Effects of the Toxic Non-Protein Amino Acid β-Methylamino-L-Alanine (BMAA) on Intracellular Amino Acid Levels in Neuroblastoma Cells
Abstract
:1. Introduction
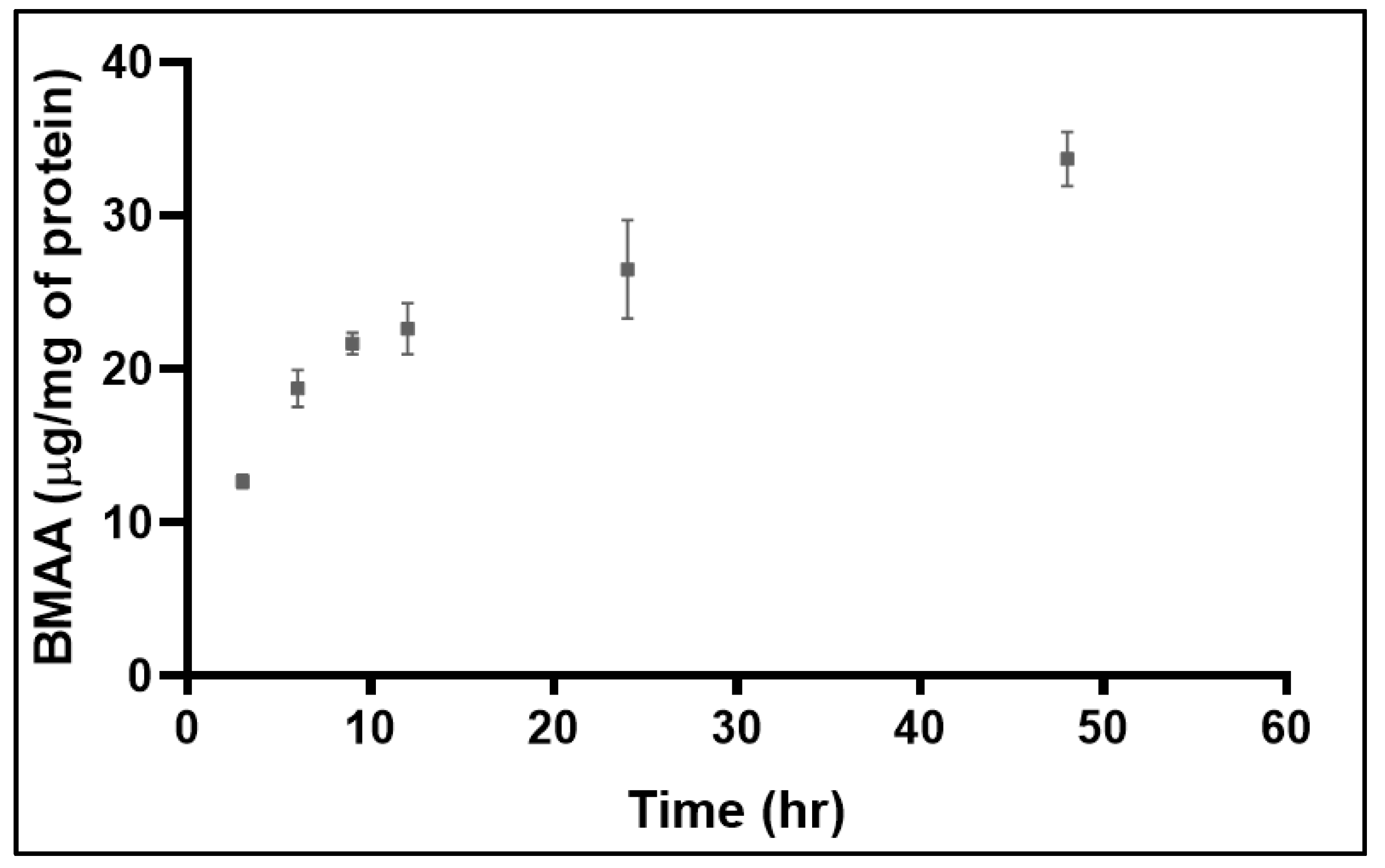
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Cell Culture and Treatment
5.2. Amino Acid Extraction
5.3. Protein Assay
5.4. HILIC-TQMS
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ajroud-Driss, S.; Siddique, T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 679–684. [Google Scholar] [CrossRef]
- Al-Chalabi, A.; Calvo, A.; Chio, A.; Colville, S.; Ellis, C.M.; Hardiman, O.; Heverin, M.; Howard, R.S.; Huisman, M.H.; Keren, N.; et al. Analysis of amyotrophic lateral sclerosis as a multistep process: A population-based modelling study. Lancet. Neurol. 2014, 13, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Al-Chalabi, A.; Hardiman, O. The epidemiology of ALS: A conspiracy of genes, environment and time. Nat. Rev. Neurol. 2013, 9, 617–628. [Google Scholar] [CrossRef]
- McComas, A.J.; Upton, A.R.; Sica, R.E. Motoneurone disease and ageing. Lancet 1973, 2, 1477–1480. [Google Scholar] [CrossRef]
- Violi, J.P.; Mitrovic, S.M.; Colville, A.; Main, B.J.; Rodgers, K.J. Prevalence of beta-methylamino-L-alanine (BMAA) and its isomers in freshwater cyanobacteria isolated from eastern Australia. Ecotoxicol. Environ. Saf. 2019, 172, 72–81. [Google Scholar] [CrossRef]
- Torbick, N.; Ziniti, B.; Stommel, E.; Linder, E.; Andrew, A.; Caller, T.; Haney, J.; Bradley, W.; Henegan, P.L.; Shi, X. Assessing Cyanobacterial Harmful Algal Blooms as Risk Factors for Amyotrophic Lateral Sclerosis. Neurotox. Res. 2018, 33, 199–212. [Google Scholar] [CrossRef]
- Caller, T.A.; Doolin, J.W.; Haney, J.F.; Murby, A.J.; West, K.G.; Farrar, H.E.; Ball, A.; Harris, B.T.; Stommel, E.W. A cluster of amyotrophic lateral sclerosis in New Hampshire: A possible role for toxic cyanobacteria blooms. Amyotroph. Lateral Scler. 2009, 10 (Suppl. S2), 101–108. [Google Scholar] [CrossRef]
- Cawte, J.; Kilburn, C.; Florence, M. Motor neurone disease of the western Pacific: Do the foci extend to Australia? Neurotoxicology 1989, 10, 263–270. [Google Scholar]
- Garruto, R.M.; Gajdusek, D.C.; Chen, K.M. Amyotrophic lateral sclerosis and parkinsonism-dementia among Filipino migrants to Guam. Ann. Neurol. 1981, 10, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Goutman, S.A.; Savelieff, M.G.; Jang, D.G.; Hur, J.; Feldman, E.L. The amyotrophic lateral sclerosis exposome: Recent advances and future directions. Nat. Rev. Neurol. 2023, 19, 617–634. [Google Scholar] [CrossRef]
- Longinetti, E.; Pupillo, E.; Belometti, C.; Bianchi, E.; Poloni, M.; Fang, F.; Beghi, E. Geographical clusters of amyotrophic lateral sclerosis and the Bradford Hill criteria. Amyotroph. Lateral Scler. Front. Degener. 2022, 23, 329–343. [Google Scholar] [CrossRef]
- Newell, M.E.; Adhikari, S.; Halden, R.U. Systematic and state-of the science review of the role of environmental factors in Amyotrophic Lateral Sclerosis (ALS) or Lou Gehrig’s Disease. Sci. Total Environ. 2022, 817, 152504. [Google Scholar] [CrossRef]
- Cox, P.A.; Banack, S.A.; Murch, S.J.; Rasmussen, U.; Tien, G.; Bidigare, R.R.; Metcalf, J.S.; Morrison, L.F.; Codd, G.A.; Bergman, B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc. Natl. Acad. Sci. USA 2005, 102, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Reveillon, D.; Abadie, E.; Sechet, V.; Masseret, E.; Hess, P.; Amzil, Z. beta-N-methylamino-l-alanine (BMAA) and isomers: Distribution in different food web compartments of Thau lagoon, French Mediterranean Sea. Mar. Environ. Res. 2015, 110, 8–18. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Codd, G.A. Cyanobacteria, neurotoxins and water resources: Are there implications for human neurodegenerative disease? Amyotroph. Lateral Scler. 2009, 10 (Suppl. S2), 74–78. [Google Scholar] [CrossRef] [PubMed]
- Koreiviene, J.; Anne, O.; Kasperoviciene, J.; Burskyte, V. Cyanotoxin management and human health risk mitigation in recreational waters. Environ. Monit. Assess. 2014, 186, 4443–4459. [Google Scholar] [CrossRef]
- Arnold, A.; Edgren, D.C.; Palladino, V.S. Amyotrophic lateral sclerosis; fifty cases observed on Guam. J. Nerv. Ment. Dis. 1953, 117, 135–139. [Google Scholar] [CrossRef]
- Kurland, L.T.; Mulder, D.W. Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution, with special reference to the Mariana Islands, including clinical and pathologic observations. Neurology 1954, 4, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Nunn, P.B. 50 years of research on alpha-amino-beta-methylaminopropionic acid (beta-methylaminoalanine). Phytochemistry 2017, 144, 271–281. [Google Scholar] [CrossRef]
- Polsky, F.I.; Nunn, P.B.; Bell, E.A. Distribution and toxicity of alpha-amino-beta-methylaminopropionic acid. Fed. Proc. 1972, 31, 1473–1475. [Google Scholar]
- Cheng, R.; Banack, S.A. Previous studies underestimate BMAA concentrations in cycad flour. Amyotroph. Lateral Scler. 2009, 10 (Suppl. S2), 41–43. [Google Scholar] [CrossRef] [PubMed]
- Violi, J.P.; Facey, J.A.; Mitrovic, S.M.; Colville, A.; Rodgers, K.J. Production of beta-methylamino-L-alanine (BMAA) and Its Isomers by Freshwater Diatoms. Toxins 2019, 11, 512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Eriksson, J.; Lage, S.; Jonasson, S.; Shams, S.; Mehine, M.; Ilag, L.L.; Rasmussen, U. Diatoms: A novel source for the neurotoxin BMAA in aquatic environments. PLoS ONE 2014, 9, e84578. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H. Nutrient and other environmental controls of harmful cyanobacterial blooms along the freshwater-marine continuum. Adv. Exp. Med. Biol. 2008, 619, 217–237. [Google Scholar] [CrossRef]
- Mitrovic, S.M.; Hardwick, L.; Dorani, F. Use of flow management to mitigate cyanobacterial blooms in the Lower Darling River, Australia. J. Plankton Res. 2010, 33, 229–241. [Google Scholar] [CrossRef]
- Garamszegi, S.P.; Banack, S.A.; Duque, L.L.; Metcalf, J.S.; Stommel, E.W.; Cox, P.A.; Davis, D.A. Detection of beta-N-methylamino-l-alanine in postmortem olfactory bulbs of Alzheimer’s disease patients using UHPLC-MS/MS: An autopsy case-series study. Toxicol. Rep. 2023, 10, 87–96. [Google Scholar] [CrossRef]
- Hu, C.; Rzymski, P. Non-Photosynthetic Melainabacteria (Cyanobacteria) in Human Gut: Characteristics and Association with Health. Life 2022, 12, 476. [Google Scholar] [CrossRef] [PubMed]
- Karamyan, V.T.; Speth, R.C. Animal models of BMAA neurotoxicity: A critical review. Life Sci. 2008, 82, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Davis, D.A.; Mash, D.C.; Metcalf, J.S.; Banack, S.A. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proc. Biol. Sci. 2016, 283, 20152397. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Cox, P.A.; Banack, S.A.; Lecusay, P.D.; Garamszegi, S.P.; Hagan, M.J.; Powell, J.T.; Metcalf, J.S.; Palmour, R.M.; Beierschmitt, A.; et al. l-Serine Reduces Spinal Cord Pathology in a Vervet Model of Preclinical ALS/MND. J. Neuropathol. Exp. Neurol. 2020, 79, 393–406. [Google Scholar] [CrossRef]
- Dunlop, R.A.; Cox, P.A.; Banack, S.A.; Rodgers, K.J. The Non-Protein Amino Acid BMAA Is Misincorporated into Human Proteins in Place of l-Serine Causing Protein Misfolding and Aggregation. PLoS ONE 2013, 8, e75376. [Google Scholar] [CrossRef]
- Main, B.J.; Dunlop, R.A.; Rodgers, K.J. The use of l-serine to prevent beta-methylamino-l-alanine (BMAA)-induced proteotoxic stress in vitro. Toxicon 2016, 109, 7–12. [Google Scholar] [CrossRef]
- Dunlop, R.A.; Powell, J.; Guillemin, G.J.; Cox, P.A. Mechanisms of L-Serine Neuroprotection in vitro Include ER Proteostasis Regulation. Neurotox. Res. 2018, 33, 123–132. [Google Scholar] [CrossRef]
- Dunlop, R.A.; Powell, J.T.; Metcalf, J.S.; Guillemin, G.J.; Cox, P.A. L-Serine-Mediated Neuroprotection Includes the Upregulation of the ER Stress Chaperone Protein Disulfide Isomerase (PDI). Neurotox. Res. 2018, 33, 113–122. [Google Scholar] [CrossRef]
- Quinn, A.W.; Phillips, C.R.; Violi, J.P.; Steele, J.R.; Johnson, M.S.; Westerhausen, M.T.; Rodgers, K.J. beta-Methylamino-L-alanine-induced protein aggregation in vitro and protection by L-serine. Amino Acids 2021, 53, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Engskog, M.K.; Ersson, L.; Haglof, J.; Arvidsson, T.; Pettersson, C.; Brittebo, E. beta-N-Methylamino-L-alanine (BMAA) perturbs alanine, aspartate and glutamate metabolism pathways in human neuroblastoma cells as determined by metabolic profiling. Amino Acids 2017, 49, 905–919. [Google Scholar] [CrossRef]
- Violi, J.P.; Bishop, D.P.; Padula, M.P.; Westerhausen, M.T.; Rodgers, K.J. Acetonitrile adduct analysis of underivatised amino acids offers improved sensitivity for hydrophilic interaction liquid chromatography tandem mass-spectrometry. J. Chromatogr. A 2021, 1655, 462530. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.; Korolchuk, V.I.; Sarkar, S. Amino acids and autophagy: Cross-talk and co-operation to control cellular homeostasis. Amino Acids 2015, 47, 2065–2088. [Google Scholar] [CrossRef]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef] [PubMed]
- Vabulas, R.M.; Hartl, F.U. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 2005, 310, 1960–1963. [Google Scholar] [CrossRef] [PubMed]
- Van Onselen, R.; Downing, T.G. BMAA-protein interactions: A possible new mechanism of toxicity. Toxicon 2018, 143, 74–80. [Google Scholar] [CrossRef]
- Glover, W.B.; Mash, D.C.; Murch, S.J. The natural non-protein amino acid N-beta-methylamino-L-alanine (BMAA) is incorporated into protein during synthesis. Amino Acids 2014, 46, 2553–2559. [Google Scholar] [CrossRef]
- Han, N.C.; Bullwinkle, T.J.; Loeb, K.F.; Faull, K.F.; Mohler, K.; Rinehart, J.; Ibba, M. The mechanism of beta-N-methylamino-l-alanine inhibition of tRNA aminoacylation and its impact on misincorporation. J. Biol. Chem. 2020, 295, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaie, L.; Klomp, L.W.; Berger, R.; de Koning, T.J. L-serine synthesis in the central nervous system: A review on serine deficiency disorders. Mol. Genet. Metab. 2010, 99, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, O.D.; Berkers, C.R.; Mason, S.M.; Zheng, L.; Blyth, K.; Gottlieb, E.; Vousden, K.H. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature 2013, 493, 542–546. [Google Scholar] [CrossRef]
- Maddocks, O.D.K.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; van den Broek, N.J.F.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017, 544, 372–376. [Google Scholar] [CrossRef]
- Engskog, M.K.; Karlsson, O.; Haglof, J.; Elmsjo, A.; Brittebo, E.; Arvidsson, T.; Pettersson, C. The cyanobacterial amino acid beta-N-methylamino-l-alanine perturbs the intermediary metabolism in neonatal rats. Toxicology 2013, 312, 6–11. [Google Scholar] [CrossRef]
- Holecek, M. Roles of malate and aspartate in gluconeogenesis in various physiological and pathological states. Metabolism 2023, 145, 155614. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.A.; Allen, A.E.; Liu, S.; Liberti, M.V.; Liu, P.; Liu, X.; Dai, Z.; Gao, X.; Wang, Q.; Liu, Y.; et al. Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism. Nat. Commun. 2018, 9, 5442. [Google Scholar] [CrossRef] [PubMed]
- Esaki, K.; Sayano, T.; Sonoda, C.; Akagi, T.; Suzuki, T.; Ogawa, T.; Okamoto, M.; Yoshikawa, T.; Hirabayashi, Y.; Furuya, S. L-Serine Deficiency Elicits Intracellular Accumulation of Cytotoxic Deoxysphingolipids and Lipid Body Formation. J. Biol. Chem. 2015, 290, 14595–14609. [Google Scholar] [CrossRef] [PubMed]
- Brassier, A.; Valayannopoulos, V.; Bahi-Buisson, N.; Wiame, E.; Hubert, L.; Boddaert, N.; Kaminska, A.; Habarou, F.; Desguerre, I.; Van Schaftingen, E.; et al. Two new cases of serine deficiency disorders treated with l-serine. Eur. J. Paediatr. Neurol. 2016, 20, 53–60. [Google Scholar] [CrossRef]
- Van der Crabben, S.N.; Verhoeven-Duif, N.M.; Brilstra, E.H.; Van Maldergem, L.; Coskun, T.; Rubio-Gozalbo, E.; Berger, R.; de Koning, T.J. An update on serine deficiency disorders. J. Inherit. Metab. Dis. 2013, 36, 613–619. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Dunlop, R.A.; Powell, J.T.; Banack, S.A.; Cox, P.A. L-Serine: A Naturally-Occurring Amino Acid with Therapeutic Potential. Neurotox. Res. 2018, 33, 213–221. [Google Scholar] [CrossRef]
- Razak, M.A.; Begum, P.S.; Viswanath, B.; Rajagopal, S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxid. Med. Cell Longev. 2017, 2017, 1716701. [Google Scholar] [CrossRef]
- Dos Santos Fagundes, I.; Rotta, L.N.; Schweigert, I.D.; Valle, S.C.; de Oliveira, K.R.; Huth Kruger, A.; Souza, K.B.; Souza, D.O.; Perr, M.L. Glycine, serine, and leucine metabolism in different regions of rat central nervous system. Neurochem. Res. 2001, 26, 245–249. [Google Scholar] [CrossRef]
- Baronio, D.; Gonchoroski, T.; Castro, K.; Zanatta, G.; Gottfried, C.; Riesgo, R. Histaminergic system in brain disorders: Lessons from the translational approach and future perspectives. Ann. Gen. Psychiatry 2014, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Merel, S.; Walker, D.; Chicana, R.; Snyder, S.; Baures, E.; Thomas, O. State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 2013, 59, 303–327. [Google Scholar] [CrossRef]
- Panula, P.; Airaksinen, M.S.; Pirvola, U.; Kotilainen, E. A histamine-containing neuronal system in human brain. Neuroscience 1990, 34, 127–132. [Google Scholar] [CrossRef]
- Holecek, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- De Munck, E.; Munoz-Saez, E.; Miguel, B.G.; Solas, M.T.; Ojeda, I.; Martinez, A.; Gil, C.; Arahuetes, R.M. beta-N-methylamino-l-alanine causes neurological and pathological phenotypes mimicking Amyotrophic Lateral Sclerosis (ALS): The first step towards an experimental model for sporadic ALS. Environ. Toxicol. Pharmacol. 2013, 36, 243–255. [Google Scholar] [CrossRef]
- Liu, X.; Rush, T.; Zapata, J.; Lobner, D. beta-N-methylamino-l-alanine induces oxidative stress and glutamate release through action on system Xc(-). Exp. Neurol. 2009, 217, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.J.; Hume, P.M.; Dunlop, R.A.; Dean, R.T. Biosynthesis and turnover of DOPA-containing proteins by human cells. Free Radic. Biol. Med. 2004, 37, 1756–1764. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Banack, S.A.; Murch, S.J. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. USA 2003, 100, 13380–13383. [Google Scholar] [CrossRef] [PubMed]
| Time (h) | Ala | Arg | Asn | Asp | Cys | Glu | Gln | Gly | His | Ile | leu | Lys | Phe | Pro | Ser | Thr | Trp | Tyr | Val |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 130 ± 6.6 | 110 ± 3.5 | 140 ± 11 | 110 ± 3.9 | ND | 110 ± 3.6 | 100 ± 3.0 | 130 ± 8.3 | 120 ± 10 | 100 ± 4.4 | 100 ± 2.1 | 120 ± 3.3 | 110 ± 3.2 | 110 ± 3.2 | 100 ± 10 | 120 ± 4.2 | 100 ± 3.0 | 140 ± 10 | 110 ± 4.3 |
| 3 | 230 ± 12 *** | 140 ± 3.9 ** | 120 ± 13 | 140 ± 3.7 *** | ND | 150 ± 4.0 *** | 84 ± 2.2 * | 1200 ± 260 ** | 89 ± 5.7 | 91 ± 2.9 | 92 ± 2.2 | 140 ± 3.0 ** | 100 ± 2.6 | 97 ± 1.9 | 120 ± 9.3 | 160 ± 7.7 * | 94 ± 6.1 | 59 ± 3.5 * | 180 ± 8.5 * |
| 6 | 95 ± 5.4 | 130 ± 8.1 | 84 ± 8.0 | 93 ± 4.3 | 110 ± 5.2 | 91 ± 4.2 | 93 ± 4.7 | 89 ± 6.9 | 110 ± 4.8 | 110 ± 4.8 | 99 ± 4.2 | 130 ± 5.8 | 91 ± 3.9 | 95 ± 4.0 | 100 ± 9.2 | 83 ± 5.9 | 95 ± 3.3 | 110 ± 9.1 | 110 ± 6.0 |
| 9 | 62 ± 4.5 * | 74 ± 4.0 * | 23 ± 3.8 * | 70 ± 4.2 * | 88 ± 3.6 | 73 ± 4.2 * | 65 ± 4.3 * | 63 ± 4.3 | 74 ± 2.5 * | 48 ± 3.9 ** | 81 ± 3.2 | 83 ± 2.8 * | 66 ± 5.8 * | 74 ± 3.9 * | 27 ± 3.7 ** | 53 ± 2.4 * | 90 ± 2.2 | 59 ± 3.9 ** | 62 ± 1.7 ** |
| 12 | 97 ± 4.9 | 110 ± 7.2 | 95 ± 6.0 | 100 ± 4.6 | 100 ± 6.2 | 110 ± 5.7 | 110 ± 6.7 | 110 ± 6.9 | 85 ± 5.5 | 88 ± 5.0 | 86 ± 3.7 | 100 ± 6.2 | 88 ± 4.4 | 110 ± 6.4 | 66 ± 3.8 * | 100 ± 5.3 | 100 ± 8.3 | 100 ± 5.7 | 93 ± 4.6 |
| 24 | 95 ± 6.1 | 80 ± 5.8 | 75 ± 6.0 | 100 ± 7.1 | 100 ± 7.8 | 100 ± 7.7 | 88 ± 6.1 | 100 ± 6.3 | 74 ± 4.3 * | 91 ± 7.2 | 86 ± 6.9 | 88 ± 5.7 | 91 ± 7.1 | 92 ± 7.0 | 62 ± 5.2 * | 90 ± 5.9 | 100 ± 5.3 | 77 ± 5.8 | 81 ± 6.1 |
| 48 | 73 ± 5.2 * | 76 ± 4.4 | 59 ± 3.1 * | 70 ± 4.1 * | 110 ± 8.3 | 87 ± 7.4 | 86 ± 8.7 | 110 ± 17 | 74 ± 3.6 * | 66 ± 4.0 * | 82 ± 3.7 | 85 ± 4.3 | 71 ± 5.2 * | 80.0 ± 3.9 | 60 ± 4.5 * | 81 ± 4.3 | 100 ± 8.0 | 69 ± 3.1 * | 74 ± 3.5 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Violi, J.P.; Pu, L.; Pravadali-Cekic, S.; Bishop, D.P.; Phillips, C.R.; Rodgers, K.J. Effects of the Toxic Non-Protein Amino Acid β-Methylamino-L-Alanine (BMAA) on Intracellular Amino Acid Levels in Neuroblastoma Cells. Toxins 2023, 15, 647. https://doi.org/10.3390/toxins15110647
Violi JP, Pu L, Pravadali-Cekic S, Bishop DP, Phillips CR, Rodgers KJ. Effects of the Toxic Non-Protein Amino Acid β-Methylamino-L-Alanine (BMAA) on Intracellular Amino Acid Levels in Neuroblastoma Cells. Toxins. 2023; 15(11):647. https://doi.org/10.3390/toxins15110647
Chicago/Turabian StyleVioli, Jake P., Lisa Pu, Sercan Pravadali-Cekic, David P. Bishop, Connor R. Phillips, and Kenneth J. Rodgers. 2023. "Effects of the Toxic Non-Protein Amino Acid β-Methylamino-L-Alanine (BMAA) on Intracellular Amino Acid Levels in Neuroblastoma Cells" Toxins 15, no. 11: 647. https://doi.org/10.3390/toxins15110647
APA StyleVioli, J. P., Pu, L., Pravadali-Cekic, S., Bishop, D. P., Phillips, C. R., & Rodgers, K. J. (2023). Effects of the Toxic Non-Protein Amino Acid β-Methylamino-L-Alanine (BMAA) on Intracellular Amino Acid Levels in Neuroblastoma Cells. Toxins, 15(11), 647. https://doi.org/10.3390/toxins15110647






