Anaerobes and Toxins, a Tradition of the Institut Pasteur
Abstract
1. Introduction
2. “Poisons” before the Pasteur’s Era
3. Anaerobes and Toxins in Pasteur’s Era
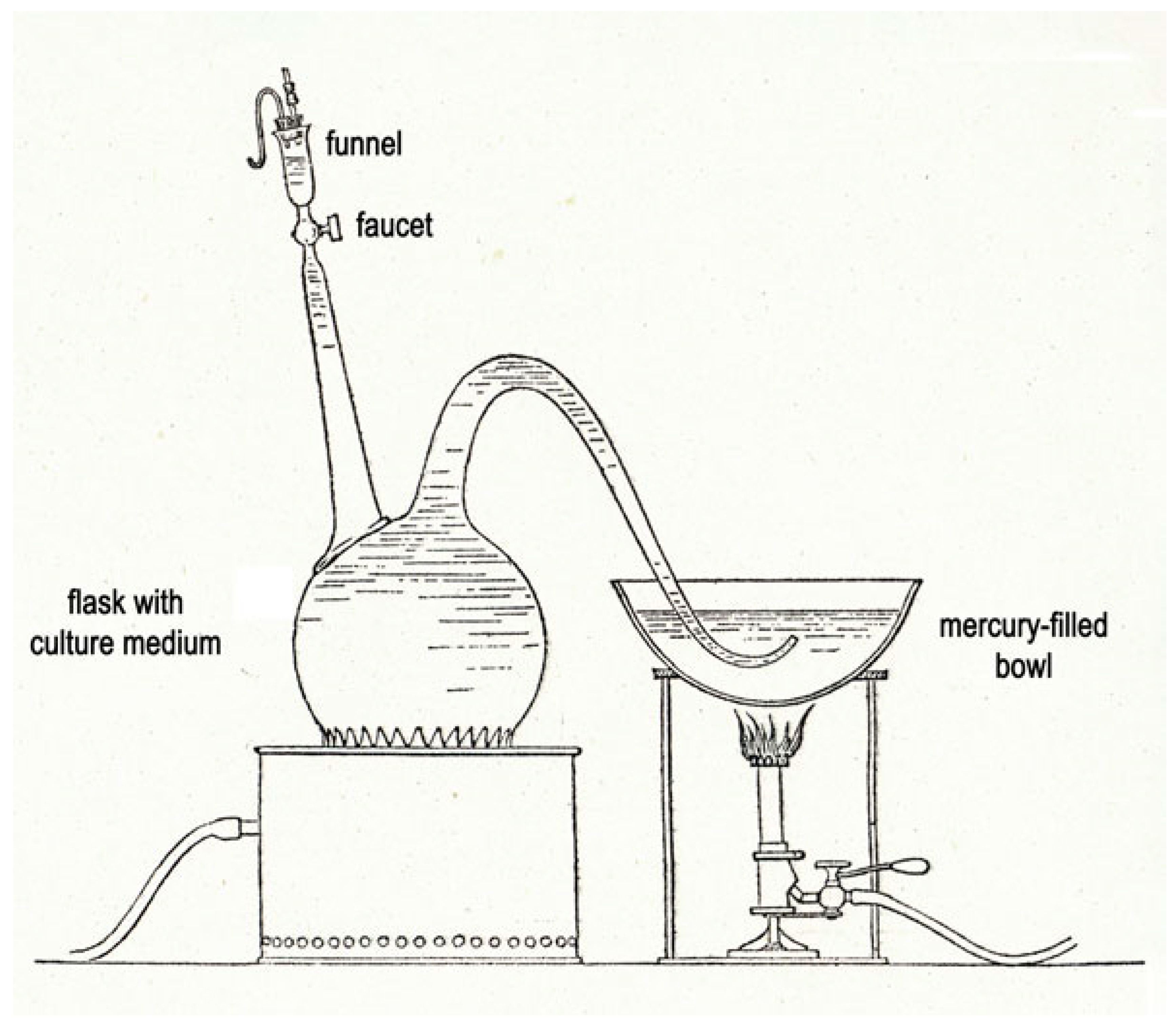
4. Period 1900–1940: The “Microbie Technique”
5. Period 1941–1968: The “Service des Anaérobies”
6. Period 1969–2000s: Towards the Genetics of Anaerobes and Molecular Mode of Action of Toxins
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bisset, N.G. Arrow and dart poisons. J. Ethnopharmacol. 1989, 25, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.N.; Gilbert, S.G. Historical milestones and discoveries that shaped the toxicology sciences. EXS 2009, 99, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. Historical links between toxinology and immunology. Pathog. Dis. 2018, 76, 4923027. [Google Scholar] [CrossRef] [PubMed]
- Brieger, L. Weitere Untersuchungen über Ptomaine; August Hirschwald: Berlin, Germany, 1885; Volume 1. [Google Scholar]
- Schwartz, M. Dr. Jekyll and Mr. Hyde: A short history of anthrax. Mol. Asp. Med. 2009, 30, 347–355. [Google Scholar] [CrossRef]
- Pollitzer, R.; Swaroop, S.; Burrows, W. Cholera. Monogr. Ser. World Health Organ. 1959, 58, 1001–1019. [Google Scholar]
- Collier, R.J. Diphtheria toxin: Mode of action and structure. Bacteriol. Rev. 1975, 39, 54–85. [Google Scholar] [CrossRef]
- Roux, E.; Yersin, A. Contribution à l’étude de la diphtérie. Ann. Inst. Pasteur. 1888, 2, 629–661. [Google Scholar]
- Cavaillon, J.M. From Bacterial Poisons to Toxins: The Early Works of Pasteurians. Toxins 2022, 14, 759. [Google Scholar] [CrossRef]
- Berche, P. Louis Pasteur, from crystals of life to vaccination. Clin. Microbiol. Infect. 2012, 18, 1–6. [Google Scholar] [CrossRef]
- Pasteur, L. Recherches sur la putréfaction. CR Acad. Sci. 1861, LVI, 1189–1194. [Google Scholar]
- Pasteur, L.; Joubert, J.; Chamberland, C. La théorie des germes et ses applications à la médecine et à la chirurgie. Bull. Acad. Med. 1878, VII, 432–453. [Google Scholar]
- Pasteur, L. Expériences et vues nouvelles sur la nature des fermentations. CR Acad. Sci. 1861, LII, 1260–1264. [Google Scholar]
- Pasteur, L. Oeuvres de Pasteur Collected by Pasteur Vallery-Radot; Masson, Ed.; Masson et Cie: Paris, France, 1992; Volume Tome V Etudes sur la Bière, p. 412. [Google Scholar]
- Roux, E. Sur la culture des microbes anaérobies. Ann. Inst. Pasteur. 1887, 2, 49–62. [Google Scholar]
- Veillon, A.; Zuber, M. Recherches sur quelques microbes strictement anaérobies et leur rôle en pathologie. Arch. Med. Exp. 1898, 10, 517–545. [Google Scholar]
- Roux, E. André Veillon. Ann. Inst. Pasteur. 1931, XLVII, 1–3. [Google Scholar]
- van Ermengem, E. Classics in infectious diseases. A new anaerobic bacillus and its relation to botulism. E. van Ermengem. Originally published as “Ueber einen neuen anaeroben Bacillus und seine Beziehungen zum Botulismus” in Zeitschrift fur Hygiene und Infektionskrankheiten 26: 1–56, 1897. Rev. Infect. Dis. 1979, 1, 701–719. [Google Scholar]
- Du Mesnil, O. Empoisonnement par de la viande de conserve. Ann. Santé. Publique. 1875, 43, 472–478. [Google Scholar]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Human Botulism in France, 1875–2016. Toxins 2020, 12, 338. [Google Scholar] [CrossRef]
- Legroux, R.; Jeramec, C.; Levaditi, J.C. Statistique du botulisme de l’occupation 1940–1944. Bull. Acad. Med. 1945, 129, 643–645. [Google Scholar]
- Legroux, R.; Levaditi, J.C.; Jéramec, C. Le botulisme en France pendant l’occupation. Presse. Med. 1947, 57, 109–110. [Google Scholar]
- Legroux, R.; Jeramec, C. Le botulisme et les jambons salés. Bull. Acad. Natl. Med. 1944, 128, 174–178. [Google Scholar]
- Anonymous. René Legroux. Ann. Inst. Pasteur. 1951, 80, 332–336. [Google Scholar]
- Legroux, R. André-Pierre Marie. Ann. Inst. Pasteur. 1929, 43, 959. [Google Scholar]
- Legroux, R.; Levaditi, J.C. On the serotherapy of experimental botulinum intoxication of the guinea pig. Ann. Inst. Pasteur. 1946, 72, 216–221. [Google Scholar]
- Legroux, R.; Levaditi, J.C.; Lamy, R. Le botulisme experimental du cheval et la question du botulisme naturel. Ann. Inst. Pasteur. 1946, 72, 545–552. [Google Scholar]
- Legroux, R.; Lavaditi, J.C.; Jeramec, C. Influence des voies d’introduction de la toxine sur le botulisme expérimental du lapin. Ann. Inst. Pasteur. 1945, 71, 490–493. [Google Scholar]
- Weinberg, M.; Ginsbourg, B. Données Récentes sur les Microbes Anaérobies et leur Role en Pathologie; Masson et Cie: Paris, France, 1927. [Google Scholar]
- Weinberg, M.; Seguin, P. La Gangrene Gazeuse; Masson et Cie: Paris, France, 1918. [Google Scholar]
- Weinberg, M.; Nativelle, R.; Prévot, A.R. Les Microbes Anaérobies; Masson et Cie: Paris, France, 1937; p. 1186. [Google Scholar]
- Anonymous. Michel Weinberg. Ann. Inst. Pasteur. 1940, 64, 461–465. [Google Scholar]
- Behring, E.; Kitasato, S. Über das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren. Dtsch. Med. Wochenschrift. 1890, 49, 1113–1114. [Google Scholar]
- Roux, E.; Vaillard, L. Contribution à l’étude du tétanos, Prévention et Traitement par le Serum Antitoxique. Ann. Inst. Pasteur. 1893, 7, 65–140. [Google Scholar]
- Marie, A.C.; Morax, V. Sur l’absorption de la toxine tétanique. Ann. Inst. Pasteur. 1902, 16, 818–832. [Google Scholar]
- Morax, V.; Marie, A.C. Recherches sur l′absorption de la toxine tétanique. Ann. Inst. Pasteur. 1903, 17, 335–342. [Google Scholar]
- Wellhöner, H.H. Tetanus and Botulinum Neurotoxins. In Selective Neurotoxicity; Herken, H., Hucho, F., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 102, pp. 357–417. [Google Scholar]
- Bizzini, B. Axoplasmic transport and transynaptic movement of tetanus toxin. In Botulinum Neurotoxin and Tetanus Toxin; Simpson, L.L., Ed.; Academic Pres: San Diego, CA, USA, 1989; pp. 203–229. [Google Scholar]
- Surana, S.; Tosolini, A.P.; Meyer, I.F.G.; Fellows, A.D.; Novoselov, S.S.; Schiavo, G. The travel diaries of tetanus and botulinum neurotoxins. Toxicon 2018, 147, 58–67. [Google Scholar] [CrossRef]
- Ramon, G. Sur le pouvoir floculant et sur les propriétés immunisantes d’une toxine diphtérique rendue anatoxique (anatoxine). CR Acad. Sci. 1923, 177, 1338–1340. [Google Scholar]
- Ramon, G.; Descombey, M.P. Sur l’immunisation antitétanique et sur la production de l’antitoxine tétanique. CR Soc. Biol. 1925, 93, 508–509. [Google Scholar]
- Ramon, G. Floculation dans un mélange neutre toxine-antitoxine diphtériques. CR Soc. Biol. 1922, 86, 661–663. [Google Scholar]
- Ramon, G.; Descombey, M.P. Sur l’appéciation de la valeur antigène de la toxine et de l’anatoxine tétaniques par la méthode de floculation. CR Soc. Biol. 1926, 95, 434–436. [Google Scholar]
- Anonymous. André-Romain Prévot. Bull. Institut. Pasteur. 1983, 81, 1–3. [Google Scholar]
- Prévot, A.R.; Turpin, A.; Kaiser, P. Les Bactéries Anaérobies; Dunod: Paris, France, 1967; p. 2188. [Google Scholar]
- Strotter, W.H.; Wallenstein, P. From VPI to State University—President T. Marshall Hahn Jr. and the Transformation of Virginia Tech, 1962–1974; Mercer University Press: Macon, GA, USA, 2004. [Google Scholar]
- Holdeman, L.V.; Cato, E.P.; Moore, W.E.C. Anaerobe Laboratory Manual, 4th ed.; Virginia Polytechnic Institute and State University: Blacksbourg, VA, USA, 1977. [Google Scholar]
- Prévot, A.R.; Brygoo, E.R. Etude de la première souche française de Cl. botulinum D. Ann. Inst. Pasteur. 1950, 78, 274–276. [Google Scholar]
- Prévot, A.R.; Huet, M.; Tardiieux, P. Etude de vingt-cinq foyers récents de botulisme animal. Bull. Acad. Vet. Fr. 1950, 23, 481–487. [Google Scholar] [CrossRef][Green Version]
- Jacquet, J.; Prevot, A.R. Recherches sur le botulisme equin experimental. Ann. Inst. Pasteur. 1951, 81, 334–337. [Google Scholar]
- Prévot, A.R.; Huet, M. Existence in France of human botulism due to fish and to Clostridium botulinum E. Bull. Acad. Natl. Med. 1951, 135, 432–435. [Google Scholar] [PubMed]
- Demarchi, J.; Mourgues, C.; Orio, J.; Prévot, A.R. Existence of type D botulism in man. Bull. Acad. Natl. Med. 1958, 142, 580–582. [Google Scholar]
- Rasetti-Escargueil, C.; Lemichez, E.; Popoff, M.R. Public Health Risk Associated with Botulism as Foodborne Zoonoses. Toxins 2020, 12, 17. [Google Scholar] [CrossRef]
- Prévot, A.R.; Sillioc, R. Activation of botulinum D toxinogenesis by Bacillus licheniformis. Ann. Inst. Pasteur. 1959, 96, 630–632. [Google Scholar]
- Raynaud, M.; Bizzini, B.; Turpin, A. Heterogeneity of tetanus anatoxin. Ann. Inst. Pasteur. 1971, 120, 801–815. [Google Scholar]
- Raynaud, M.; Relyveld, E.H.; Turpin, A.; Mangalo, R. Preparation of highly purified diphtheric, tetanic & staphylococcic anatoxins absorbed on calcium phosphate (brushite). Ann. Inst. Pasteur. 1959, 96, 60–71. [Google Scholar]
- Raynaud, M.; Turpin, A.; Bizzini, B. Existence of tetanus toxin under several aggregation conditions. Ann. Inst. Pasteur. 1960, 99, 167–172. [Google Scholar]
- Bizzini, B.; Turpin, A.; Raynaud, M. Production et purification de la toxine tétanique. Ann. Inst. Pasteur. 1969, 116, 686–712. [Google Scholar]
- Turpin, A.; Raynaud, M. Tetanus toxin. Ann. Inst. Pasteur. 1959, 97, 718–732. [Google Scholar]
- Turpin, A.; Raynaud, M.; Rouyer, M. The purification of tetanus toxin and antitoxin. Ann. Inst. Pasteur. 1952, 82, 299–309. [Google Scholar]
- Raynaud, M.; Turpin, A.; Relyveld, E.H.; Corvazier, R.; Girard, O. Preparation of tetanus antitoxin by immunization of horses with tetanus anatoxins of high purity. Ann. Inst. Pasteur. 1959, 96, 649–658. [Google Scholar]
- Brefort, G.; Magot, M.; Ionesco, H.; Sebald, M. Characterization and transferability of Clostridium perfringens plasmids. Plasmid 1977, 1, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Dublanchet, A.; Sebald, M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J. Infect Dis. 1979, 139, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Sebald, M.; Fayolle, F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature 1979, 278, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Truffaut, N.; Hubert, J.; Reysset, G. Construction of shuttle vectors useful for transforming Clostridium acetobutylicum. FEMS Microbiol. Lett. 1989, 49, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Reysset, G.; Haggoud, A.; Sebald, M. Genetics of resistance of î to 5-nitroimidazole. Clin Infect Dis. 1993, 16, S401–S403. [Google Scholar] [CrossRef]
- Trinh, S.; Haggoud, A.; Reysset, G.; Sebald, M. Plasmids pIP419 and pIP421 from Bacteroides: 5-nitroimidazole resistance genes and their upstream insertion sequence elements. Microbiology 1995, 141, 927–935. [Google Scholar] [CrossRef]
- Sebald, M.; Saimot, G. Toxémie botulique. Intérêt de sa mise en évidence dans le diagnostic du botulisme humain de type B. Ann. Microbiol. 1973, 124, 61–69. [Google Scholar]
- Sebald, M.; Duchemin, J.; Desenclos, J.C. Le risque botulique C chez l’homme. Bull. Epidémiol. Hebd 1997, 2, 8–9. [Google Scholar]
- Bizzini, B. Tetanus toxin. Microbiol. Rev. 1979, 43, 224–240. [Google Scholar] [CrossRef]
- Bizzini, B.; Stoeckel, K.; Schwab, M. An antigenic polypeptide fragment isolated from tetanus toxin: Chemical characterization, binding to gangliosides and retrograde axonal transport in various neuron systems. J. Neurochem. 1977, 28, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. Joseph Alouf (1929–2014). FEMS Microbiol. Lett. 2014, 355, 90–91. [Google Scholar] [CrossRef] [PubMed]
- The Comprehensive Sourcebook of Bacterial Protein Toxins, 4th ed.; Alouf, J., Ladant, D., Popoff, M.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Mock, M.; Fouet, A. Anthrax. Annu. Rev. Microbiol. 2001, 55, 647–671. [Google Scholar] [CrossRef] [PubMed]
- Mock, M.; Ullmann, A. Calmodulin-activated bacterial adenylate cyclases as virulence factors. TIMS 1993, 1, 187–192. [Google Scholar] [CrossRef]
- Kaczorek, M.; Delpeyroux, F.; Chenciner, N.; Streeck, R.E.; Murphy, J.R.; Boquet, P.; Tiollais, P. Nucleotide sequence and expression of the diphtheria tox228 gene in Escherichia coli. Science 1983, 221, 855–858. [Google Scholar] [CrossRef]
- Lemichez, E.; Bomsel, M.; Devilliers, G.; vanderSpek, J.; Murphy, J.R.; Lukianov, E.V.; Olsnes, S.; Boquet, P. Membrane translocation of diphtheria toxin fragment A exploits early to late endosome trafficking machinery. Mol. Microbiol. 1997, 23, 445–457. [Google Scholar] [CrossRef]
- Boquet, P.; Duflot, E. Tetanus toxin fragment forms channels in lipid vesicles at low pH. Proc. Natl. Acad. Sci. USA 1982, 79, 7614–7618. [Google Scholar] [CrossRef]
- Roa, M.; Boquet, P. Interaction of tetanus toxin with lipid vesicles at low pH. J. Biol. Chem. 1985, 260, 6827–6835. [Google Scholar] [CrossRef]
- Gill, D.M.; Merens, R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: Basis of the activation of adenylate cyclase. Proc. Natl. Acad. Sci. USA 1978, 75, 3050–3054. [Google Scholar] [CrossRef]
- Aktories, K.; Bärmann, M.; Ohishi, I.; Tsuyama, S.; Jakobs, K.H.; Habermann, E. Botulinum C2 toxin ADP-ribosylates actin. Nature 1986, 322, 390–392. [Google Scholar] [CrossRef]
- Aktories, K.; Weller, U.; Chhatwal, G.S. Clostridium botulinum type C produces a novel ADP-ribosyltransferase distinct from botulinum C2 toxin. FEBS Lett. 1987, 212, 109–113. [Google Scholar] [CrossRef]
- Narumiya, S.; Sekine, A.; Fujiwara, M. Substrate for botulinum ADP-ribosyltransferase, Gb, has an amino acid sequence homologous to a putative rho gene product. J. Biol. Chem. 1988, 263, 17255–17257. [Google Scholar] [CrossRef] [PubMed]
- Chardin, P.; Boquet, P.; Madaule, P.; Popoff, M.R.; Rubin, E.J.; Gill, D.M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero Cells. EMBO J. 1989, 8, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Nobes, C.D.; Hall, A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 1995, 81, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.F.; Self, A.J.; Garrett, M.D.; Just, I.; Korus, A.; Aktories, K.; Hall, A. Microinjection of recombinant p21RHO induces rapid changes in cell morphology. J. Cell Biol. 1990, 111, 1001–1007. [Google Scholar] [CrossRef]
- Popoff, M.R.; Boquet, P. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochem. Biophys. Res. Commun. 1988, 152, 1361–1368. [Google Scholar] [CrossRef]
- Popoff, M.R.; Rubin, E.J.; Gill, D.M.; Boquet, P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 1988, 56, 2299–2306. [Google Scholar] [CrossRef]
- Gerding, D.N.; Johnson, S.; Rupnik, M.; Aktories, K. Clostridium difficile binary toxin CDT: Mechanism, epidemiology, and potential clinical importance. Gut Microbes. 2014, 5, 15–27. [Google Scholar] [CrossRef]
- Perelle, S.; Gibert, M.; Boquet, P.; Popoff, M.R. Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli. Infect. Immun. 1993, 61, 5147–5156, Correction in Escherichia coli. Infect. Immun. 1995, 5163, 4967. [Google Scholar] [CrossRef]
- Stiles, B.G.; Wigelsworth, D.J.; Popoff, M.R.; Barth, H. Clostridial binary toxins: Iota and C2 family portraits. Front. Cell. Infect. Microbiol. 2011, 1, 2–13. [Google Scholar] [CrossRef]
- Binz, T.; Kurazono, H.; Wille, M.; Frevert, J.; Wernars, K.; Niemann, H. The complete sequence of botulinum neurotoxin type A and comparison with other clostridial neurotoxins. J. Biol. Chem. 1990, 265, 9153–9158. [Google Scholar] [CrossRef] [PubMed]
- Eisel, U.; Jarausch, W.; Goretzki, K.; Henschen, A.; Engels, J.; Weller, U.; Hudel, M.; Habermann, E.; Niemann, H. Tetanus toxin: Primary structure, expression in E. coli, and homology with botulinum toxins. EMBO J. 1986, 5, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R.; Marvaud, J.C. Structural and genomic features of clostridial neurotoxins. In The Comprehensive Sourcebook of Bacterial Protein Toxins, 2nd ed.; Alouf, J.E., Freer, J.H., Eds.; Academic Press: London, UK, 1999; Volume 2, pp. 174–201. [Google Scholar]
- Rossetto, O.; Deloye, F.; Poulain, B.; Pellizzari, R.; Schiavo, G.; Montecucco, C. The metallo-proteinase activity of tetanus and botulism neurotoxins. J. Physiol. Paris 1995, 89, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hauser, D.; Eklund, M.W.; Boquet, P.; Popoff, M.R. Organization of the botulinum neurotoxin C1 gene and its associated non-toxic protein genes in Clostridium botulinum C468. Mol. Gen. Genet. 1994, 243, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Popoff, M.R.; Bouvet, P. Clostridial toxins. Future Microbiol. 2009, 4, 1021–1064. [Google Scholar] [CrossRef]
- Cossart, P.; Boquet, P.; Normark, S.; Rappuoli, R. Cellular Microbiology; ASM Press: Washington, DC, USA, 2000. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popoff, M.R.; Legout, S. Anaerobes and Toxins, a Tradition of the Institut Pasteur. Toxins 2023, 15, 43. https://doi.org/10.3390/toxins15010043
Popoff MR, Legout S. Anaerobes and Toxins, a Tradition of the Institut Pasteur. Toxins. 2023; 15(1):43. https://doi.org/10.3390/toxins15010043
Chicago/Turabian StylePopoff, Michel R., and Sandra Legout. 2023. "Anaerobes and Toxins, a Tradition of the Institut Pasteur" Toxins 15, no. 1: 43. https://doi.org/10.3390/toxins15010043
APA StylePopoff, M. R., & Legout, S. (2023). Anaerobes and Toxins, a Tradition of the Institut Pasteur. Toxins, 15(1), 43. https://doi.org/10.3390/toxins15010043





