Current Knowledge of Individual and Combined Toxicities of Aflatoxin B1 and Fumonisin B1 In Vitro
Abstract
:1. Introduction
2. Overview of the Toxic Effects of AFB1 In Vitro
| Organs | Cells | Exposure Time (Hour) | Concentration (μM) | Effects | References |
|---|---|---|---|---|---|
| Liver | HepG2 cells | 24 | 32.0 | Inducing cell death, DNA strand breaks, ROS generation, nuclear changes, cell cycle arrests, and apoptotic body formation | [33] |
| HepG2 cells | 24 | 13.0 | Promoting MDA release, inhibiting cell growth, causing DNA migration, and increasing the level of ERK1/2-P (A) in the MAPK pathway | [41] | |
| HepG2 cells | 24 | 100.0 | Decreasing the expression of the p53 protein | [43] | |
| HepG2 cells | 24 | 105.0 | Suppressing p53 protein expression, and causing mitochondrial damage, nuclear condensation, and a loss of cell-to-cell contact | [45] | |
| HepG2 cells | 24 | 16.9 | Increasing ROS and ΔΨm damage, and the expression of p53 | [44] | |
| HepG2 cells | 24 | 10.0 | Inducing ROS production and DNA oxidation | [50] | |
| HepG2 cells | 24 | 30.0 | Increasing GST activity, to induce ROS | [36] | |
| L-O2 cell | 24 | 192.0 | Reducing ΔΨm, and increasing ROS generation | [51] | |
| HepG2 cells | 24 | 5.0 | Causing oxidative stress, and increasing GST activities | [52] | |
| HepG2 cells | 24 | 30.0 | Inducing DNA damage and more significant amounts of ROS | [37] | |
| HepG2 cells | 24 | 32.0 | Inducing oxidative stress, energy metabolism, DNA damage, and cell apoptosis | [34] | |
| HepG2 cells | 24 | 50.0 | Inducing DNA fragmentation and ROS | [53] | |
| HepG2 cells | 24 | 10.0 | Ameliorating DNA damage and p53-mediated apoptosis | [42] | |
| HepG2 cells | 24 | 10.0 | Causing ROS production and DNA damage | [54] | |
| HepG2 cells | 24 | 10.0 | Inducing oxidative lipid damage | [55] | |
| HL7702 cells | 24 | 10.0 | Inducing oxidative stress and DNA damage | [56] | |
| HepG2 cells | 24 | 10.0 | Inducing ROS and DNA strand break, downregulating the Nrf2/HO-1 pathway | [57] | |
| HepG2 cells | 24 | 4.0 | Altering the GSH content, GPx, and SOD activity | [58] | |
| HepG2 cells | 24 | 3.0 | Inducing P450 activities and DNA damage | [46] | |
| HepG2 cells | 24 | 48.4 | Increasing ROS generation and MMP disruption, inducing mitochondrial dysfunction, and inhibiting ATP production | [31] | |
| L-O2 cell | 36 | 40.0 | Inducing autophagy by regulating the EGFR/PI3K-AKT/mTOR signaling pathway | [32] | |
| HepG2 cells | 48 | 10.0 | Decreasing the activity of GST, increasing the P450 3A4 activity, and inducing oxidative stress | [59] | |
| L-O2 cell | 48 | 8.0 | Inducing the expression of P450 and the nuclear translocation of AHR | [48] | |
| BFH12 cells | 48 | 0.1 | Causing lipid peroxidation, reducing the antioxidant activity of the NAD(H): quinone oxidoreductase 1, and increasing the cytochrome P450 3A activity | [47] | |
| HepG2 cells | 72 | 2.0 | Inducing apoptosis and cytochrome P450 1A/1B activity | [60] | |
| Intestine | Caco-2 cells | 24 | 13.0 | Promoting MDA release, inhibiting cell growth, causing DNA migration, and increasing the level of ERK1/2-P (A) in the MAPK pathway | [41] |
| Caco-2 cells | 24 | 20.0 | Leading to cellular apoptosis or necrosis: downregulating the Bcl-2 gene and upregulating the Bax, p53, caspase-3, caspase-8, and caspase- 9 genes, and seriously affecting glycine, serine, threonine, and pyruvate metabolism. | [39] | |
| Caco-2 cells | 24 | 50.0 | Inducing DNA fragmentation and ROS | [53] | |
| Caco-2 cells | 24 | 10.0 | Inducing oxidative lipid damage | [55] | |
| Caco-2 cells | 24 | 80.6 | Increasing ROS and MMP damage, disrupting the ETC, and inhibiting ATP production | [31] | |
| Caco-2 cells | 72 | 3.0 | Increasing intracellular ROS generation, and leading to membrane damage and DNA strand break. | [61] | |
| Kidney | Vero cells | 24 | 40.0 | Inducing DNA fragmentation, increasing the level of p53, and decreasing the level of bcl-2 protein | [62] |
| HEK cells | 24 | 13.0 | Promoting MDA release, inhibiting cell growth, and causing DNA migration | [41] | |
| PK-15 cells | 24 | 1.0 | Inducing ROS production and apoptosis | [63] | |
| MDCK cells | 24 | 0.8 | Inducing oxidative stress: MDA level increased, GSH level and GPX1 activity decreased. | [64] | |
| HEK 293 cells | 48 | 1.6 | Activating oxidative stress | [65] | |
| Bronchial epithelial | BEAS-2B cells | 12 | 1.5 | Inducing mutation by the attenuation of DNA adduct and p53-mediated | [66] |
| BEAS-2B cells | 24 | 0.1 | Inducing apoptosis by inhibiting the CYP enzyme, and increasing DNA adduct | [67] | |
| BEAS-2B cells | 24 | 1.5 | Decreasing both 1A2-expressing and 3A4-expressing CYPs | [68] | |
| Genital system | sperm cells | 4 | 1.0 | Decreasing MMP, and inducing fragmented DNA | [69] |
| Bone marrow | SK-N-SH cells | 24 | 12.8 | Promoting MDA release, inhibiting cell growth, and causing DNA migration | [41] |
| Mammary gland | MAC-T cells | 24 | 12.8 | Increasing ROS production, decreasing MMP, and inducing apoptosis, by reducing three anti-stress genes (Nrf2, SOD2, and HSP70) of the Nrf2 pathway | [70] |
| Bone | MSCs and CD34+ cells | 24 | 10.0 | Inducing DNA damage | [71] |
| Colon | HCT-116 cells | 24 | 10.0 | Increasing the expression of p53 | [72] |
| Brain | NHA-SV40LT cells | 48 | 50.0 | Inducing cytosolic and mitochondrial calcium changes and ROS generation, and changes in AKT and ERK1/2 MAPK signaling | [40] |
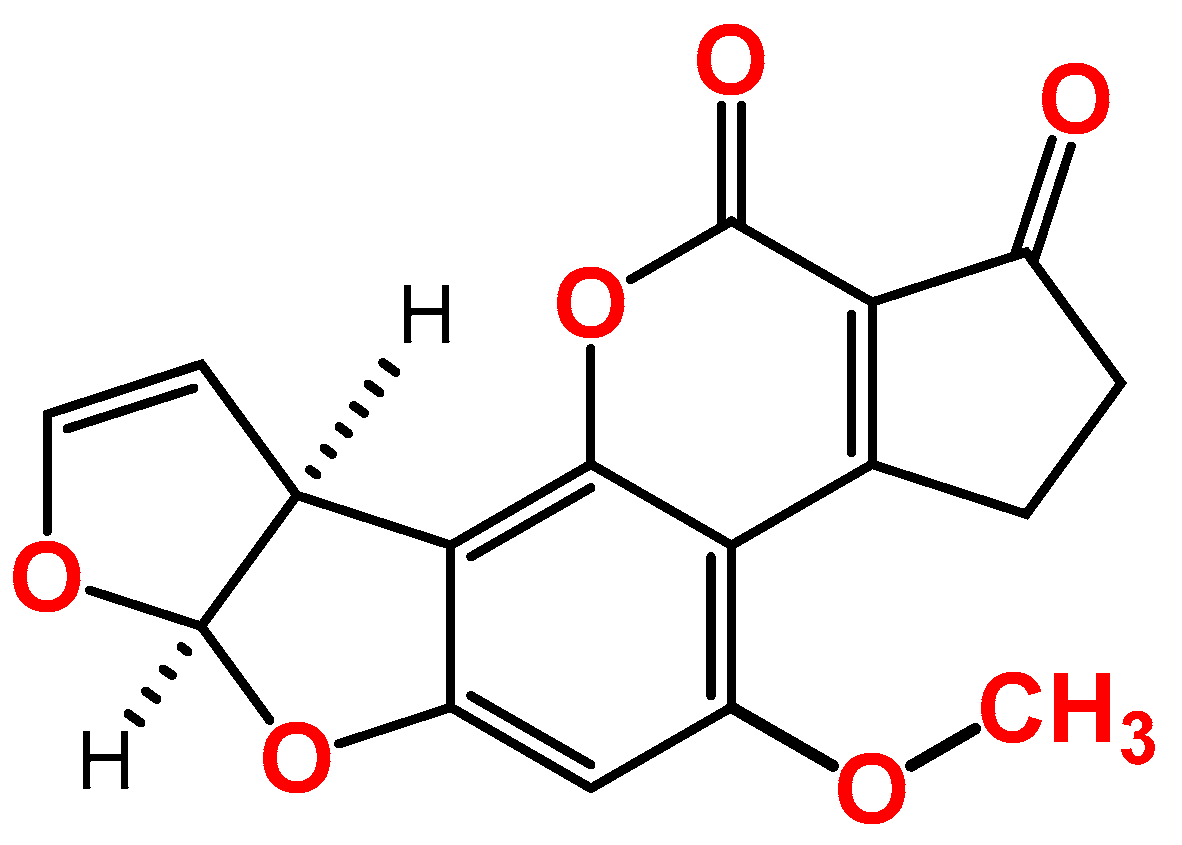
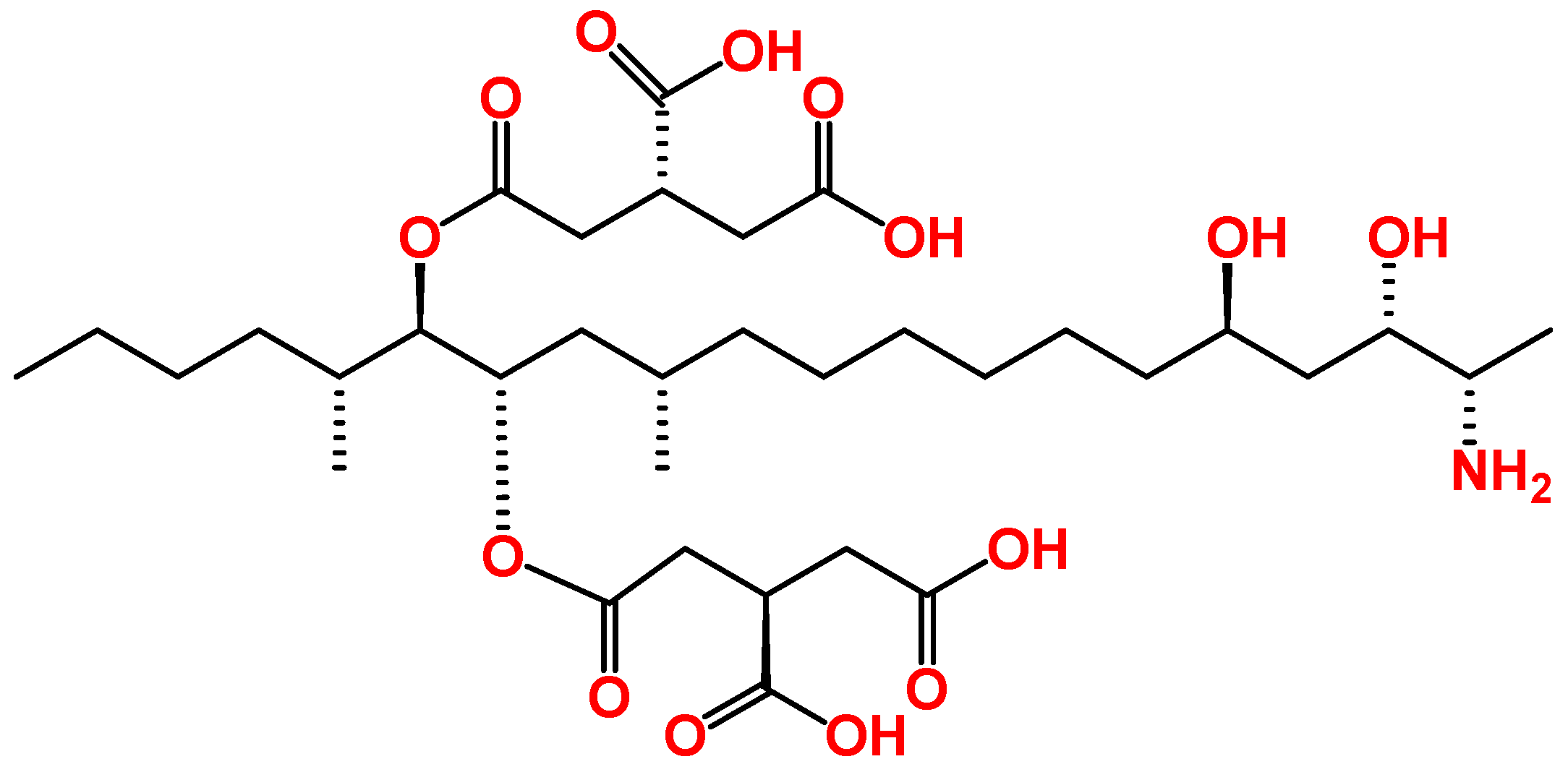
3. Overview of the Toxic Effects of FB1 In Vitro
| Organs | Cells | Exposure Time (Hour) | Concentration (μM) | Effects | References |
|---|---|---|---|---|---|
| Liver | HepG2 cells | 6 | 50.0 | Reducing ceramide levels, elevating the expression of ABCA1 (a cholesterol efflux promoter) in an LXR-dependent mechanism, and disrupting lipid homeostasis | [85] |
| HepG2 cells | 24 | 50.0 | Inducing autophagy via the generation of ROS, ER stress, the phosphorylation of JNK, suppressing mTOR, and activating LC3I/II | [80] | |
| HepG2 cells | 24 | 200.0 | Inhibiting sphingolipid biosynthesis and upregulating the anti-apoptotic Birc-8/ILP-2 gene and protein expression to induce apoptosis | [86] | |
| HepG2 cells | 24 | 35.0 | Inducing ROS generation, MMP damage, and mitochondrial dysfunction | [31] | |
| Intestine | HT-29 cells | 12 | 69.0 | Inducing lipid peroxidation | [79] |
| Caco-2 cells | 24 | 20.0 | Inhibiting DNA synthesis | [81] | |
| Caco-2 cells | 24 | 560.7 | Increasing ROS and MMP damage, disrupting the ETC, and inhibiting ATP production | [31] | |
| Caco-2 cells | 48 | 20.0 | Inhibiting sphingolipid biosynthesis | [87] | |
| LLC-PK1 cells | 48 | 50.0 | Inhibiting cell proliferation, and decreasing TEER | [88] | |
| Bone | SH-SY5Y cells | 24 | 50.0 | Leading to a sustained deregulation of calcium homeostasis and, presumably, to cell death | [89] |
| Colon | HT-29 cells | 24 | 50.0 | Inhibiting ceramide synthesis and sphingolipids | [90] |
| Brain | U-118MG cells | 48 | 100.0 | Causing ROS production and lipid peroxidation, and lowering GSH levels | [82] |
| Esophagus | SNO cells | 48 | 20.0 | Increasing lipid peroxidation, decreasing GSH, altering mitochondrial membrane depolarization, and depleting ATP | [84] |
| Endothelia | HUVEC cells | 48 | 50.0 | Inducing lipid peroxidation and ROS | [83] |
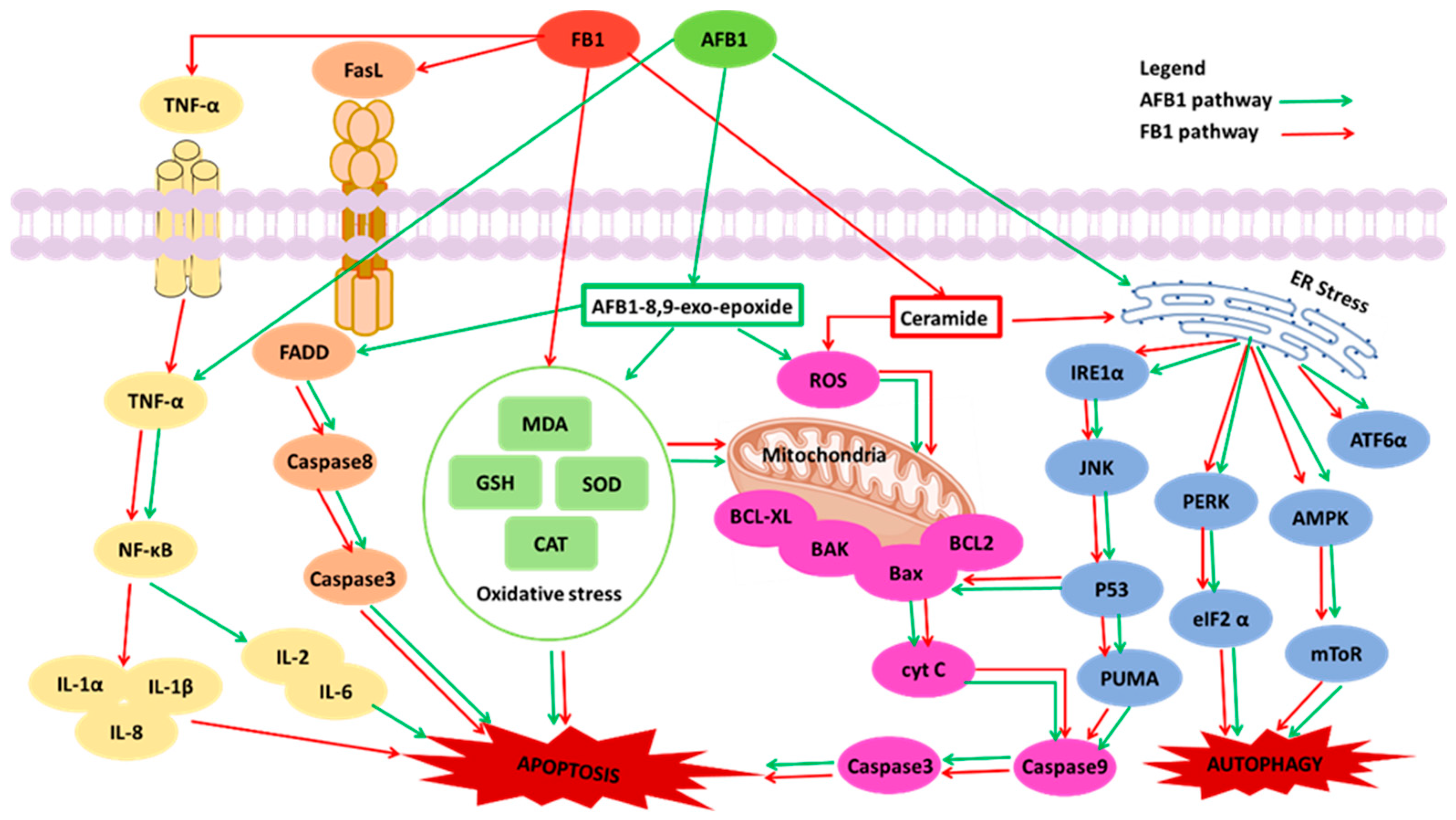
4. Combined Toxicity of AFB1 and FB1 in Human Cells
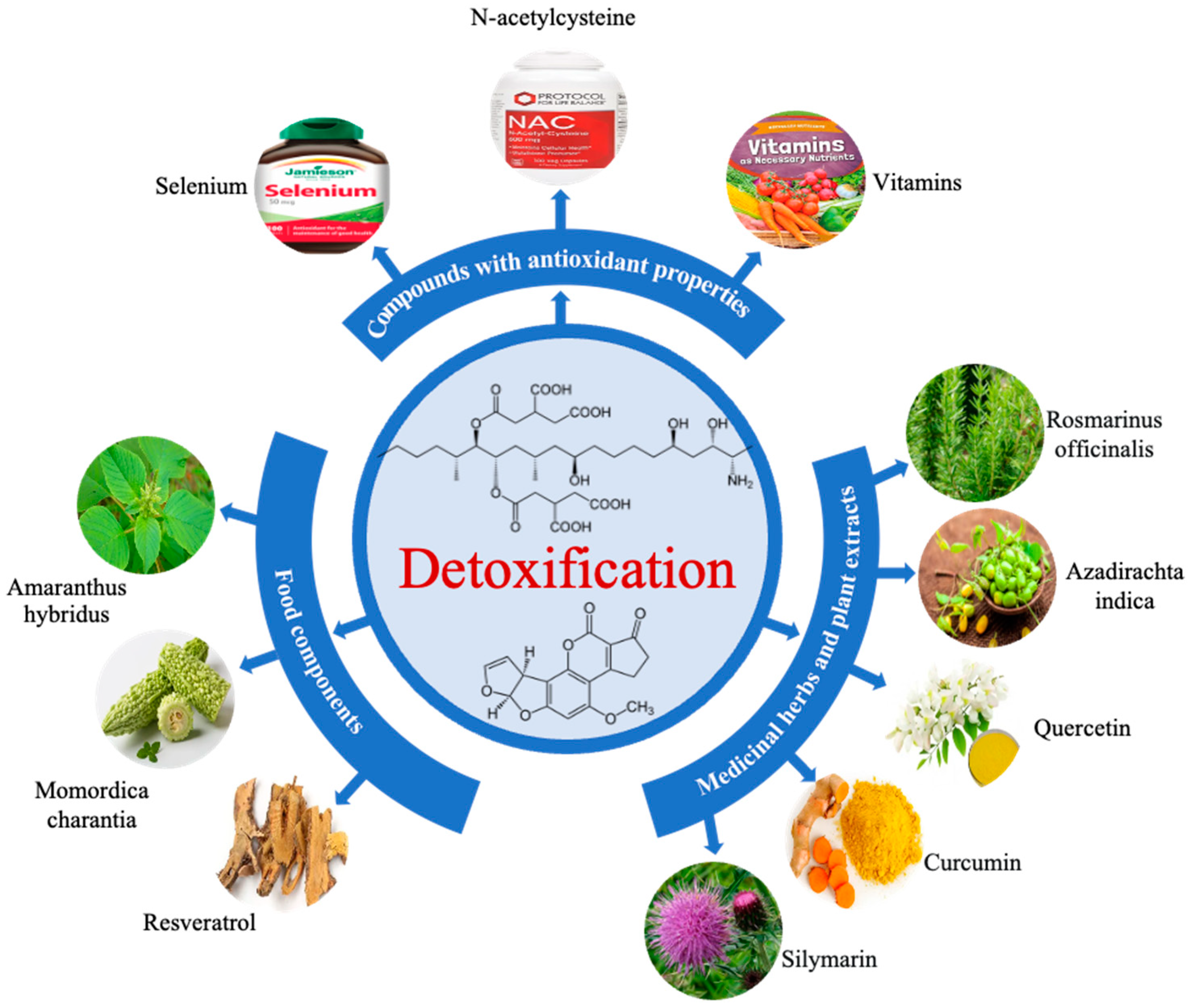
5. Mycotoxin Mitigation
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Milicevic, D.; Nedeljkovic-Trailovic, J.; Masic, Z. Mycotoxins in food chain: Risk assessment and importance for public health. Tehnol. Mesa 2014, 55, 22–38. [Google Scholar] [CrossRef]
- Gurikar, C.; Shivaprasad, D.P.; Sabillón, L.; Gowda, N.A.N.; Siliveru, K. Impact of mycotoxins and their metabolites associated with food grains. Grain Oil Sci. Technol. 2023, 6, 1–9. [Google Scholar] [CrossRef]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Abrahantes, J.C.; Dorne, J.L.; Battilani, P.; Toscano, P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Mohanta, K.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A.; et al. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Perspect. 2005, 113, 1763–1767. [Google Scholar] [CrossRef]
- Wouters, A.T.B.; Casagrande, R.A.; Wouters, F.; Watanabe, T.T.N.; Boabaid, F.M.; Cruz, C.E.F.; Driemeier, D. An outbreak of aflatoxin poisoning in dogs associated with aflatoxin B1-contaminated maize products. J. Vet. Diagn. Investig. 2013, 25, 282–287. [Google Scholar] [CrossRef]
- IARC. Aflatoxin: Scientific Background, Control, and Implications; IARC (International Agency for Research on Cancer): Paris, France, 2012. [Google Scholar]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006 (Text with EEA relevance). Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Chen, J.; Wen, J.; Tang, Y.T.; Shi, J.C.; Mu, G.D.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Gelderblom, W.C.A.; Jaskiewicz, K.; Marasas, W.F.O.; Thiel, P.G.; Horak, R.M.; Vleggaar, R.; Kriek, N.P.J. Fumonisins—Novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1998, 54, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Rosiles, M.R.; Bautista, J.; Fuentes, V.O.; Ross, F. An outbreak of Equine leukoencephalomalacia at Oaxaca, Mexico, sssociated with Fumonisin B1. J. Vet. Med. A Physiol. Pathol. Clin. Med. 1998, 45, 299–302. [Google Scholar] [CrossRef] [PubMed]
- IARC. International Agency for Research on Cancer IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Paris, France, 2002; Volume 96, pp. 1–390. [Google Scholar]
- Shetty, P.H.; Bhat, R.V. Natural Occurrence of fumonisin B1 and its co-occurrence with aflatoxin B1 in Indian sorghum, maize, and Poultry Feeds. J. Agric. Food Chem. 1997, 45, 2170–2173. [Google Scholar] [CrossRef]
- Alizadeh, A.M.; Roshandel, G.; Roudbarmohammadi, S.; Roudbary, M.; Sohanaki, H.; Ghiasian, S.A.; Taherkhani, A.; Semnani, S.; Aghasi, M. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in Northeastern Iran. Asian Pac. J. Cancer Prev. 2012, 13, 2625–2628. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.K.; Barregard, L.; Bignami, M.; Bruschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B. Appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, 5172. [Google Scholar]
- Sydenham, E.W.; Gelderblom, W.C.A.; Thiel, P.G.; Marasas, W.F.O. Evidence for the natural occurrence of fumonisin B1, a mycotoxin produced by Fusarium moniliforme, in corn. J. Agric. Food Chem. 1990, 38, 285–290. [Google Scholar] [CrossRef]
- Massomo, S.M.S. Aspergillus flavus and aflatoxin contamination in the maize value chain and what needs to be done in Tanzania. Sci. Afr. 2020, 10, e00606. [Google Scholar] [CrossRef]
- Fels-Klerx, H.J.V.D.; Liu, C.; Battilani, P. Modelling climate change impacts on mycotoxin contamination. World Mycotoxin J. 2016, 9, 717–726. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van Der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef] [PubMed]
- Leggieri, M.C.; Toscano, P.; Battilani, P. Predicted aflatoxin b1 increase in europe due to climate change: Actions and reactions at global level. Toxins 2021, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Akello, J.; Ortega-Beltran, A.; Katati, B.; Atehnkeng, J.; Augusto, J.; Mwila, C.M.; Mahuku, G.; Chikoye, D.; Bandyopadhyay, R. Prevalence of aflatoxin-and fumonisin-producing fungi associated with cereal crops grown in zimbabwe and their associated risks in a climate change scenario. Foods 2021, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- MacArthur Clark, J. The 3Rs in research: A contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 2018, 120, S1–S7. [Google Scholar] [CrossRef]
- Knierim, U.; Van Dongen, S.; Forkman, B.; Tuyttens, F.A.M.; Špinka, M.; Campo, J.L.; Weissengruber, G.E. Fluctuating asymmetry as an animal welfare indicator—A review of methodology and validity. Physiol. Behav. 2007, 92, 398–421. [Google Scholar] [CrossRef]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and functional characterization of human hepatoma HepG2 cells. In Protocols in In Vitro Hepatocyte Research; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; Volume 1250, pp. 77–93. [Google Scholar]
- Ding, X.; Hu, X.; Chen, Y.; Xie, J.; Ying, M.; Wang, Y.; Yu, Q. Differentiated Caco-2 cell models in food-intestine interaction study: Current applications and future trends. Trends Food Sci. Technol. 2021, 107, 455–465. [Google Scholar] [CrossRef]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure, and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Renehan, A.G.; Booth, C.; Potteri, C.S. What is apoptosis, and why is it important? BMJ 2001, 322, 1536–1538. [Google Scholar] [CrossRef]
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Rajkovic, A. Bioenergetic status of the intestinal and hepatic cells after short term exposure to fumonisin B1 and aflatoxin B1. Int. J. Mol. Sci. 2022, 23, 6945. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, W.; Lv, P.; Meng, W.; Mao, G.; Gong, C.; Chen, Y.; Wei, Y.; He, X.; Zhao, J.; et al. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death Dis. 2020, 11, 6. [Google Scholar] [CrossRef]
- Yang, X.; Lv, Y.; Huang, K.; Luo, Y.; Xu, W. Zinc inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in human hepatocytes (HepG2 cells). Food Chem. Toxicol. 2016, 92, 17–25. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, C.; Yang, X.; Zhang, B.; He, X.; Xu, W.; Huang, K. Proteomics reveals the alleviation of zinc towards aflatoxin B1-induced cytotoxicity in human hepatocyes (HepG2 cells). Ecotoxicol. Environ. Saf. 2020, 198, 110596. [Google Scholar] [CrossRef] [PubMed]
- Inal, M.E.; Kanbak, G.; Sunal, E. Antioxidant enzyme activities and malondialdehyde levels related to aging. Clin. Chim. Acta 2001, 305, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Schwaiger, S.; Cervellati, R.; Stuppner, H.; Speroni, E.; Guerra, M.C. In vitro evaluation of the chemoprotective action mechanisms of leontopodic acid against aflatoxin B1 and deoxynivalenol-induced cell damage. J. Appl. Toxicol. 2009, 29, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Corcuera, L.A.; Arbillaga, L.; Vettorazzi, A.; Azqueta, A.; López de Cerain, A. Ochratoxin A reduces aflatoxin B1 induced DNA damage detected by the comet assay in Hep G2 cells. Food Chem. Toxicol. 2011, 49, 2883–2889. [Google Scholar] [CrossRef]
- Fang, E.F.; Scheibye-Knudsen, M.; Chua, K.F.; Mattson, M.P.; Croteau, D.L.; Bohr, V.A. Nuclear DNA damage signalling to mitochondria in ageing. Nat. Rev. Mol. Cell Biol. 2016, 17, 308–321. [Google Scholar] [CrossRef]
- Ji, J.; Wang, Q.; Wu, H.; Xia, S.; Guo, H.; Blaženović, I.; Zhang, Y.; Sun, X. Insights into cellular metabolic pathways of the combined toxicity responses of Caco-2 cells exposed to deoxynivalenol, zearalenone and Aflatoxin B1. Food Chem. Toxicol. 2019, 126, 106–112. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.Y.; You, S.; Song, G.; Lim, W. Neurotoxic effects of aflatoxin B1 on human astrocytes in vitro and on glial cell development in zebrafish in vivo. J. Hazard. Mater. 2020, 386, 121639. [Google Scholar] [CrossRef]
- Zheng, N.; Zhang, H.; Li, S.; Wang, J.; Liu, J.; Ren, H.; Gao, Y. Lactoferrin inhibits aflatoxin B1- and aflatoxin M1-induced cytotoxicity and DNA damage in Caco-2, HEK, Hep-G2, and SK-N-SH cells. Toxicon 2018, 150, 77–85. [Google Scholar] [CrossRef]
- Li, C.H.; Li, W.Y.; Hsu, I.N.; Liao, Y.Y.; Yang, C.Y.; Taylor, M.C.; Liu, Y.F.; Huang, W.H.; Chang, H.H.; Huang, H.; et al. Recombinant aflatoxin-degrading f420h2-dependent reductase from mycobacterium smegmatis protects mammalian cells from aflatoxin toxicity. Toxins 2019, 11, 259. [Google Scholar] [CrossRef]
- Liu, R.; Jin, Q.; Huang, J.; Liu, Y.; Wang, X.; Zhou, X.; Mao, W.; Wang, S. In vitro toxicity of aflatoxin B 1 and its photodegradation products in HepG2 cells. J. Appl. Toxicol. 2012, 32, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Zhang, G. Proapoptotic activity of aflatoxin B1 and sterigmatocystin in HepG2 cells. Toxicol. Rep. 2014, 1, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Odhav, B.; Bhoola, K. Aflatoxin B1-induced toxicity in HepG2 cells inhibited by carotenoids: Morphology, apoptosis and DNA damage. J. Biol. Chem. 2006, 387, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Desaulniers, D.; Cummings-Lorbetskie, C.; Leingartner, K.; Xiao, G.H.; Zhou, G.; Parfett, C. Effects of vanadium (sodium metavanadate) and aflatoxin-B1 on cytochrome p450 activities, DNA damage and DNA methylation in human liver cell lines. Toxicol. Vitr. 2021, 70, 105036. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Discovering the protective effects of resveratrol on aflatoxin b1-induced toxicity: A whole transcriptomic study in a bovine hepatocyte cell line. Antioxidants 2021, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ma, Y.; Liang, J.; Wei, Z.; Li, M.; Zhang, Y.; Liu, M.; He, H.; Qu, C.; Cai, J.; et al. AHR mediates the aflatoxin B1 toxicity associated with hepatocellular carcinoma. Signal Transduct. Target. Ther. 2021, 6, 299. [Google Scholar] [CrossRef]
- Nebert, D.W.; Dalton, T.P. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat. Rev. Cancer 2006, 6, 947–960. [Google Scholar] [CrossRef]
- Costa, S.; Utan, A.; Speroni, E.; Cervellati, R.; Piva, G.; Prandini, A.; Guerra, M.C. Carnosic acid from rosemary extracts: A potential chemoprotective agent against aflatoxin B1. An in vitro study. J. Appl. Toxicol. 2007, 27, 152–159. [Google Scholar] [CrossRef]
- Zhang, B.; Dai, Y.; Zhu, L.; He, X.; Huang, K.; Xu, W. Single-cell sequencing reveals novel mechanisms of aflatoxin B1-induced hepatotoxicity in S phase-arrested L02 cells. Cell Biol. Toxicol. 2020, 36, 603–608. [Google Scholar] [CrossRef]
- Chan, H.T.; Chan, C.; Ho, J.W. Inhibition of glycyrrhizic acid on aflatoxin B1-induced cytotoxicity in hepatoma cells. Toxicology 2003, 188, 211–217. [Google Scholar] [CrossRef]
- Guerra, M.C.; Galvano, F.; Bonsi, L.; Speroni, E.; Costa, S.; Renzulli, C.; Cervellati, R. Cyanidin-3-O-β-glucopyranoside, a natural free-radical scavenger against aflatoxin B1- and ochratoxin A-induced cell damage in a human hepatoma cell line (Hep G2) and a human colonic adenocarcinoma cell line (CaCo-2). Br. J. Nutr. 2005, 94, 211–220. [Google Scholar] [CrossRef]
- Singto, T.; Tassaneeyakul, W.; Porasuphatana, S. Protective effects of purple waxy corn on aflatoxin B1-induced oxidative stress and micronucleus in HepG2 cells. Indian. J. Pharm. Sci. 2020, 82, 506–513. [Google Scholar] [CrossRef]
- Halbin, K.J. Low level of ochratoxin A enhances aflatoxin B1 induced cytotoxicity and lipid peroxydation in both human intestinal (Caco-2) and hepatoma (HepG2) cells lines. Int. J. Food Sci. Nutr. 2013, 2, 294. [Google Scholar] [CrossRef]
- Liu, W.; Wang, L.; Zheng, C.; Liu, L.; Wang, J.; Li, D.; Tan, Y.; Zhao, X.; He, L.; Shu, W. Microcystin-LR increases genotoxicity induced by aflatoxin B1 through oxidative stress and DNA base excision repair genes in human hepatic cell lines. Environ. Pollut. 2018, 233, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Vipin, A.V.; Raksha Rao, K.; Nawneet Kumar, K.; Anu Appaiah, A.; Venkateswaran, G. Protective effects of phenolics rich extract of ginger against aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 2017, 91, 415–424. [Google Scholar]
- Tadee, A.; Mahakunakorn, P.; Porasuphatana, S. Oxidative stress and genotoxicity of co-exposure to chlorpyrifos and aflatoxin B1 in HepG2 cells. Toxicol. Ind. Health 2020, 36, 336–345. [Google Scholar] [CrossRef]
- Lee, J.K.; Choi, E.H.; Lee, K.G.; Chun, H.S. Alleviation of aflatoxin B1-induced oxidative stress in HepG2 cells by volatile extract from Allii Fistulosi Bulbus. Life Sci. 2005, 77, 2896–2910. [Google Scholar] [CrossRef]
- Zhou, Q.; Xie, H.; Zhang, L.; Stewart, J.K.; Gu, X.X.; Ryan, J.J. Cis-terpenones as an effective chemopreventive agent against aflatoxin B1-induced cytotoxicity and TCDD-induced P450 1A/B activity in HepG2 cells. Chem. Res. Toxicol. 2006, 19, 1415–1419. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, N.; Liu, J.; Li, F.D.; Li, S.L.; Wang, J.Q. Aflatoxin B1 and aflatoxin M1 induced cytotoxicity and DNA damage in differentiated and undifferentiated Caco-2 cells. Food Chem. Toxicol. 2015, 83, 54–60. [Google Scholar] [CrossRef]
- El Golli-Bennour, E.; Kouidhi, B.; Bouslimi, A.; Abid-Essefi, S.; Hassen, W.; Bacha, H. Cytotoxicity and genotoxicity induced by aflatoxin B1, ochratoxin A, and their combination in cultured vero cells. J. Biochem. Mol. Toxicol. 2010, 24, 42–50. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, N.; Qi, D. In vitro investigation of individual and combined cytotoxic effects of aflatoxin B1 and other selected mycotoxins on the cell line porcine kidney 15. Exp. Toxicol. Pathol. 2013, 65, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Parveen, F.; Nizamani, Z.A.; Gan, F.; Chen, X.; Shi, X.; Kumbhar, S.; Zeb, A.; Huang, K. Protective effect of selenomethionine on aflatoxin b1-induced oxidative stress in MDCK cells. Biol. Trace Elem. Res. 2014, 157, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xing, L.; Zhang, M.; Wang, J.; Zheng, N. The toxic effects of aflatoxin B1 and aflatoxin M1 on kidney through regulating L-proline and downstream apoptosis. Biomed. Res. Int. 2018, 2018, 9074861. [Google Scholar] [CrossRef] [PubMed]
- Van Vleet, T.R.; Watterson, T.L.; Klein, P.J.; Coulombe, R.A. Aflatoxin B1 alters the expression of p53 in cytochrome p450-expressing human lung cells. Toxicol. Sci. 2006, 89, 399–407. [Google Scholar] [CrossRef]
- Yang, X.J.; Lu, H.Y.; Li, Z.Y.; Bian, Q.; Qiu, L.L.; Li, Z.; Liu, Q.; Li, J.; Wang, X.; Wang, S.L. Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology 2012, 300, 138–148. [Google Scholar] [CrossRef]
- Van Vleet, T.R.; Klein, P.J.; Coulombe, R.A. Metabolism and cytotoxicity of aflatoxin B1 in cytochrome P-450-expressing human lung cells. J. Toxicol. Environ. Health A 2002, 65, 853–867. [Google Scholar] [CrossRef]
- Komsky-Elbaz, A.; Saktsier, M.; Roth, Z. Aflatoxin B1 impairs sperm quality and fertilization competence. Toxicology 2008, 393, 42–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Y.; Yu, H.; Shan, A.; Shen, J.; Zhou, C.; Zhao, Y.; Fang, H.; Wang, X.; Wang, J.; et al. Resveratrol inhibits aflatoxin B1-induced oxidative stress and apoptosis in bovine mammary epithelial cells and is involved the Nrf2 signaling pathway. Toxicon 2019, 164, 10–15. [Google Scholar] [CrossRef]
- Ghaderi, M.; Allameh, A.; Soleimani, M.; Rastegar, H.; Ahmadi-Ashtiani, H.R. A comparison of DNA damage induced by aflatoxin B1 in hepatocyte-like cells, their progenitor mesenchymal stem cells and CD34+ cells isolated from umbilical cord blood. Mutat. Res. Genet. Toxicol. Environ. Mutagen 2011, 719, 14–20. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.H.; Do, K.H.; Kim, D.; Moon, Y. Interference with mutagenic aflatoxin B1-induced checkpoints through antagonistic action of ochratoxin A in intestinal cancer cells: A molecular explanation on potential risk of crosstalk between carcinogens. Oncotarget 2016, 7, 39627–39639. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of fumonisins, their modified forms and hidden forms in feed. EFSA J. 2018, 16, e05242. [Google Scholar]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Fugio, L.B.; Coeli-Lacchini, F.B.; Leopoldino, A.M. Sphingolipids and mitochondrial dynamic. Cells 2020, 9, 581. [Google Scholar] [CrossRef] [PubMed]
- Lumsangkul, C.; Chiang, H.I.; Lo, N.W.; Fan, Y.K.; Ju, J.C. Developmental toxicity of mycotoxin fumonisin B1 in animal embryogenesis: An overview. Toxins 2019, 11, 114. [Google Scholar] [CrossRef]
- Stiban, J.; Tidhar, R.; Futerman, A.H. Ceramide synthases: Roles in cell physiology and signaling. Adv. Exp. Med. Biol. 2010, 688, 60–71. [Google Scholar] [PubMed]
- Zitomer, N.C.; Mitchell, T.; Voss, K.A.; Bondy, G.S.; Pruett, S.T.; Garnier-Amblard, E.C.; Liebeskind, L.S.; Park, H.; Wang, E.; Sulllards, M.C.; et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine. A novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J. Biol. Chem. 2009, 284, 4786–4795. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Garbetta, A.; D’Antuono, I.; Cardinali, A.; Martino, N.A.; Debellis, L.; Visconti, A. Toxic mechanisms induced by fumonisin B1 mycotoxin on human intestinal cell line. Arch. Environ. Contam. Toxicol. 2014, 67, 115–123. [Google Scholar] [CrossRef]
- Singh, M.P.; Kang, S.C. Endoplasmic reticulum stress-mediated autophagy activation attenuates fumonisin B1 induced hepatotoxicity in vitro and in vivo. Food Chem. Toxicol. 2017, 110, 371–382. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Stockmann-Juvala, H.; Mikkola, J.; Naarala, J.; Loikkanen, J.; Elovaara, E.; Savolainen, K. Fumonisin B1-induced toxicity and oxidative damage in U-118MG glioblastoma cells. Toxicology 2004, 202, 173–183. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Liu, J.L.; Zhang, J.H.; Zhang, S.C.; Ouyang, Y.; Huang, J.T.; Peng, X.Y.; Zeng, Z.; Hu, Z.Q. Fumonisin B1 affects the biophysical properties, migration and cytoskeletal structure of human umbilical vein endothelial cells. Cell Biochem. Biophys. 2020, 78, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.B.; Phulukdaree, A.; Chuturgoon, A.A. Fumonisin B1 induces oxidative stress in oesophageal (SNO) cancer cells. Toxicon 2018, 141, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Abdul, N.S.; Chuturgoon, A.A. Fumonisin B1 regulates LDL receptor and ABCA1 expression in an LXR dependent mechanism in liver (HepG2) cells. Toxicon 2021, 190, 58–64. [Google Scholar] [CrossRef]
- Chuturgoon, A.A.; Phulukdaree, A.; Moodley, D. Fumonisin B1 inhibits apoptosis in HepG2 cells by inducing Birc-8/ILP-2. Toxicol. Lett. 2015, 235, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Stevens, V.L.; Tang, J. Fumonisin B1-induced sphingolipid depletion inhibits vitamin uptake via the glycosylphosphatidylinositol-anchored folate receptor. J. Biol. Chem. 1997, 272, 18020–18025. [Google Scholar] [CrossRef]
- Bouhet, S.; Hourcade, E.; Loiseau, N.; Fikry, A.; Martinez, S.; Roselli, M.; Galtier, P.; Mengheri, E.; Oswald, I.P. The mycotoxin fumonisin B1 alters the proliferation and the barrier function of porcine intestinal epithelial cells. Toxicol. Sci. 2004, 77, 165–171. [Google Scholar] [CrossRef]
- Domijan, A.M.; Abramov, A.Y. Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis—Implication to mechanism of cell toxicity. Int. J. Biochem. Cell Biol. 2011, 43, 897–904. [Google Scholar] [CrossRef]
- Merrill, A.H.; Sullards, M.C.; Wang, E.; Voss, K.A.; Riley, R.T. Sphingolipid metabolism: Roles in signal transduction and disruption by fumonisins. Environ. Health Perspect. 2001, 109 (Suppl. S2), 283–289. [Google Scholar]
- Du, M.; Liu, Y.; Zhang, G. Interaction of aflatoxin B1 and fumonisin B1 in HepG2 cell apoptosis. Food Biosci. 2017, 20, 131–140. [Google Scholar] [CrossRef]
- Mary, V.S.; Arias, S.L.; Otaiza, S.N.; Velez, P.A.; Rubinstein, H.R.; Theumer, M.G. The aflatoxin B1-fumonisin B1 toxicity in BRL-3A hepatocytes is associated to induction of cytochrome P450 activity and arachidonic acid metabolism. Environ. Toxicol. 2017, 32, 1711–1724. [Google Scholar] [CrossRef]
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Filip, V.N.; Rajkovic, A. New insights into the combined toxicity of aflatoxin B1 and fumonisin B1 in HepG2 cells using Seahorse respirometry analysis and RNA transcriptome sequencing. Environ. Int. 2023, 175, 107945. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.B.; Williams, D.E.; Spitsbergen, J.M.; Ross, P.F.; Bacon, C.W.; Meredith, F.I.; Riley, R.T. Fumonisin B1 promotes aflatoxin B1 and N-methyl-N′-nitronitrosoguanidine-initiated liver tumors in rainbow trout. Toxicol. Appl. Pharmacol. 2001, 172, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.; Matute, J.; Gelineau-Van Waes, J.; Maddox, J.R.; Gregory, S.G.; Ashley-Koch, A.E.; Showker, J.L.; Voss, K.A.; Riley, R.T. Human health implications from co-exposure to aflatoxins and fumonisins in maize-based foods in Latin America: Guatemala as a case study. World Mycotoxin J. 2015, 8, 143–159. [Google Scholar] [CrossRef]
- McKean, C.; Tang, L.; Tang, M.; Billam, M.; Wang, Z.; Theodorakis, C.W.; Kendall, R.J.; Wang, J.S. Comparative acute and combinative toxicity of aflatoxin B1 and fumonisin B1 in animals and human cells. Food Chem. Toxicol. 2006, 44, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Hew, Y.C.; Ong, C.N. Inhibition of aflatoxinB1-induced cell injury by selenium: An in vitro study. Hum. Exp. Toxicol. 1995, 14, 55–60. [Google Scholar] [CrossRef]
- Liao, S.; Shi, D.; Clemons-Chevis, C.L.; Guo, S.; Su, R.; Qiang, P.; Tang, Z. Protective role of Selenium on aflatoxin B1-induced hepatic dysfunction and apoptosis of liver in ducklings. Biol Trace Elem Res. 2014, 162, 296–301. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Ma, L.B.; Zhang, W.P.; Khalil, M.M.; Karrow, N.A.; Qi, D.S.; Sun, L.H. Dietary se deficiency dysregulates metabolic and cell death signaling in aggravating the afb1 hepatotoxicity of chicks. Food Chem. Toxicol. 2021, 149, 8. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Zhang, M.; Yang, L.; Cheng, B.; Li, J.; Shan, A. Individual and combined effects of Fusarium toxins on apoptosis in PK15 cells and the protective role of N-acetylcysteine. Food Chem. Toxicol. 2018, 111, 27–43. [Google Scholar] [CrossRef]
- Valdivia, A.G.; Martinez, A.; Damian, F.J.; Quezada, T.; Ortiz, R.; Martinez, C.; Llamas, J.; Rodríguez, M.L.; Yamamoto, L.; Jaramillo, F.; et al. Efficacy of N-acetylcysteine to reduce the effects of aflatoxin B1 intoxication in broiler chickens. Poult. Sci. 2011, 80, 727–734. [Google Scholar] [CrossRef]
- Alpsoy, L.; Yildirim, A.; Agar, G. The antioxidant effects of vitamin A, C, and E on aflatoxin B1-induced oxidative stress in human lymphocytes. Toxicol. Ind. Health 2009, 25, 121–127. [Google Scholar] [CrossRef]
- Turkez, H.; Geyikoglu, F. Boric acid: A potential chemoprotective agent against aflatoxin b 1 toxicity in human blood. Cytotechnology 2010, 62, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Zhang, Y.J.; Blaner, W.S.; Santella, R.M. Influence of vitamins a, c, and e and beta-carotene on aflatoxin b1 binding to dna in woodchuck hepatocytes. Cancer 2015, 73, 596–604. [Google Scholar] [CrossRef]
- Mobio, T.A.; Baudrimont, M.; Sanni, A.; Shier, T.W.; Saboureau, D.; Dano, S.D.; Ueno, Y.; Steyn, P.S.; Creppy, E.E. Prevention by vitamin e of dna fragmentation and apoptosis induced by fumonisin B1 in C6 glioma cells. Arch Toxikol. 2000, 74, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.M.; Pieters, R.; van der Walt, A.M.; Bezuidenhout, C.C.; Abdel-Azeim, S.H.; Abdel-Wahhab, M.A. Protective effect of traditional african vegetable (Amaranthus hybridus) against aflatoxin B1 and/or fumonisin b1 in a rat hepatoma cell-line. Toxicol. Lett. 2013, 221, S159. [Google Scholar] [CrossRef]
- Ibrahim, M.I.M.; Pieters, R.; Abdel-Aziem, S.H.; Walt, A.M.V.D.; Bezuidenhout, C.C.; Giesy, J.P.; Abdel-Wahhab, M.A. Protective effects of Amaranthus hybridus against aflatoxin b1 and fumonisin b1 induced genotoxicity in h4iieluc cells. Hepatoma Res. 2015, 1, 11. [Google Scholar]
- Offord, E.A.; Mace, K.; Avanti, O.; Pfeifer, A.M. Mechanism involved in the chemoprotective effects of rosemary extract studied in human liver and bronchial cells. Cancer Lett. 1997, 114, 275–281. [Google Scholar] [CrossRef]
- Balentine, D.A.; Albano, M.C.; Nair, M.G. Role of Medicinal Plants, Herbs, and Spices in Protecting Human Health. Nutr. Rev. 2009, 57, 41–45. [Google Scholar] [CrossRef]
- Kusamran, W.R.; Ratanavila, A.; Tepsuwan, A. Effect of neem flowers, Thai and Chinese bitter gourd fruits and sweet basil leaves on hepatic monooxygenases and glutathione S-transferase activities, and in vitro metabolic activation of chemical carcinogens in rats. Food Chem. Toxicol. 1998, 36, 475–484. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Bardhi, A.; Barbarossa, A.; Montanucci, L.; Zaghini, A.; Dacasto, M. Discovering the protective effects of quercetin on aflatoxin b1-induced toxicity in bovine foetal hepatocyte-derived cells (BFH12). Toxins 2023, 15, 555. [Google Scholar] [CrossRef]
- Elbasuni, S.S.; Ibrahim, S.S.; Elsabagh, R.; Nada, M.O.; Elshemy, M.A.; Ismail, A.K.; Mansour, H.M.; Ghamry, H.I.; Ibrahim, S.F.; Alsaati, I.; et al. The Preferential Therapeutic Potential of Chlorella vulgaris against Aflatoxin-Induced Hepatic Injury in Quail. Toxins 2022, 14, 843. [Google Scholar] [CrossRef]
- Ledur, P.C.; Santurio, J.M. Cytoprotective effects of curcumin and silymarin on pk-15 cells exposed to ochratoxin a, fumonisin b1 and deoxynivalenol. Toxicon 2020, 85, 97–103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; F. Abdallah, M.; Chen, X.; Rajkovic, A. Current Knowledge of Individual and Combined Toxicities of Aflatoxin B1 and Fumonisin B1 In Vitro. Toxins 2023, 15, 653. https://doi.org/10.3390/toxins15110653
Chen X, F. Abdallah M, Chen X, Rajkovic A. Current Knowledge of Individual and Combined Toxicities of Aflatoxin B1 and Fumonisin B1 In Vitro. Toxins. 2023; 15(11):653. https://doi.org/10.3390/toxins15110653
Chicago/Turabian StyleChen, Xiangrong, Mohamed F. Abdallah, Xiangfeng Chen, and Andreja Rajkovic. 2023. "Current Knowledge of Individual and Combined Toxicities of Aflatoxin B1 and Fumonisin B1 In Vitro" Toxins 15, no. 11: 653. https://doi.org/10.3390/toxins15110653
APA StyleChen, X., F. Abdallah, M., Chen, X., & Rajkovic, A. (2023). Current Knowledge of Individual and Combined Toxicities of Aflatoxin B1 and Fumonisin B1 In Vitro. Toxins, 15(11), 653. https://doi.org/10.3390/toxins15110653







