Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors
Abstract
1. Introduction
2. Results
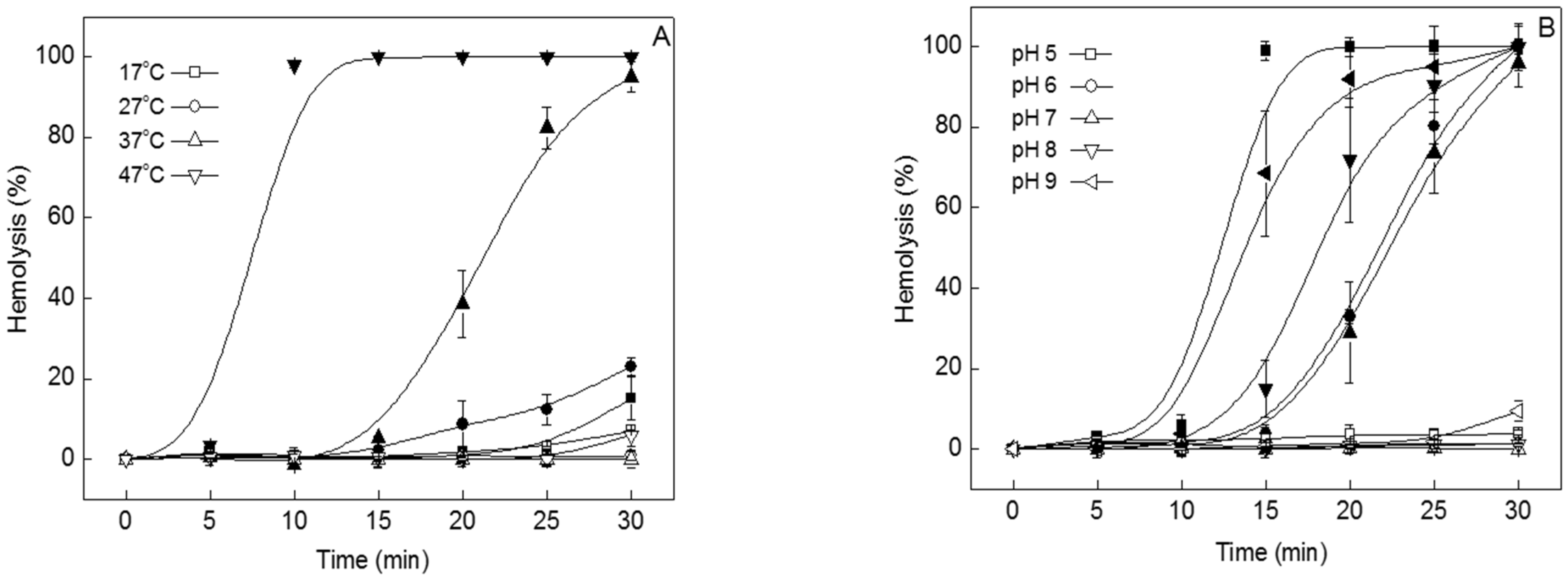
2.1. Temperature and pH Dependence of TIF 16 Effects
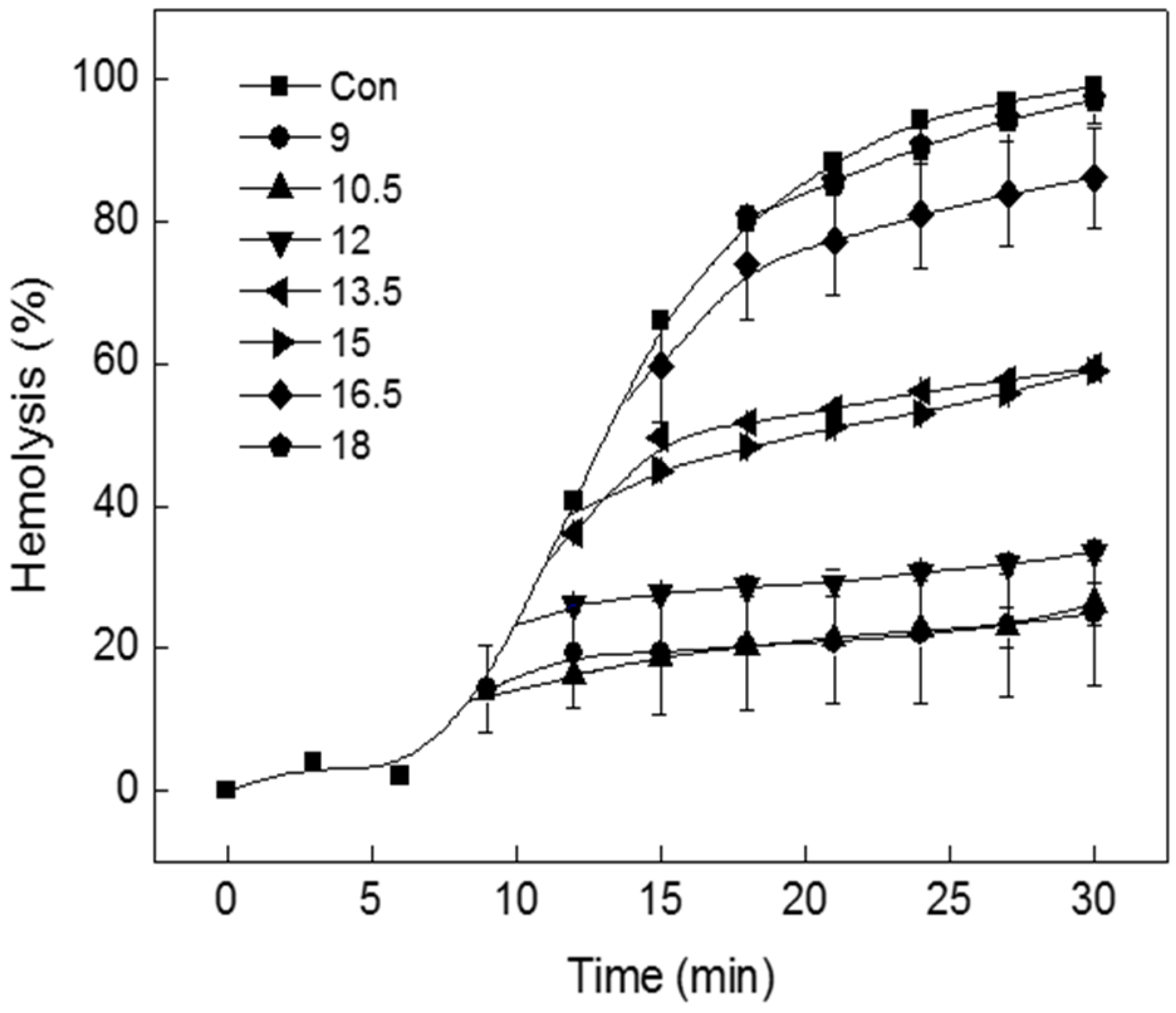
2.2. Effect of TIF 16 on Pretreated Tolaasin Hemolysis
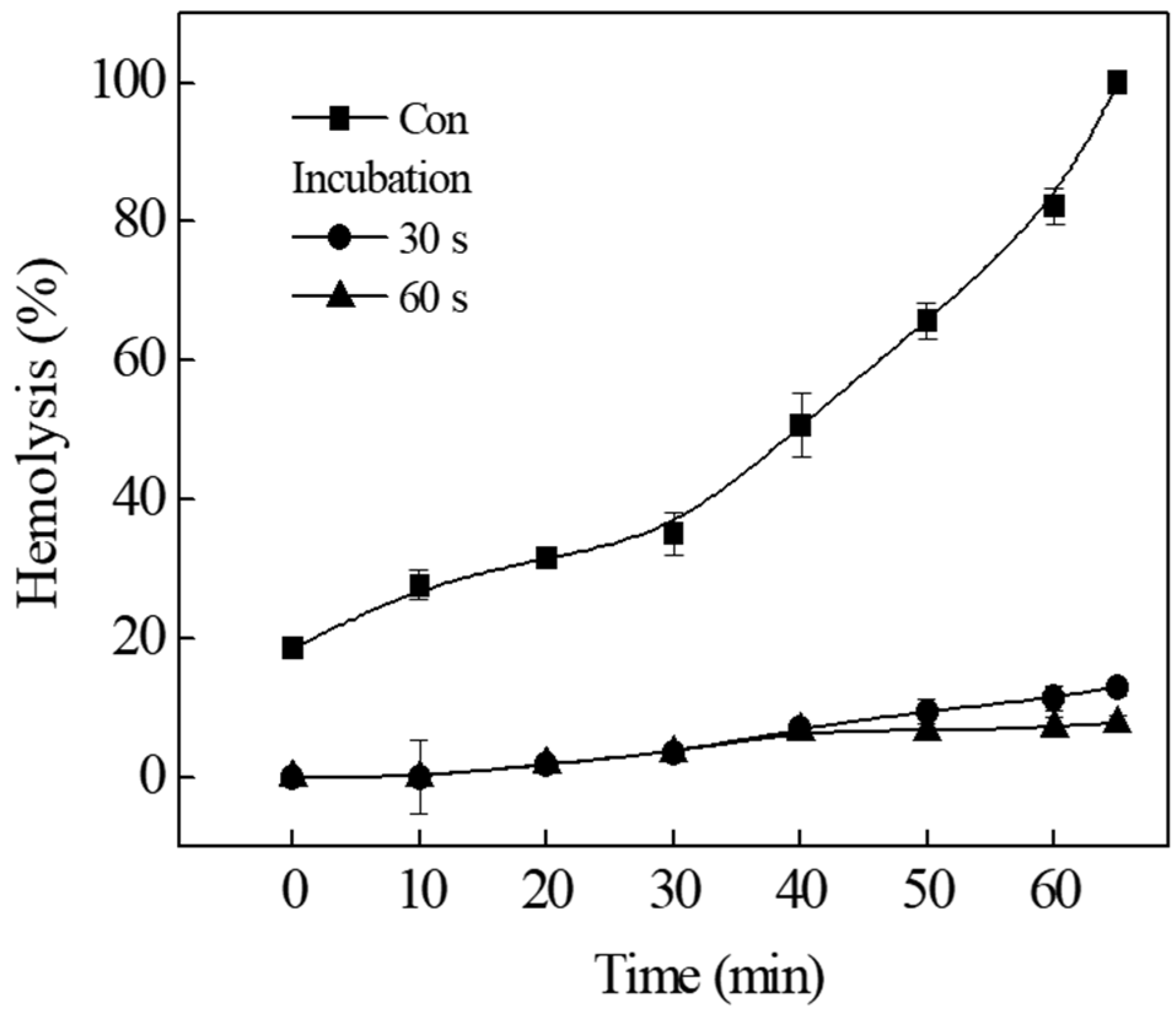
2.3. Irreversible Binding of TIF 16 to Erythrocyte Membrane
2.4. Inhibition of Tolaasin Channel by TIF 16
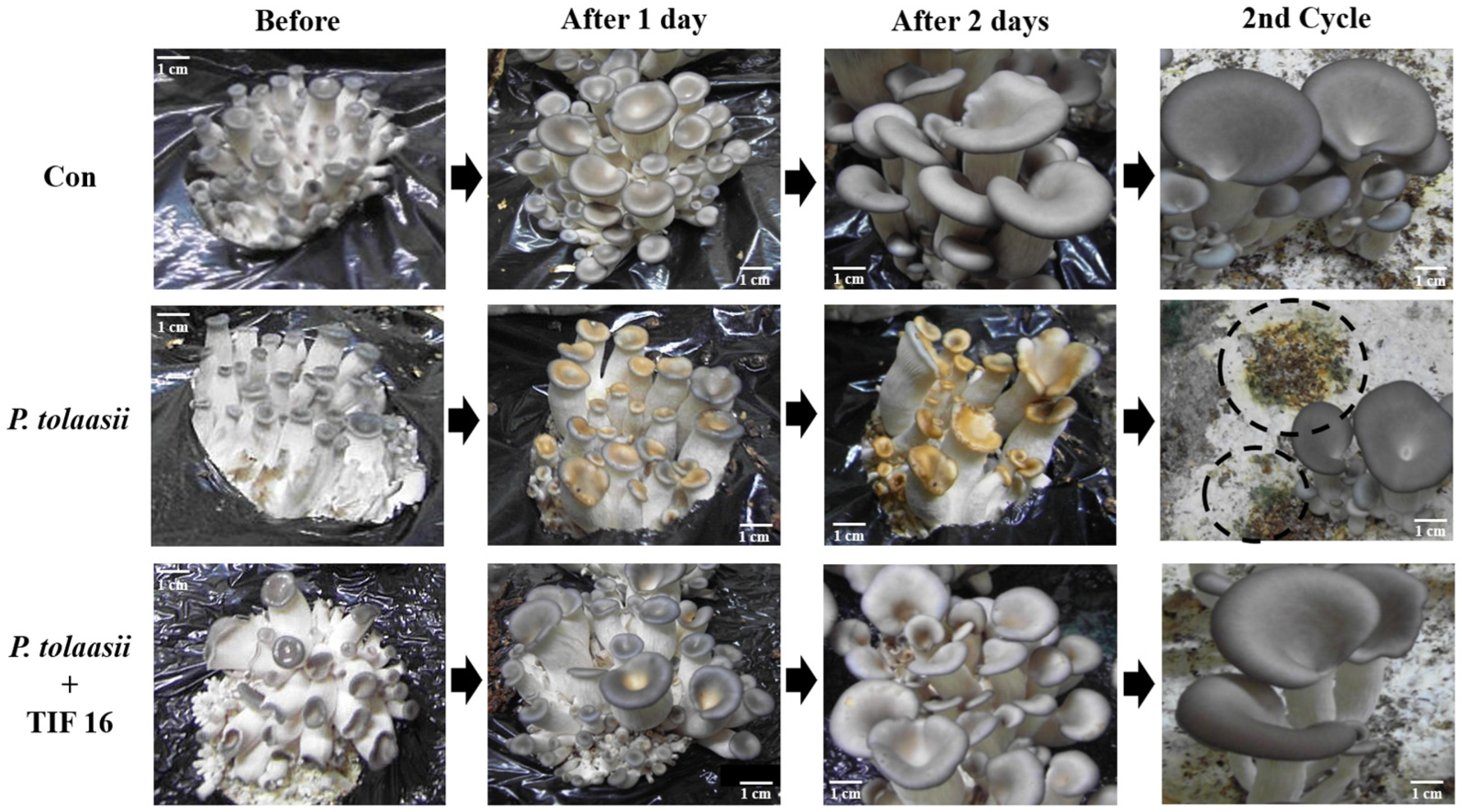
2.5. Effect of TIF 16 on Oyster Mushroom Cultivation
3. Discussion
4. Materials and Methods
4.1. Purification of Tolaasin and Its Hemolytic Activity
4.2. Effect of TIF 16 on Tolaasin Channel
4.3. Effects of TIF 16 on Oyster Mushroom Cultivation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolaas, A.G. A bacterial disease of cultivated mushrooms. Phytopathology 1915, 5, 51–54. [Google Scholar]
- Peng, J.T. Resistance to Disease in Agaricus bisporus (lange) Imbach. Ph.D. Thesis, University of Leeds, Leeds, UK, 1986. [Google Scholar]
- Hutchison, M.I.; Johnstone, K. Evidence for the involvement of the surface active properties of the extracellular toxin tolaasin in the manifestation of brown blotch disease symptoms by Pseudomonas tolaasii on Agaricus bisporus. Physiol. Mol. Plant. Pathol. 1993, 42, 273–384. [Google Scholar] [CrossRef]
- Otsuka, Y.; Ishikawa, T.; Takahashi, C.; Masuda, M. A short peptide derived from the ZorO toxin functions as an effective antimicrobial. Toxins 2019, 11, 392. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, Y.K. Two types of ion channel formation of tolaasin, a Pseudomonas peptide toxin. FEMS Microbiol. Lett. 2003, 221, 221–226. [Google Scholar] [CrossRef]
- Rainey, P.B.; Brodey, C.L.; Johnstone, K. Biological properties and spectrum of activity of tolaasin, a lipodepsipeptide toxin produced by the mushroom pathogen Pseudomonas tolaasii. Physiol. Mol. Plant. Pathol. 1991, 39, 57–70. [Google Scholar] [CrossRef]
- Wong, W.C.; Preece, T.F. Pseudomonas tolaasii in cultivated mushroom (Agaricus bisporus) crops: Effects of sodium hypochlorite on the bacterium and on blotch disease severity. J. Appl. Microbiol. 1985, 58, 259–267. [Google Scholar]
- Geels, F.P.; van Griensven, L.D.; Rutjens, A.J. Science and Cultivation of Edible Mushrooms. In Mushroom Science XIII; Maher, M.J., Ed.; Balkema: Rotterdam, The Netherland, 1991; pp. 437–442. [Google Scholar]
- Tajalipour, S.; Hassanzadeh, N.; Jolfaee, H.K.; Heydari, A.; Hasemi, A. Biological control of mushroom brown blotch disease using antagonistic bacteria. Biocontrol. Sci. Technol. 2014, 24, 473–484. [Google Scholar] [CrossRef]
- Geels, F.P. Pseudomonas tolaasii control by kasugamycin in cultivated mushroom (Agaricus bisporus). J. Appl. Microbiol. 1995, 79, 38–42. [Google Scholar]
- Yun, Y.B.; Kim, M.H.; Han, J.H.; Kim, Y.K. Suppression of brown blotch disease by tolaasin inhibitory factors. J. Appl. Biol. Chem. 2017, 60, 179–184. [Google Scholar] [CrossRef]
- Kim, S.T.; Choi, T.K.; Kim, Y.K. pH-dependent cytotoxicity of a peptide toxin, tolaasin. J. Korean Soc. Appl. Biol. Chem. 2007, 50, 257–261. [Google Scholar]
- Sun, D.; Yu, Y.; Xue, X.; Pan, M.; Wen, M.; Li, S.; Qu, Q.; Li, X.; Zhang, L.; Li, X.; et al. Cryo-EM structure of the ASIC1a-mambalgin-1 complex reveals that the peptide toxin mambalgin-1 inhibits acid-sensing ion channels through an unusual allosteric effect. Cell Discov. 2018, 4, 27. [Google Scholar] [CrossRef]
- Shewell, L.K.; Harvey, R.M.; Higgins, M.A.; Day, C.J.; Hartley-Tassell, L.E.; Chen, A.Y.; Gillen, C.M.; James, D.N.A.; Alonzo, F.; Torres, V.J.; et al. The cholesterol-dependent cytolysins pneumolysin and streptolysin O require binding to red blood cell glycans for hemolytic activity. Proc. Natl. Acad. Sci. USA 2014, 111, 5312–5320. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Moller, A.C.; Arpin, N.; Olivier, J.M.; Wichers, H.J. Induction of a tyrosinase mRNA in Agaricus bisporus upon treatment with a tolaasin preparation from Pseudomonas tolaasii. Physico. Mol. Plant Pathol. 2001, 58, 95–99. [Google Scholar] [CrossRef]
- Statt, S.; Ruan, J.W.; Hung, L.Y.; Chang, C.Y.; Huang, C.T.; Lim, J.H.; Li, J.D.; Wu, R.; Kao, C.Y. Statin-conferred enhanced cellular resistance against bacterial pore-forming toxins in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2015, 53, 689–702. [Google Scholar] [CrossRef]
- Omersa, N.; Podobnik, M.; Anderluh, G. Inhibition of pore-forming proteins. Toxins 2019, 11, 545. [Google Scholar] [CrossRef]
- Ganpule, S.; Vijaya, A.K.; Sukova, A.; Preta, G. Membrane cholesterol content and lipid organization influence melittin and pneumolysin pore-forming activity. Toxins 2022, 14, 346. [Google Scholar] [CrossRef]
- Cho, K.H.; Wang, H.S.; Kim, Y.K. Temperature-dependent hemolytic activity of membrane pore-forming peptide toxin, tolaasin. J. Pept. Sci. 2010, 16, 85–90. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific Opinion on the re-evaluation of polyglycerol esters of fatty acids (E 475) as a food additive. EFSA J. 2017, 15, 5089. [Google Scholar]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A membrane-active peptide with diverse functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef]
- Klocek, G.; Schulthess, T.; Shai, Y.; Seelig, J. Thermodynamics of melittin binding to lipid bilayers, aggregation and pore formation. Biochemistry 2009, 48, 2586–2596. [Google Scholar] [CrossRef]
- Mihajlovic, M.; Lazaridis, T. Antimicrobial peptides in toroidal and cylindrical pores. Biochim. Biophys. Acta Biomembr. 2010, 1798, 1485–1493. [Google Scholar] [CrossRef] [PubMed]
- Kanemitsu, S.; Morita, K.; Tominaga, Y.; Nishimura, K.; Yashiro, T.; Sakurai, H.; Yamamoto, Y.; Kurisaki, I.; Tanaka, S.; Matsui, M.; et al. Inhibition of melittin activity using a small molecule with an indole ring. J. Phys. Chem. B 2022, 126, 5793–5802. [Google Scholar] [CrossRef] [PubMed]
- Zakharian, E. Recording of ion channel activity in planar lipid bilayer experiments. Methods Mol. Biol. 2013, 998, 109–118. [Google Scholar] [PubMed]
 ) and H.P. mean closed state and holding potential, respectively.
) and H.P. mean closed state and holding potential, respectively.
 ) and H.P. mean closed state and holding potential, respectively.
) and H.P. mean closed state and holding potential, respectively.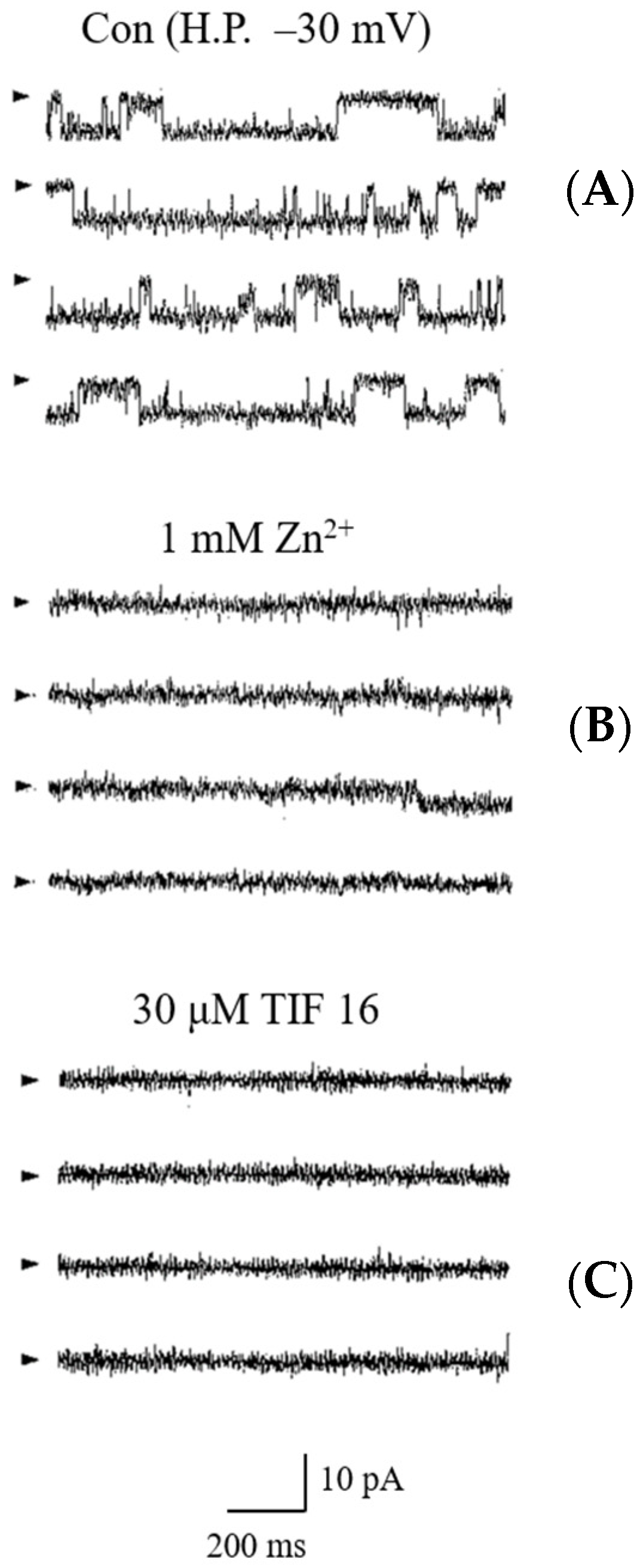
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.-B.; Cho, K.-H.; Kim, Y.-K. Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors. Toxins 2023, 15, 66. https://doi.org/10.3390/toxins15010066
Yun Y-B, Cho K-H, Kim Y-K. Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors. Toxins. 2023; 15(1):66. https://doi.org/10.3390/toxins15010066
Chicago/Turabian StyleYun, Yeong-Bae, Kwang-Hyun Cho, and Young-Kee Kim. 2023. "Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors" Toxins 15, no. 1: 66. https://doi.org/10.3390/toxins15010066
APA StyleYun, Y.-B., Cho, K.-H., & Kim, Y.-K. (2023). Inhibition of Tolaasin Cytotoxicity Causing Brown Blotch Disease in Cultivated Mushrooms Using Tolaasin Inhibitory Factors. Toxins, 15(1), 66. https://doi.org/10.3390/toxins15010066




