 106.0 (73.1) | 102.5 (82.5) | 113.3 (71.6) | 0.458 |
| Patients Previously Treated with Onabotulinumtoxin-A (n 51) | Onabotulinumtoxin-A Naïve Patients (n 77) | p Value | |
|---|---|---|---|
| Monthly headache days (MHDs) T3 | 10.9 (7.7) | 11.4 (8.6) | 0.451 |
| Monthly migraine days (MMDs) T3 | 3.3 (3.7) | 5.2 (5.0) | 0.017 * |
| Pain intensity (using NRS) T3 | 5.9 (1.0) | 6.6 (2.0) | 0.013 * |
| Mean analgesic consumption T3 | 8.1 (6.1) | 8.9 (9.3) | 0.105 |
| MIDAS score T3 | 23.3 (21.0) | 37.4 (39.6) | 0.013 * |
| Patients Who Received at Least 3 Onabotulinumtoxin-A Administrations (n 37) | Patients Who Received Less than 3 Onabotulinumtoxin-A Administrations (n 14) | p Value | |
|---|---|---|---|
| Monthly headache days (MHDs) | 9.8 (7.2) | 13.6 (8.5) | 0.468 |
| Monthly migraine days (MMDs) | 2.1 (2.7) | 6.5 (4.4) | 0.024 * |
| Pain intensity (using NRS) | 5.7 (1.1) | 6.3 (0.9) | 0.197 |
| Mean analgesic consumption | 7.2 (6.0) | 10.4 (6.1) | 0.995 |
| MIDAS score | 23.4 (21.7) | 22.6 (19.0) | 0.467 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccardi, G.; Schiano di Cola, F.; Caratozzolo, S.; Di Pasquale, M.; Bolchini, M.; Padovani, A.; Rao, R. Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine. Toxins 2023, 15, 677. https://doi.org/10.3390/toxins15120677
Ceccardi G, Schiano di Cola F, Caratozzolo S, Di Pasquale M, Bolchini M, Padovani A, Rao R. Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine. Toxins. 2023; 15(12):677. https://doi.org/10.3390/toxins15120677
Chicago/Turabian StyleCeccardi, Giulia, Francesca Schiano di Cola, Salvatore Caratozzolo, Michele Di Pasquale, Marco Bolchini, Alessandro Padovani, and Renata Rao. 2023. "Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine" Toxins 15, no. 12: 677. https://doi.org/10.3390/toxins15120677
APA StyleCeccardi, G., Schiano di Cola, F., Caratozzolo, S., Di Pasquale, M., Bolchini, M., Padovani, A., & Rao, R. (2023). Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine. Toxins, 15(12), 677. https://doi.org/10.3390/toxins15120677




