Knowledge about Snake Venoms and Toxins from Colombia: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
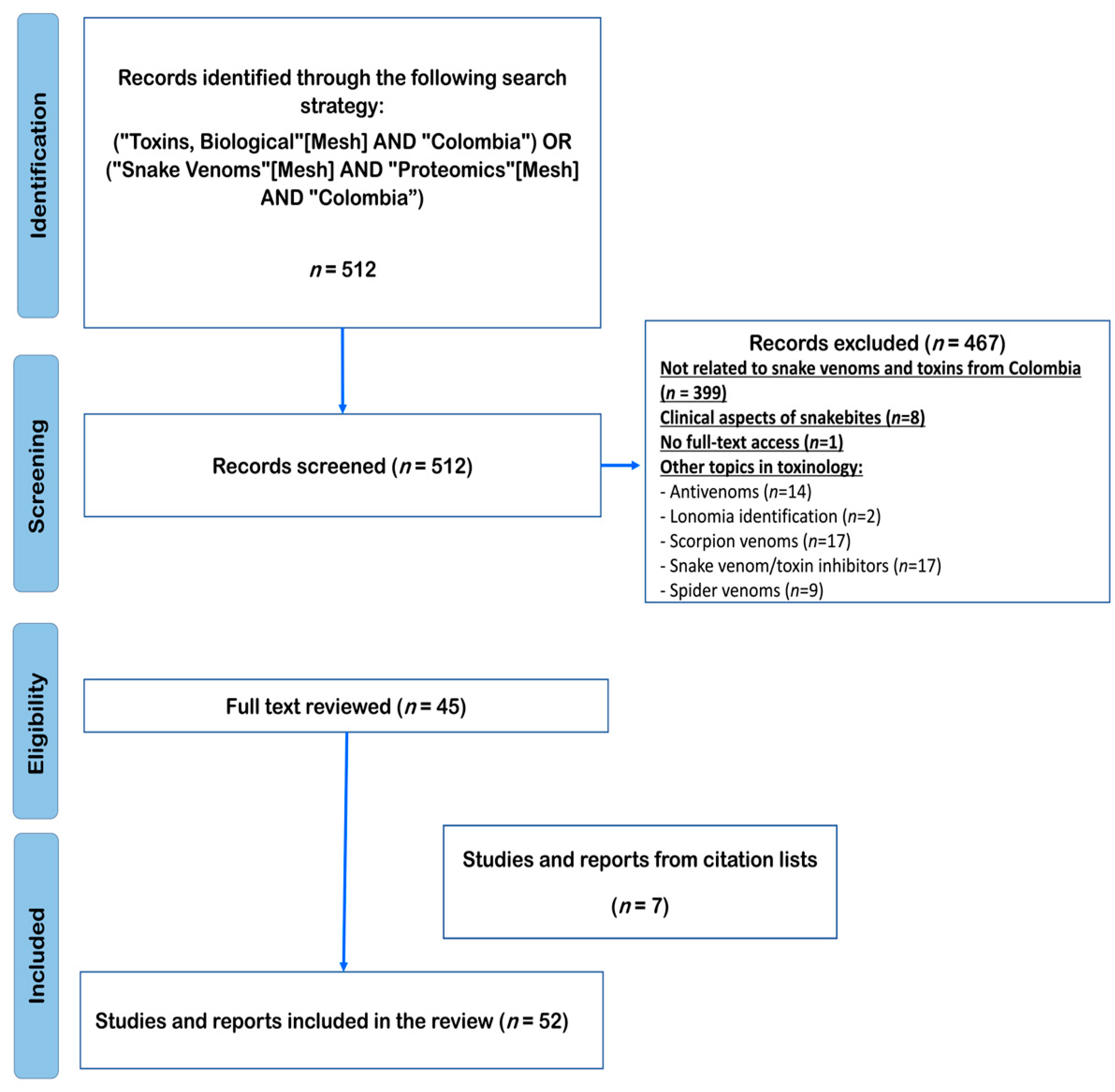
2.1. Eligibility Criteria, Search Strategies, and Information Sources
2.2. Study Selection
2.3. Data Collection Process and Data Items
2.4. Graphics
3. Results and Discussion
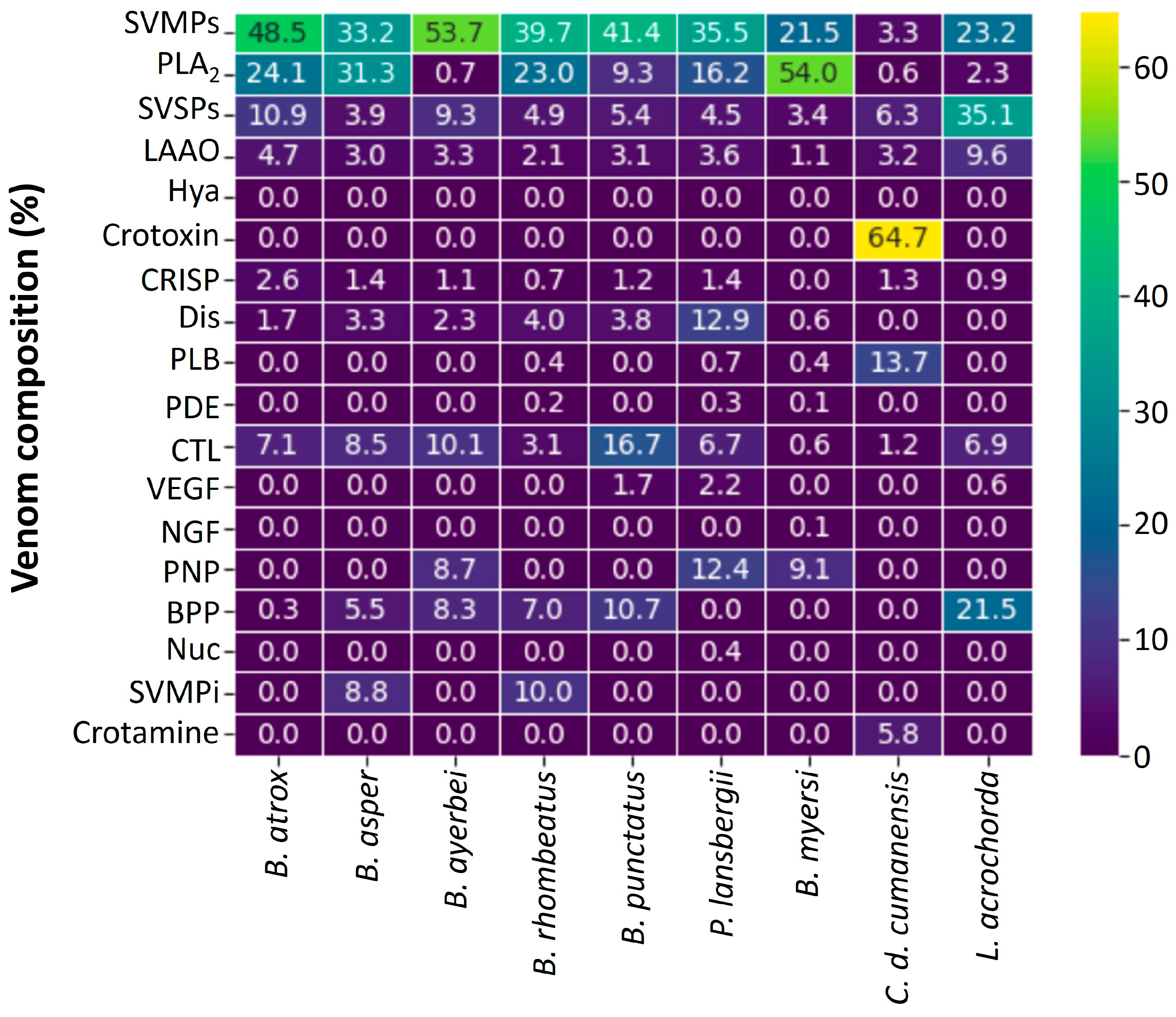
3.1. Proteomic Profiles of Snake Venoms from Colombia
3.2. Biological Activities of Viperidae and Elapidae Venoms from Colombia
3.3. Comments on Specific Genus/Species
3.3.1. Bothrops spp.
3.3.2. Lachesis acrochorda
3.3.3. Crotalus durissus cumanensis
3.3.4. Bothriechis schelegelii
3.3.5. Porthidium spp.
3.3.6. Micrurus spp.
3.4. Colubrid Venoms
3.5. Potential Applications of Colombian Snake Venoms
3.5.1. Antibacterial Activity
3.5.2. Antiplasmodial Activity
3.5.3. Cytotoxicity on Tumoral Cell Lines
3.5.4. Distribution of the Species Described in This Systematic Review
3.5.5. Reported Findings after the Final Date of the Systematic Search
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The Chemistry of Snake Venom and Its Medicinal Potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef]
- Warrell, D.A. Snake Bite. Lancet 2010, 375, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite Envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Kasturiratne, A.; Wickremasinghe, A.R.; de Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; de Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef] [PubMed]
- Pereañez Jiménez, J.A.; Vargas Muñoz, L.J. Toxinas de serpientes con alto potencial terapéutico y su uso en la biomedicina. Iatreia 2009, 22, 382–391. [Google Scholar] [CrossRef]
- O’Shea, M. Snakes of the World: A Guide to Every Family; Princeton University Press: Princeton, NJ, USA, 2023; ISBN 978-0-691-24066-4. [Google Scholar]
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Hošek, J. The Reptile Database. Available online: http://www.reptile-database.org/ (accessed on 24 August 2023).
- Lynch, J.D. El contexto de las serpientes de Colombia con un análisis de las amenazas en contra de su conservación. Rev. Acad. Colomb. Cienc. Exactas Físicas Nat. 2012, 36, 435–449. [Google Scholar]
- Otero-Patiño, R. Snake Bites in Colombia. In Clinical Toxinology in Australia, Europe, and Americas; Vogel, C.-W., Seifert, S.A., Tambourgi, D.V., Eds.; Toxinology; Springer: Dordrecht, The Netherlands, 2018; pp. 3–50. ISBN 978-94-017-7438-3. [Google Scholar]
- León-Núñez, L.J.; Camero-Ramos, G.; Gutiérrez, J.M. Epidemiology of Snakebites in Colombia (2008–2016). Rev. Salud Publica 2020, 22, 280–287. [Google Scholar] [CrossRef]
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating Toxin Diversity and Abundance in Snake Venom Proteomes. Front. Pharmacol. 2021, 12, 768015. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.K. A Current Perspective on Snake Venom Composition and Constituent Protein Families. Arch. Toxicol. 2023, 97, 133–153. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Preciado, L.M.; Romero, L.E. Toxinology in Colombia: Contributions of programa de ofidismo/escorpionismo and other research groups. Vitae 2019, 26, 120–134. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Salazar-Valenzuela, D.; Pla, D.; Lomonte, B.; Guerrero-Vargas, J.A.; Ayerbe, S.; Gibbs, H.L.; Calvete, J.J. Venom Variation in Bothrops asper Lineages from North-Western South America. J. Proteom. 2020, 229, 103945. [Google Scholar] [CrossRef]
- Mora-Obando, D.; Guerrero-Vargas, J.A.; Prieto-Sánchez, R.; Beltrán, J.; Rucavado, A.; Sasa, M.; Gutiérrez, J.M.; Ayerbe, S.; Lomonte, B. Proteomic and Functional Profiling of the Venom of Bothrops ayerbei from Cauca, Colombia, Reveals Striking Interspecific Variation with Bothrops asper Venom. J. Proteom. 2014, 96, 159–172. [Google Scholar] [CrossRef]
- Núñez, V.; Cid, P.; Sanz, L.; De La Torre, P.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Calvete, J.J. Snake Venomics and Antivenomics of Bothrops atrox Venoms from Colombia and the Amazon Regions of Brazil, Perú and Ecuador Suggest the Occurrence of Geographic Variation of Venom Phenotype by a Trend towards Paedomorphism. J. Proteom. 2009, 73, 57–78. [Google Scholar] [CrossRef]
- Fernández Culma, M.; Andrés Pereañez, J.; Núñez Rangel, V.; Lomonte, B. Snake Venomics of Bothrops punctatus, a Semiarboreal Pitviper Species from Antioquia, Colombia. PeerJ 2014, 2, e246. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Preciado, L.M.; Fernández, J.; Camacho, E.; Lomonte, B.; Castro, F.; Cañas, C.A.; Galvis, C.; Castaño, S. Snake Venomics, Experimental Toxic Activities and Clinical Characteristics of Human Envenomation by Bothrocophias myersi (Serpentes: Viperidae) from Colombia. J. Proteom. 2020, 220, 103758. [Google Scholar] [CrossRef]
- Jiménez-Charris, E.; Montealegre-Sanchez, L.; Solano-Redondo, L.; Mora-Obando, D.; Camacho, E.; Castro-Herrera, F.; Fierro-Pérez, L.; Lomonte, B. Proteomic and Functional Analyses of the Venom of Porthidium lansbergii lansbergii (Lansberg’s Hognose Viper) from the Atlantic Department of Colombia. J. Proteom. 2015, 114, 287–299. [Google Scholar] [CrossRef]
- Madrigal, M.; Sanz, L.; Flores-Díaz, M.; Sasa, M.; Núñez, V.; Alape-Girón, A.; Calvete, J.J. Snake Venomics across Genus Lachesis. Ontogenetic Changes in the Venom Composition of Lachesis stenophrys and Comparative Proteomics of the Venoms of Adult Lachesis melanocephala and Lachesis acrochorda. J. Proteom. 2012, 77, 280–297. [Google Scholar] [CrossRef]
- Quintana-Castillo, J.C.; Vargas, L.J.; Segura, C.; Estrada-Gómez, S.; Bueno-Sánchez, J.C.; Alarcón, J.C. Characterization of the Venom of C. d. cumanesis of Colombia: Proteomic Analysis and Antivenomic Study. Toxins 2018, 10, 85. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Cid, P.; de la Torre, P.; Flores-Díaz, M.; Dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake Venomics of the Central American Rattlesnake Crotalus simus and the South American Crotalus durissus Complex Points to Neurotoxicity as an Adaptive Paedomorphic Trend along Crotalus Dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef]
- Boldrini-França, J.; Corrêa-Netto, C.; Silva, M.M.S.; Rodrigues, R.S.; De La Torre, P.; Pérez, A.; Soares, A.M.; Zingali, R.B.; Nogueira, R.A.; Rodrigues, V.M.; et al. Snake Venomics and Antivenomics of Crotalus durissus Subspecies from Brazil: Assessment of Geographic Variation and Its Implication on Snakebite Management. J. Proteom. 2010, 73, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Batista da Cunha, D.; Pupo Silvestrini, A.V.; Gomes da Silva, A.C.; Maria de Paula Estevam, D.; Pollettini, F.L.; de Oliveira Navarro, J.; Alves, A.A.; Remédio Zeni Beretta, A.L.; Annichino Bizzacchi, J.M.; Pereira, L.C.; et al. Mechanistic Insights into Functional Characteristics of Native Crotamine. Toxicon 2018, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Lomonte, B.; Saviola, A.J.; Calderón Celis, F.; Ruiz Encinar, J. Quantification of Snake Venom Proteomes by Mass Spectrometry-Considerations and Perspectives. Mass. Spectrom. Rev. 2023. [Google Scholar] [CrossRef]
- Jiménez-Charris, E.; Montoya-Gómez, A.; Torres, J.K.; Gómez-Díaz, M.; Bolívar-García, W. First Functional and Proteomic Analysis of Bothrops asper Snake Venom from Gorgona Island-Colombia, and Its Comparative Characterization with Two Colombian Southwest Ecoregions. Biochimie 2022, 194, 19–27. [Google Scholar] [CrossRef]
- Montoya-Gómez, A.; Osorno-Valencia, D.; Gómez-Díaz, M.; Bolívar-García, W.; Jiménez-Charris, E. Proteomic and Functional Analyses of Lachesis acrochorda Snake Venom from the Valle Del Cauca Department of Colombia. Acta Trop. 2023, 241, 106895. [Google Scholar] [CrossRef] [PubMed]
- Rey-Suárez, P.; Núñez, V.; Gutiérrez, J.M.; Lomonte, B. Proteomic and Biological Characterization of the Venom of the Redtail Coral Snake, Micrurus mipartitus (Elapidae), from Colombia and Costa Rica. J. Proteom. 2011, 75, 655–667. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Núñez, V.; Fernández, J.; Lomonte, B. Integrative Characterization of the Venom of the Coral Snake Micrurus dumerilii (Elapidae) from Colombia: Proteome, Toxicity, and Cross-Neutralization by Antivenom. J. Proteom. 2016, 136, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Sanz, L.; Quesada-Bernat, S.; Ramos, T.; Casais-E-Silva, L.L.; Corrêa-Netto, C.; Silva-Haad, J.J.; Sasa, M.; Lomonte, B.; Calvete, J.J. New Insights into the Phylogeographic Distribution of the 3FTx/PLA2 Venom Dichotomy across Genus Micrurus in South America. J. Proteom. 2019, 200, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Rey-Suárez, P.; Fernández, J.; Sasa, M.; Pla, D.; Vargas, N.; Bénard-Valle, M.; Sanz, L.; Corrêa-Netto, C.; Núñez, V.; et al. Venoms of Micrurus Coral Snakes: Evolutionary Trends in Compositional Patterns Emerging from Proteomic Analyses. Toxicon 2016, 122, 7–25. [Google Scholar] [CrossRef]
- Saldarriaga, M.M.; Otero, R.; Núñez, V.; Toro, M.F.; Díaz, A.; Gutiérrez, J.M. Ontogenetic Variability of Bothrops atrox and Bothrops asper Snake Venoms from Colombia. Toxicon 2003, 42, 405–411. [Google Scholar] [CrossRef]
- Otero, R.; Guillermo Osorio, R.; Valderrama, R.; Augusto Giraldo, C. Pharmacologic and enzymatic effects of snake venoms from Antioquia and Choco (Colombia). Toxicon 1992, 30, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Mora-Obando, D.; Pla, D.; Lomonte, B.; Guerrero-Vargas, J.A.; Ayerbe, S.; Calvete, J.J. Antivenomics and in vivo Preclinical Efficacy of Six Latin American Antivenoms towards South-Western Colombian Bothrops asper Lineage Venoms. PLoS Negl. Trop. Dis. 2021, 15, e0009073. [Google Scholar] [CrossRef] [PubMed]
- De Arco-Rodríguez, B.; Montealegre-Sánchez, L.; Solano-Redondo, L.; Castro-Herrera, F.; Ortega, J.G.; Castillo, A.; Vargas-Zapata, C.; Jiménez-Charris, E. Phylogeny and Toxicological Assessments of Two Porthidium lansbergii lansbergii Morphotypes from the Caribbean Region of Colombia. Toxicon 2019, 166, 56–65. [Google Scholar] [CrossRef]
- Prezotto-Neto, J.P.; Kimura, L.F.; Alves, A.F.; Gutiérrez, J.M.; Otero, R.; Suárez, A.M.; Santoro, M.L.; Barbaro, K.C. Biochemical and Biological Characterization of Bothriechis schlegelii Snake Venoms from Colombia and Costa Rica. Exp. Biol. Med. 2016, 241, 2075–2085. [Google Scholar] [CrossRef]
- Cañas, C.A. Biological and Medical Aspects Related to South American Rattlesnake Crotalus durissus (Linnaeus, 1758): A View from Colombia. Toxins 2022, 14, 875. [Google Scholar] [CrossRef] [PubMed]
- Cañas, C.A.; Castro-Herrera, F.; Castaño-Valencia, S. Clinical Syndromes Associated with Viperidae Family Snake Envenomation in Southwestern Colombia. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Cañas, C.A.; Vallejo, A. Envenomation by Bothrops punctatus in Southwestern Colombia. Toxicon 2016, 124, 94–96. [Google Scholar] [CrossRef]
- Otero, R.; Furtado, M.F.; Gonçalves, C.; Núñez, V.; García, M.E.; Osorio, R.G.; Romero, M.; Gutiérrez, J.M. Comparative Study of the Venoms of Three Subspecies of Lachesis muta (Bushmaster) from Brazil, Colombia and Costa Rica. Toxicon 1998, 36, 2021–2027. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef]
- Otero-Patiño, R.; Segura, A.; Herrera, M.; Angulo, Y.; León, G.; Gutiérrez, J.M.; Barona, J.; Estrada, S.; Pereañez, A.; Quintana, J.C.; et al. Comparative Study of the Efficacy and Safety of Two Polyvalent, Caprylic Acid Fractionated [IgG and F(Ab’)2] Antivenoms, in Bothrops asper Bites in Colombia. Toxicon 2012, 59, 344–355. [Google Scholar] [CrossRef]
- Otero, R.; Gutiérrez, J.; Beatriz Mesa, M.; Duque, E.; Rodríguez, O.; Luis Arango, J.; Gómez, F.; Toro, A.; Cano, F.; María Rodríguez, L.; et al. Complications of Bothrops, Porthidium, and Bothriechis Snakebites in Colombia. A Clinical and Epidemiological Study of 39 Cases Attended in a University Hospital. Toxicon 2002, 40, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Cañas, C.A.; Castro-Herrera, F.; Castaño-Valencia, S. Envenomation by the Red-Tailed Coral Snake (Micrurus mipartitus) in Colombia. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Posada Arias, S.; Rey-Suárez, P.; Pereáñez, J.A.; Acosta, C.; Rojas, M.; Delazari Dos Santos, L.; Ferreira, R.S.; Núñez, V. Isolation and Functional Characterization of an Acidic Myotoxic Phospholipase A2 from Colombian Bothrops asper Venom. Toxins 2017, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Quintana, J.C.; Alarcón, J.C.; Núñez, V. Isolation and Functional Characterization of a Basic Phospholipase A2 from Colombian Bothrops asper Venom. Vitae 2014, 21, 38–48. [Google Scholar] [CrossRef]
- Núñez, V.; Arce, V.; Gutiérrez, J.M.; Lomonte, B. Structural and Functional Characterization of Myotoxin I, a Lys49 Phospholipase A2 Homologue from the Venom of the Snake Bothrops atrox. Toxicon 2004, 44, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B. Lys49 Myotoxins, Secreted Phospholipase A2-like Proteins of Viperid Venoms: A Comprehensive Review. Toxicon 2023, 224, 107024. [Google Scholar] [CrossRef]
- Patiño, A.C.; Pereañez, J.A.; Núñez, V.; Benjumea, D.M.; Fernandez, M.; Rucavado, A.; Sanz, L.; Calvete, J.J. Isolation and Biological Characterization of Batx-I, a Weak Hemorrhagic and Fibrinogenolytic PI Metalloproteinase from Colombian Bothrops atrox Venom. Toxicon 2010, 56, 936–943. [Google Scholar] [CrossRef]
- Angel-Camilo, K.L.; Guerrero-Vargas, J.A.; de Carvalho, E.F.; Lima-Silva, K.; de Siqueira, R.J.B.; Freitas, L.B.N.; de Sousa, J.A.C.; Mota, M.R.L.; Santos, A.A.D.; da Neves-Ferreira, A.G.C.; et al. Disorders on Cardiovascular Parameters in Rats and in Human Blood Cells Caused by Lachesis acrochorda Snake Venom. Toxicon 2020, 184, 180–191. [Google Scholar] [CrossRef]
- Céspedes, N.; Castro, F.; Jiménez, E.; Montealegre, L.; Castellanos, A.; Cañas, C.A.; Arévalo-Herrera, M.; Herrera, S. Biochemical Comparison of Venoms from Young Colombian Crotalus durissus cumanensis and Their Parents. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 268–284. [Google Scholar] [CrossRef]
- Arévalo-Páez, M.; Rada-Vargas, E.; Betancur-Hurtado, C.; Renjifo, J.M.; Renjifo-Ibáñez, C. Neuromuscular Effect of Venoms from Adults and Juveniles of Crotalus durissus cumanensis (Humboldt, 1811) from Guajira, Colombia. Toxicon 2017, 139, 41–44. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, A.; Vega, N.; Reyes-Montaño, E.; Corzo, G.; Neri-Castro, E.; Clement, H.; Ruiz-Gómez, F. Intraspecific Differences in the Venom of Crotalus durissus cumanensis from Colombia. Toxins 2022, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Gómez, I.D.; Patiño, A.C. Relationship between the Structure and the Enzymatic Activity of Crotoxin Complex and Its Phospholipase A2 Subunit: An in Silico Approach. J. Mol. Graph. Model. 2012, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, S.C.; Hyslop, S.; Fontes, M.R.M.; Prado-Franceschi, J.; Zambelli, V.O.; Magro, A.J.; Brigatte, P.; Gutierrez, V.P.; Cury, Y. Crotoxin: Novel Activities for a Classic Beta-Neurotoxin. Toxicon 2010, 55, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Núñez, V.; Huancahuire-Vega, S.; Marangoni, S.; Ponce-Soto, L.A. Biochemical and Biological Characterization of a PLA2 from Crotoxin Complex of Crotalus durissus cumanensis. Toxicon 2009, 53, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Patiño, A.C.; Pereañez, J.A.; Gutiérrez, J.M.; Rucavado, A. Biochemical and Biological Characterization of Two Serine Proteinases from Colombian Crotalus durissus cumanensis Snake Venom. Toxicon 2013, 63, 32–43. [Google Scholar] [CrossRef]
- Vargas, L.J.; Quintana, J.C.; Pereañez, J.A.; Núñez, V.; Sanz, L.; Calvete, J. Cloning and Characterization of an Antibacterial L-Amino Acid Oxidase from Crotalus durissus cumanensis Venom. Toxicon 2013, 64, 1–11. [Google Scholar] [CrossRef]
- Montealegre-Sánchez, L.; Montoya-Gómez, A.; Jiménez-Charris, E. Individual Variations in the Protein Profiles and Functional Activities of the Eyelash Palm Pit-Viper (Bothriechis schlegelii) Venom from the Colombian Southwest Region. Acta Trop. 2021, 223, 106113. [Google Scholar] [CrossRef]
- Vargas Muñoz, L.J.; Estrada-Gomez, S.; Núñez, V.; Sanz, L.; Calvete, J.J. Characterization and cDNA Sequence of L-Amino Acid Oxidase with Antibacterial Activity. Int. J. Biol. Macromol. 2014, 69, 200–207. [Google Scholar] [CrossRef]
- Jiménez-Charris, E.; González-Duque, D.; Moreno, M.C.; Solano-Redondo, L.; Montoya-Gómez, A.; Montealegre-Sánchez, L.; Buriticá, E. Evaluation of the Systemic Alterations Triggers by Porthidium lansbergii lansbergii Snake Venom. Acta Trop. 2021, 222, 106047. [Google Scholar] [CrossRef]
- Jiménez-Charris, E.; Montealegre-Sánchez, L.; Solano-Redondo, L.; Castro-Herrera, F.; Fierro-Pérez, L.; Lomonte, B. Divergent Functional Profiles of Acidic and Basic Phospholipases A2 in the Venom of the Snake Porthidium Lansbergii Lansbergii. Toxicon 2016, 119, 289–298. [Google Scholar] [CrossRef]
- Vargas, L.J.; Londoño, M.; Quintana, J.C.; Rua, C.; Segura, C.; Lomonte, B.; Núñez, V. An Acidic Phospholipase A2 with Antibacterial Activity from Porthidium nasutum Snake Venom. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 161, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Renjifo, C.; Smith, E.N.; Hodgson, W.C.; Renjifo, J.M.; Sanchez, A.; Acosta, R.; Maldonado, J.H.; Riveros, A. Neuromuscular Activity of the Venoms of the Colombian Coral Snakes Micrurus dissoleucus and Micrurus mipartitus: An Evolutionary Perspective. Toxicon 2012, 59, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Rey-Suárez, P.; Floriano, R.S.; Rostelato-Ferreira, S.; Saldarriaga-Córdoba, M.; Núñez, V.; Rodrigues-Simioni, L.; Lomonte, B. Mipartoxin-I, a Novel Three-Finger Toxin, Is the Major Neurotoxic Component in the Venom of the Redtail Coral Snake Micrurus mipartitus (Elapidae). Toxicon 2012, 60, 851–863. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Núñez, V.; Saldarriaga-Córdoba, M.; Lomonte, B. Primary Structures and Partial Toxicological Characterization of Two Phospholipases A2 from Micrurus mipartitus and Micrurus dumerilii Coral Snake Venoms. Biochimie 2017, 137, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Cardona-Ruda, A.; Rey-Suárez, P.; Núñez, V. Anti-Neurotoxins from Micrurus mipartitus in the Development of Coral Snake Antivenoms. Toxins 2022, 14, 265. [Google Scholar] [CrossRef] [PubMed]
- Rey-Suárez, P.; Acosta, C.; Torres, U.; Saldarriaga-Córdoba, M.; Lomonte, B.; Núñez, V. MipLAAO, a New L-Amino Acid Oxidase from the Redtail Coral Snake Micrurus mipartitus. PeerJ 2018, 6, e4924. [Google Scholar] [CrossRef]
- Romero-Giraldo, L.E.; Pulido, S.; Berrío, M.A.; Flórez, M.F.; Rey-Suárez, P.; Nuñez, V.; Pereañez, J.A. Heterologous Expression and Immunogenic Potential of the Most Abundant Phospholipase A2 from Coral Snake Micrurus dumerilii to Develop Antivenoms. Toxins 2022, 14, 825. [Google Scholar] [CrossRef]
- Rey-Suárez, P.; Saldarriaga-Córdoba, M.; Torres, U.; Marin-Villa, M.; Lomonte, B.; Núñez, V. Novel Three-Finger Toxins from Micrurus dumerilii and Micrurus mipartitus Coral Snake Venoms: Phylogenetic Relationships and Characterization of Clarkitoxin-I-Mdum. Toxicon 2019, 170, 85–93. [Google Scholar] [CrossRef]
- Gómez-Robles, J.; Rey-Suárez, P.; Pereañez, J.A.; Lomonte, B.; Núñez, V. Antibodies against a Single Fraction of Micrurus dumerilii Venom Neutralize the Lethal Effect of Whole Venom. Toxicol. Lett. 2023, 374, 77–84. [Google Scholar] [CrossRef]
- Torres-Bonilla, K.A.; Schezaro-Ramos, R.; Floriano, R.S.; Rodrigues-Simioni, L.; Bernal-Bautista, M.H.; Alice da Cruz-Höfling, M. Biological Activities of Leptodeira annulata (Banded Cat-Eyed Snake) Venom on Vertebrate Neuromuscular Preparations. Toxicon 2016, 119, 345–351. [Google Scholar] [CrossRef]
- Torres-Bonilla, K.A.; Panunto, P.C.; Pereira, B.B.; Zambrano, D.F.; Herrán-Medina, J.; Bernal, M.H.; Hyslop, S. Toxinological Characterization of Venom from Leptodeira annulata (Banded Cat-Eyed Snake; Dipsadidae, Imantodini). Biochimie 2020, 174, 171–188. [Google Scholar] [CrossRef]
- Torres-Bonilla, K.A.; Floriano, R.S.; Schezaro-Ramos, R.; Rodrigues-Simioni, L.; da Cruz-Höfling, M.A. A Survey on Some Biochemical and Pharmacological Activities of Venom from Two Colombian Colubrid Snakes, Erythrolamprus bizona (Double-Banded Coral Snake Mimic) and Pseudoboa neuwiedii (Neuwied’s False Boa). Toxicon 2017, 131, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Torres-Bonilla, K.A.; Andrade-Silva, D.; Serrano, S.M.T.; Hyslop, S. Biochemical Characterization of Venom from Pseudoboa neuwiedii (Neuwied’s False Boa; Xenodontinae; Pseudoboini). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 213, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.C.; Chacón, A.M.; Vargas, L.; Segura, C.; Gutiérrez, J.M.; Alarcón, J.C. Antiplasmodial Effect of the Venom of Crotalus durissus cumanensis, Crotoxin Complex and Crotoxin B. Acta Trop. 2012, 124, 126–132. [Google Scholar] [CrossRef]
- Castillo, J.C.Q.; Vargas, L.J.; Segura, C.; Gutiérrez, J.M.; Pérez, J.C.A. In Vitro Antiplasmodial Activity of Phospholipases A2 and a Phospholipase Homologue Isolated from the Venom of the Snake Bothrops asper. Toxins 2012, 4, 1500–1516. [Google Scholar] [CrossRef]
- Bonilla-Porras, A.R.; Vargas, L.J.; Jimenez-Del-Rio, M.; Nuñez, V.; Velez-Pardo, C. Purification of Nasulysin-1: A New Toxin from Porthidium nasutum Snake Venom That Specifically Induces Apoptosis in Leukemia Cell Model through Caspase-3 and Apoptosis-Inducing Factor Activation. Toxicon 2016, 120, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Charris, E.; Lopes, D.S.; Gimenes, S.N.C.; Teixeira, S.C.; Montealegre-Sánchez, L.; Solano-Redondo, L.; Fierro-Pérez, L.; de Melo Rodrigues Ávila, V. Antitumor Potential of Pllans-II, an Acidic Asp49-PLA2 from Porthidium lansbergii lansbergii Snake Venom on Human Cervical Carcinoma HeLa Cells. Int. J. Biol. Macromol. 2019, 122, 1053–1061. [Google Scholar] [CrossRef]
- Montoya-Gómez, A.; Rivera Franco, N.; Montealegre-Sanchez, L.I.; Solano-Redondo, L.M.; Castillo, A.; Mosquera-Escudero, M.; Jiménez-Charris, E. Pllans-II Induces Cell Death in Cervical Cancer Squamous Epithelial Cells via Unfolded Protein Accumulation and Endoplasmic Reticulum Stress. Molecules 2022, 27, 6491. [Google Scholar] [CrossRef]
- Montealegre-Sánchez, L.; Gimenes, S.N.C.; Lopes, D.S.; Teixeira, S.C.; Solano-Redondo, L.; de Melo Rodrigues, V.; Jiménez-Charris, E. Antitumoral Potential of Lansbermin-I, a Novel Disintegrin from Porthidium lansbergii lansbergii Venom on Breast Cancer Cells. Curr. Top. Med. Chem. 2019, 19, 2069–2078. [Google Scholar] [CrossRef]
- Bedoya-Medina, J.; Mendivil-Perez, M.; Rey-Suarez, P.; Jimenez-Del-Rio, M.; Núñez, V.; Velez-Pardo, C. L-Amino Acid Oxidase Isolated from Micrurus mipartitus Snake Venom (MipLAAO) Specifically Induces Apoptosis in Acute Lymphoblastic Leukemia Cells Mostly via Oxidative Stress-Dependent Signaling Mechanism. Int. J. Biol. Macromol. 2019, 134, 1052–1062. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, A.; Franco-Vásquez, A.M.; Bolívar-Barbosa, J.A.; Vega, N.; Reyes-Montaño, E.; Arreguín-Espinosa, R.; Carbajal-Saucedo, A.; Angarita-Sierra, T.; Ruiz-Gómez, F. Unveiling the Venom Composition of the Colombian Coral Snakes Micrurus helleri, M. medemi, and M. sangilensis. Toxins 2023, 15, 622. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereañez, J.A.; Preciado, L.M.; Rey-Suárez, P. Knowledge about Snake Venoms and Toxins from Colombia: A Systematic Review. Toxins 2023, 15, 658. https://doi.org/10.3390/toxins15110658
Pereañez JA, Preciado LM, Rey-Suárez P. Knowledge about Snake Venoms and Toxins from Colombia: A Systematic Review. Toxins. 2023; 15(11):658. https://doi.org/10.3390/toxins15110658
Chicago/Turabian StylePereañez, Jaime Andrés, Lina María Preciado, and Paola Rey-Suárez. 2023. "Knowledge about Snake Venoms and Toxins from Colombia: A Systematic Review" Toxins 15, no. 11: 658. https://doi.org/10.3390/toxins15110658
APA StylePereañez, J. A., Preciado, L. M., & Rey-Suárez, P. (2023). Knowledge about Snake Venoms and Toxins from Colombia: A Systematic Review. Toxins, 15(11), 658. https://doi.org/10.3390/toxins15110658




