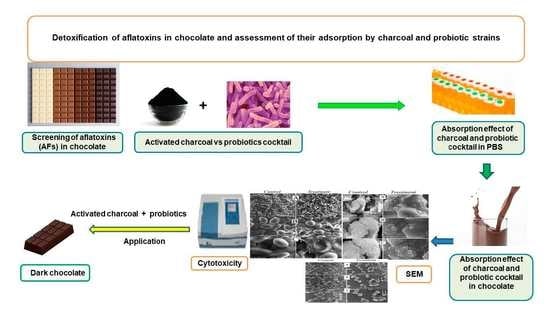
Evaluation of the Effectiveness of Charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as Aflatoxin Adsorbents in Chocolate
Abstract
1. Introduction
2. Results
2.1. Screening of Aflatoxins in Various Chocolate Kinds
2.2. Aflatoxin Adsorption Efficiency in Phosphate Buffer Solution
2.3. Aflatoxin Adsorption Efficiency in Fortified Dark Chocolate
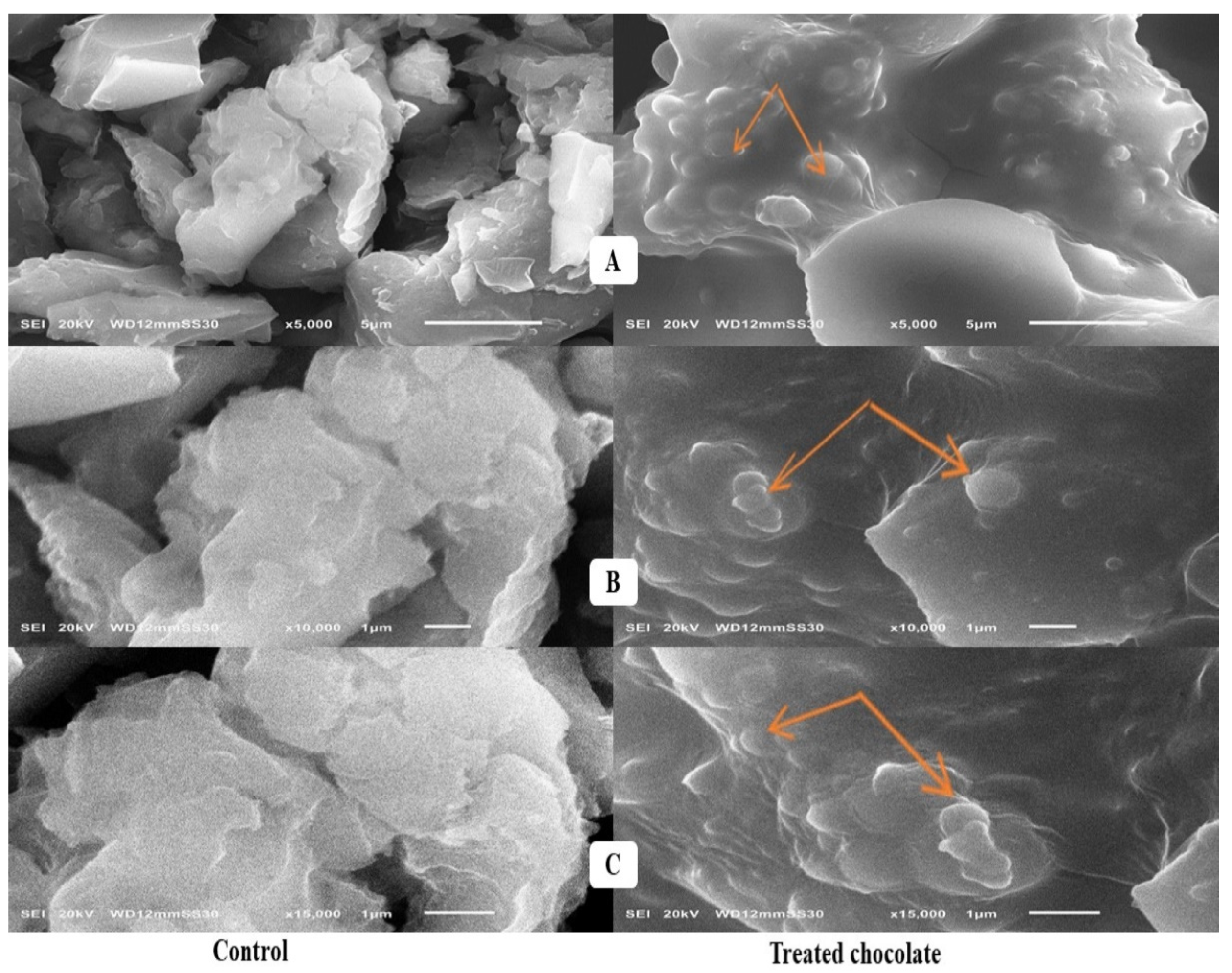
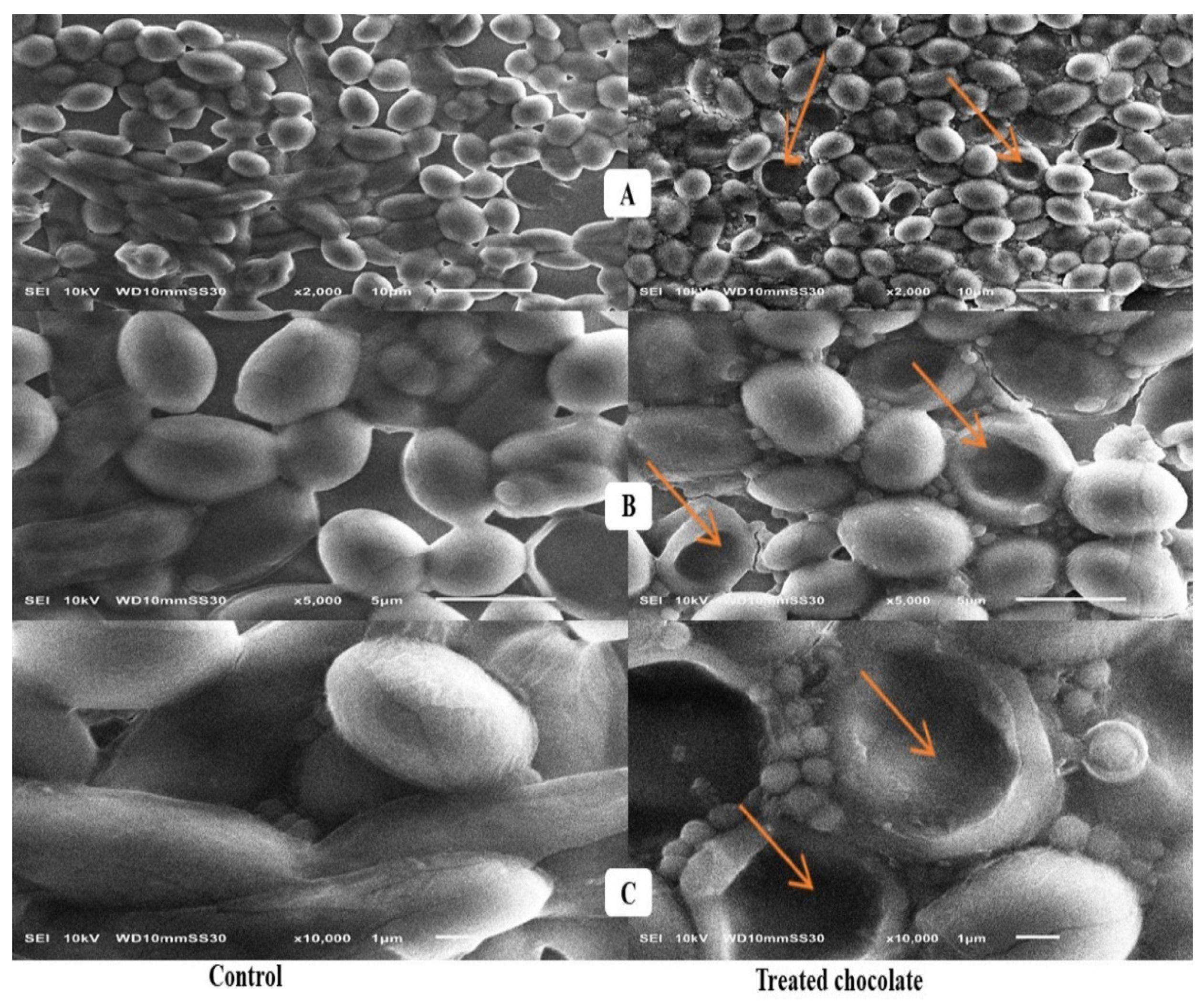
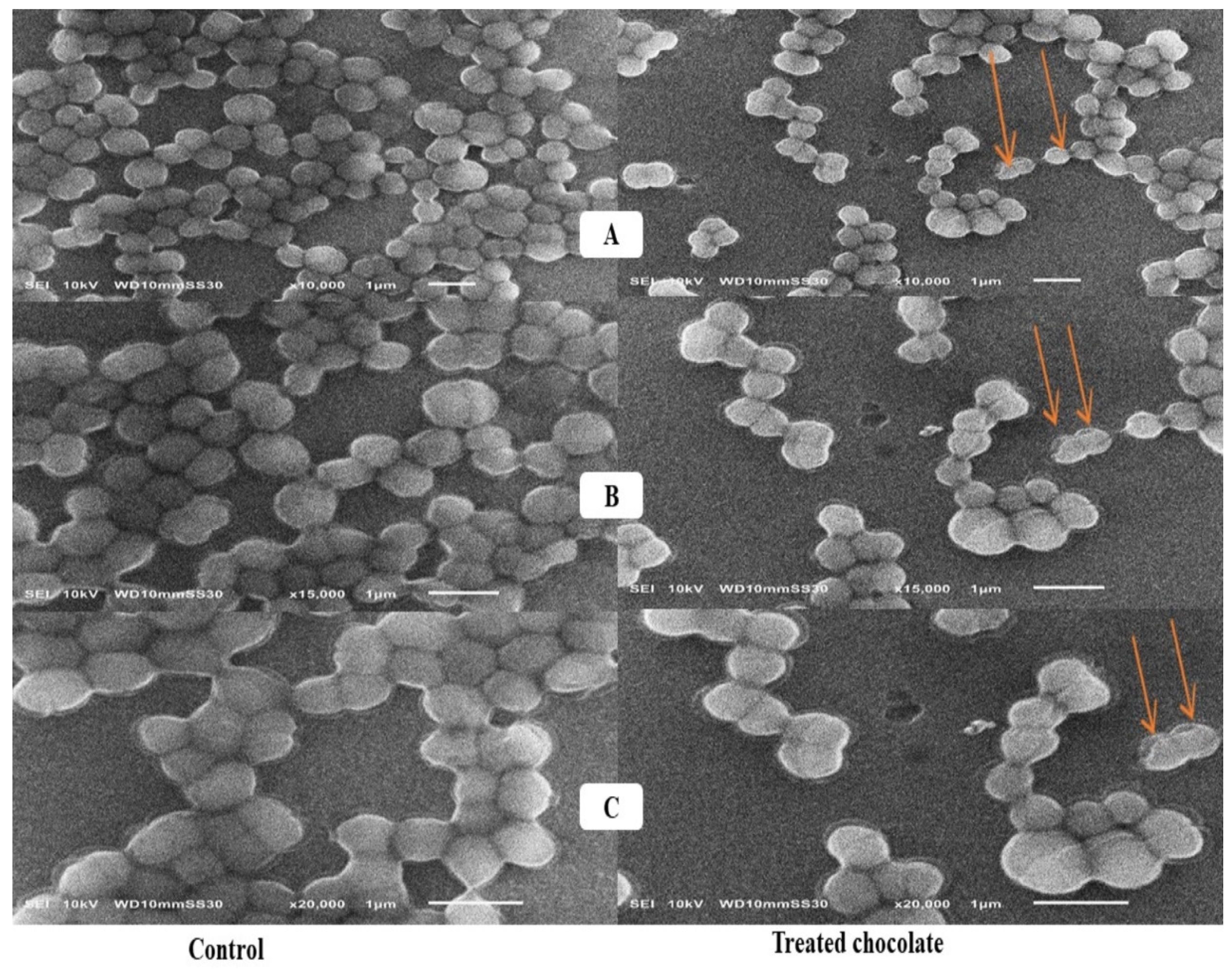
2.4. Scanning Electron Microscope Assessment
2.5. Cytotoxicity Assessment of Activated Charcoal
2.6. Sensorial Properties of Tri-Mix-Fortified Dark Chocolate
3. Discussion
3.1. Screening of Aflatoxins in Chocolates
3.2. Aflatoxin Adsorption Efficiency in Phosphate Buffer Solution
3.3. Aflatoxin Adsorption Efficiency in Supplemented Dark Chocolate
3.4. Scanning Electron Microscope Assessment
3.5. Cytotoxicity Assessment of Activated Charcoal
3.6. Sensorial Properties of Tri-Mix-Fortified Dark Chocolate
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Standards Preparation
5.3. Bacterial Strains and Cultural Conditions
5.4. Screening of Aflatoxins in Various Chocolate Types
5.5. Extraction and Immunoaffinity Chromatography (IAC) Clean-Up
5.6. HPLC Analysis
5.7. Preparation of Adsorbents in Sole, Di, and Tri-Mix Forms
5.8. Assessment of Aflatoxin Adsorption in PBS Solution
5.9. Determination of Aflatoxins Residues Using HPLC
5.10. Assessment of Aflatoxin Adsorption by Tri-Mixed Adsorbents in Dark Chocolate Model
5.11. Scanning Electron Microscope Analysis
5.12. Cytotoxicity Assessment of Activated Charcoal
5.13. Application of Tri-Mixed Adsorbents in Dark Chocolate Model
5.14. Sensory Evaluation
5.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swanson, K.M. Cocoa, Chocolate and Confectionery. In Microorganisms in Foods 8; Foods, I.C., Ed.; Springer: New York, NY, USA, 2011; pp. 241–246. [Google Scholar]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A review of recent innovative strategies for controlling mycotoxins in foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Sánchez-Santillán, P.; Cobos-Peralta, M.A.; Hernández-Sánchez, D.; Álvarado-Iglesias, A.; Espinosa-Victoria, D.; Herrera-Haro, J.G. Uso de carbón activado para conservar bacterias celulolíticas liofilizadas. Agrociencia 2016, 50, 575–582. [Google Scholar]
- Paterson, R.R.M.; Lima, N. Toxicology of mycotoxins. In Molecular, Clinical and Environmental Toxicology; Experientia Supplementum (EXS, Volume 100); Luch, A., Ed.; Birkhäuser: Basel, Switzerland, 2010; pp. 31–63. [Google Scholar]
- Li, Y.; Tian, G.; Dong, G.; Bai, S.; Han, X.; Liang, J.; Meng, J.; Zhang, H. Research progress on the raw and modified montmorillonites as adsorbents for mycotoxins: A review. Appl. Clay Sci. 2018, 163, 299–311. [Google Scholar] [CrossRef]
- Zellner, T.; Prasa, D.; Färber, E.; Hoffmann-Walbeck, P.; Genser, D.; Eyer, F. The use of activated charcoal to treat intoxications. Dtsch. Aerztebl. Int. 2019, 116, 311–317. [Google Scholar] [CrossRef]
- Dizbay-Onat, M.; Vaidya, U.K.; Lungu, C.T. Preparation of industrial sisal fiber waste derived activated carbon by chemical activation and effects of carbonization parameters on surface characteristics. Ind. Crop. Prod. 2017, 95, 583–590. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Pitt, J.I.; Taniwaki, M.H. Fungi and mycotoxins in cocoa: From farm to chocolate. Int. J. Food Microbiol. 2014, 178, 13–20. [Google Scholar] [CrossRef]
- WHO. Mycotoxins. Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 14 December 2022).
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of Oral Exposure to Aflatoxin B1-Induced Renal Dysfunction, Oxidative Stress, and Cell Apoptosis in Mice Kidney by Curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, R.; Ng, T.B.; Lai, Y.; Yang, J.; Ye, X. A new laccase of Lac 2 from the white rot fungus Cerrena unicolor 6884 and Lac 2-mediated degradation of aflatoxin B1. Toxins 2020, 12, 476. [Google Scholar] [CrossRef]
- Hamad, G.M.; Omar, S.A.; Mostafa, A.G.M.; Cacciotti, I.; Saleh, S.M.; Allam, M.G.; El-Nogoumy, B.; Abou-Alella, S.A.E.; Mehany, T. Binding and removal of polycyclic aromatic hydrocarbons in cold smoked sausage and beef using probiotic strains. Food Res. Int. 2022, 161, 111793. [Google Scholar] [CrossRef]
- Rathore, S.; Varshney, A.; Mohan, S.; Dahiya, P. An innovative approach of bioremediation in enzymatic degradation of xenobiotics. Biotechnol. Genet. Eng. Rev. 2022, 38, 1–32. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and beer. Impact of beer production process on mycotoxin contamination. A review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Muñoz, C.; Van de Voorde, D.; Tuenter, E.; Lemarcq, V.; Van de Walle, D.; Soares Maio, J.P.; Mencía, A.; Hernandez, C.E.; Comasio, A.; Sioriki, E.; et al. An in-depth multiphasic analysis of the chocolate production chain, from bean to bar, demonstrates the superiority of Saccharomyces cerevisiae over Hanseniaspora opuntiae as functional starter culture during cocoa fermentation. Food Microbiol. 2023, 109, 104115. [Google Scholar] [CrossRef] [PubMed]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef]
- Nathanail, A.V.; Gibson, B.; Han, L.; Peltonen, K.; Ollilainen, V.; Jestoi, M.; Laitila, A. The lager yeast Saccharomyces pastorianus removes and transforms Fusarium trichothecene mycotoxins during fermentation of brewer’s wort. Food Chem. 2016, 203, 448–455. [Google Scholar] [CrossRef]
- Dogi, C.; Cristofolini, A.; González Pereyra, M.L.; García, G.; Fochesato, A.; Merkis, C.; Dalcero, A.M.; Cavaglieri, L.R. Aflatoxins and Saccharomyces cerevisiae: Yeast modulates the intestinal effect of aflatoxins, while aflatoxin B1 influences yeast ultrastructure. World Mycotoxin J. 2017, 10, 171–181. [Google Scholar] [CrossRef]
- Liew, W.P.P.; Nurul-Adilah, Z.; Than, L.T.L.; Mohd-Redzwan, S. The binding efficiency and interaction of Lactobacillus casei Shirota toward aflatoxin B1. Front. Microbiol. 2018, 9, 1503. [Google Scholar] [CrossRef]
- Essia Ngang, J.J.; Yadang, G.; Sado Kamdem, S.L.; Kouebou, C.P.; Youte Fanche, S.A.; Tsochi Kougan, D.L.; Tsoungui, A.; Etoa, F.X. Antifungal properties of selected lactic acid bacteria and application in the biological control of ochratoxin A producing fungi during cocoa fermentation. Biocontrol Sci. Technol. 2015, 25, 245–259. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Standard for Chocolate and Chocolate Products; CODEX STAN; FAO/WHO: Rome, Italy, 2003. [Google Scholar]
- Kabak, B. Aflatoxins and ochratoxin A in chocolate products in Turkey. Food Addit. Contam. Part B 2019, 12, 225–230. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Pereira, J.L.; Lemes, D.P.; Nakano, F.; Taniwaki, M.H. Co-occurrence of ochratoxin a and aflatoxins in chocolate marketed in Brazil. Food Control 2012, 26, 36–41. [Google Scholar] [CrossRef]
- Turcotte, A.M.; Scott, P.M.; Tague, B. Analysis of cocoa products for ochratoxin A and aflatoxins. Mycotoxin Res. 2013, 29, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Naz, N.; Kashif, A.; Kanwal, K.; Ajaz, H. Incidence of mycotoxins in local and branded samples of chocolates marketed in Pakistan. J. Food Qual. 2017, 2017, 1947871. [Google Scholar] [CrossRef]
- Sartori, A.V.; Swensson de Mattos, J.; de Moraes, M.H.P.; da Nóbrega, A.W. Determination of Aflatoxins M1, M2, B1, B2, G1, and G2 and Ochratoxin A in UHT and Powdered Milk by Modified QuEChERS Method and Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2015, 8, 2321–2330. [Google Scholar] [CrossRef]
- Istiqomah, L.; Damayanti, E.; Arisnandhy, D.; Setyabudi, F.M.C.S.; Anwar, M. Saccharomyces cerevisiae B18 as antifungal and aflatoxin binder in vitro. AIP Conf. Proc. 2019, 2099, 020009. [Google Scholar] [CrossRef]
- Rahaie, S.; Emam-Djomeh, Z.; Razavi, S.H.; Mazaheri, M. Immobilized Saccharomyces cerevisiae as a potential aflatoxin decontaminating agent in pistachio nuts. Braz. J. Microbiol. 2010, 41, 82–90. [Google Scholar] [CrossRef]
- Palomino, M.M.; Allievi, M.C.; Gründling, A.; Sanchez-Rivas, C.; Ruzal, S.M. Osmotic stress adaptation in Lactobacillus casei BL23 leads to structural changes in the cell wall polymer lipoteichoic acid. Microbiology 2013, 159, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.; Ombarak, R.A.; Eskander, M.; Mehany, T.; Anees, F.R.; Elfayoumy, R.A.; Omar, S.A.; Lorenzo, J.M.; Abou-Alella, S.A.-E. Detection and inhibition of Clostridium botulinum in some Egyptian fish products by probiotics cell-free supernatants as bio-preservation agents. LWT 2022, 163, 113603. [Google Scholar] [CrossRef]
- Aazami, M.H.; Nasri, M.H.F.; Mojtahedi, M.; Mohammadi, S.R. In vitro aflatoxin B1 binding by the cell wall and (1→3)-β-D-glucan of Baker’s yeast. J. Food Prot. 2018, 81, 670–676. [Google Scholar] [CrossRef]
- Bueno, D.J.; Casale, C.H.; Pizzolitto, R.P.; Salvano, M.A.; Oliver, G. Physical adsorption of aflatoxin B1 by lactic acid bacteria and Saccharomyces cerevisiae: A theoretical model. J. Food Prot. 2007, 70, 2148–2154. [Google Scholar] [CrossRef]
- De Mil, T.; Devreese, M.; De Baere, S.; Van Ranst, E.; Eeckhout, M.; De Backer, P.; Croubels, S. Characterization of 27 mycotoxin binders and the relation with in vitro zearalenone adsorption at a single concentration. Toxins 2015, 7, 21–33. [Google Scholar] [CrossRef]
- Joannis-Cassan, C.; Tozlovanu, M.; Hadjeba-Medjdoub, K.; Ballet, N.; Pfohl-Leszkowicz, A. Binding of zearalenone, aflatoxin b 1, and ochratoxin a by yeast-based products: A method for quantification of adsorption performance. J. Food Prot. 2011, 74, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Ikegamai, T.; Yanagishita, H.; Kitamoto, D.; Haraya, K. Accelerated ethanol fermentation by Saccharomyces cerevisiae with addition of activated carbon. Biotechnol. Lett. 2000, 22, 1661–1665. [Google Scholar] [CrossRef]
- Peltonen, K.; El-Nezami, H.; Haskard, C.; Ahokas, J.; Salminen, S. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 2001, 84, 2152–2156. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Mendoza, A.; Guzman-De-Peña, D.; Garcia, H.S. Key role of teichoic acids on aflatoxin B1 binding by probiotic bacteria. J. Appl. Microbiol. 2009, 107, 395–403. [Google Scholar] [CrossRef]
- Shetty, P.H.; Hald, B.; Jespersen, L. Surface binding of aflatoxin B1 by Saccharomyces cerevisiae strains with potential decontaminating abilities in indigenous fermented foods. Int. J. Food Microbiol. 2007, 113, 41–46. [Google Scholar] [CrossRef]
- Gallo, A.; Masoero, F. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital. J. Anim. Sci. 2009, 9, 109–116. [Google Scholar] [CrossRef]
- Rasheed, U.; Ain, Q.U.; Yaseen, M.; Santra, S.; Yao, X.; Liu, B. Assessing the aflatoxins mitigation efficacy of blueberry pomace biosorbent in buffer, gastrointestinal fluids and modelwine. Toxins 2020, 12, 466. [Google Scholar] [CrossRef]
- Tejada, C.N.; Almanza, D.; Villabona, A.; Colpas, F.; Granados, C. Caracterización de carbón activado sintetizado a baja temperatura a partir de cáscara de cacao (Theobroma cacao) para la adsorción de amoxicilina. Ing. Compet. 2017, 19, 45–54. [Google Scholar] [CrossRef][Green Version]
- Barrientos-Velázquez, A.L.; Arteaga, S.; Dixon, J.B.; Deng, Y. The effects of pH, pepsin, exchange cation, and vitamins on aflatoxin adsorption on smectite in simulated gastric fluids. Appl. Clay Sci. 2016, 120, 17–23. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Karthick, K.; Allen, J.A.; Ghosh, O.S.N.; Chandranayaka, S.; Gupta, V.K.; Krishna, K.; Mudili, V. Application of activated carbon derived from seed shells of Jatropha curcas for decontamination of zearalenone mycotoxin. Front. Pharmacol. 2017, 8, 760. [Google Scholar] [CrossRef]
- Vilela, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Redução da acidez volátil de vinhos por células de Saccharomyces cerevisiae imobilizadas em esferas de alginato-quitosano. Enologia 2012, 38–42. Available online: https://www.researchgate.net/publication/282154567_Reducao_da_acidez_volatil_de_vinhos_por_celulas_de_Saccharomyces_cerevisiae_imobilizadas_em_esferas_de_alginato-quitosano (accessed on 29 November 2022).
- De Rossi, A.; Rigueto, C.V.T.; Dettmer, A.; Colla, L.M.; Piccin, J.S. Synthesis, characterization, and application of Saccharomyces cerevisiae/alginate composites beads for adsorption of heavy metals. J. Environ. Chem. Eng. 2020, 8, 104009. [Google Scholar] [CrossRef]
- Saha, D.; Heldt, C.L.; Gencoglu, M.F.; Vijayaragavan, K.S.; Chen, J.; Saksule, A. A study on the cytotoxicity of carbon-based materials. Mater. Sci. Eng. C 2016, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, R.O.; Usita, N.P. Utilization of Bamboo Charcoal as Additives in Cakes. Asia Pac. J. Multidiscip. Res. 2015, 3, 82–86. [Google Scholar]
- El-Nezami, H.; Kankaanpää, P.; Salminen, S.; Ahokas, J. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 1998, 61, 466–468. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mohdaly, A.A.A.; El-Nogoumy, B.A.; Ramadan, M.F.; Hassan, S.A.; Zeitoun, A.M. Detoxification of Aflatoxin B1 and Ochratoxin A Using Salvia farinacea and Azadirachta indica Water Extract and Application in Meat Products. Appl. Biochem. Biotechnol. 2021, 193, 3098–3120. [Google Scholar] [CrossRef]
- Tabari, D.G.; Kermanshahi, H.; Golian, A.; Heravi, R.M. In Vitro Binding Potentials of Bentonite, Yeast Cell Wall and Lactic Acid Bacteria for Aflatoxin B1 and Ochratoxin A. Iran. J. Toxicol. 2018, 12, 7–13. [Google Scholar] [CrossRef]
- Hamad, G.M.; Zahran, E.; Hafez, E.E. The efficacy of bacterial and yeasts strains and their combination to bind aflatoxin B1 and B2 in artificially contaminated infants food. J. Food Saf. 2017, 37, 12365. [Google Scholar] [CrossRef]
- Mirković, M.; Seratlić, S.; Kilcawley, K.; Mannion, D.; Mirković, N.; Radulović, Z. The sensory quality and volatile profile of dark chocolate enriched with encapsulated probiotic Lactobacillus plantarum bacteria. Sensors 2018, 18, 2570. [Google Scholar] [CrossRef]
- Ryan, R.M.; Deci, E.L. Self-Determination Theory: Basic Psychological Needs in Motivation, Development, and Wellness; Guilford Publications: New York, NY, USA, 2017. [Google Scholar]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef]
| Chocolate Products | Number of Samples | AFB1 | AFB2 | AFG1 | AFG2 | Total AFs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | Min–Max | Mean ± SD | ||
| Couverture chocolate | 10 | 0.015–0.542 | 0.112 ± 0.19 c | 0.006–0.262 | 0.071 ± 0.095 c | 0.014–0.096 | 0.067 ± 0.007 b | 0.013–0.086 | 0.041 ± 0.027 b,c | 0.106–0.776 | 0.290 ± 0.231 d |
| Dark chocolate | 10 | 0.06–1.87 | 0.963 ± 0.689 a | 0.076–0.972 | 0.573 ± 0.313 a | 0.036–0.098 | 0.069 ± 0.020 b | 0.037–0.062 | 0.052 ± 0.007 b | 0.247–2.669 | 1.658 ± 0.735 b |
| Milk chocolate | 10 | 0.270–1.60 | 0.966 ± 0.376 a | 0.130–0.390 | 0.239 ± 0.063 b | 0.280–0.650 | 0.425 ± 0.112 a | 0.130–0.280 | 0.178 ± 0.045 a | 1.230–2.450 | 1.808 ± 0.333 b |
| Chocolate powder | 10 | 0.08–1.85 | 1.116 ± 0.832 a | 0.280–0.820 | 0.593 ± 0.183 a | 0.230–0.560 | 0.399 ± 0.107 a | 0.120–0.240 | 0.164 ± 0.037 a | 1.460–3.060 | 2.322 ± 0.489 a |
| Bitter chocolate | 10 | 0.26–0.52 | 0.369 ± 0.079 b | 0.140–0.420 | 0.304 ± 0.099 b | 0.001–0.029 | 0.013 ± 0.009 b | 0.004–0.0360 | 0.018 ± 0.011 b,c | 0.526–0.875 | 0.704 ± 0.101 c |
| Chocolate wafer | 10 | 0.23–0.93 | 0.414 ± 0.254 b | 0.130–0.280 | 0.207 ± 0.058 b,c | 0.032–0.054 | 0.043 ± 0.008 b | 0.004–0.017 | 0.010 ± 0.005 c | 0.421–1.278 | 0.674 ± 0.274 c |
| Matrix | Time | pH | Aflatoxins Conc. (μg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | Total AFs | Adsorption% | |||
| PBS (−Ve control) | 2 h | 3.0 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 |
| 6.8 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 | ||
| 4 h | 3.0 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 | |
| 6.8 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 | ||
| PBS + AFs (+Ve control) | 2 h | 3.0 | 0.24 ± 0.01 j | 0.26 ± 0.01 i | 0.24 ± 0.03 i | 0.25 ± 0.02 j | 0.99 ± 0.01 o | 0 |
| 6.8 | 0.23 ± 0.02 j | 0.25 ± 0.01 h | 0.26 ± 0.01 j | 0.25 ± 0.01 j | 0.99 ± 0.02 o | 0 | ||
| 4 h | 3.0 | 0.25 ± 0.01 k | 0.24 ± 0.02 h | 0.25 ± 0.03 i | 0.26 ± 0.01 a | 1.00 ± 0.01 o | 0 | |
| 6.8 | 0.24 ± 0.02 j | 0.26 ± 0.01 i | 0.24 ± 0.01 i | 0.25 ± 0.01 j | 0.99 ± 0.02 o | 0 | ||
| Activated charcoal | 2 h | 3.0 | 0.12 ± 0.01 i | 0.13 ± 0.02 g | 0.12 ± 0.01 g | 0.11 ± 0.02 h | 0.48 ± 0.01 m | 52.0 ± 0.31 l |
| 6.8 | 0.11 ± 0.01 h | 0.12 ± 0.01 f | 0.13 ± 0.02 h | 0.12 ± 0.01 h | 0.48 ± 0.01 m | 52.0 ± 0.70 l | ||
| 4 h | 3.0 | 0.11 ± 0.01 h | 0.11 ± 0.01 f | 0.12 ± 0.01 g | 0.11 ± 0.01 h | 0.45 ± 0.01 l | 55.0 ± 0.42 k | |
| 6.8 | 0.12 ± 0.01 i | 0.11 ± 0.02 f | 0.13 ± 0.01 g | 0.12 ± 0.02 h | 0.48 ± 0.02 m | 52.0 ± 0.30 l | ||
| L. rhamnosus | 2 h | 3.0 | 0.13 ± 0.03 i | 0.12 ± 0.01 f | 0.12 ± 0.02 g | 0.11 ± 0.02 h | 0.48 ± 0.01 m | 52.0 ± 0.11 l |
| 6.8 | 0.12 ± 0.01 i | 0.12 ± 0.01 f | 0.13 ± 0.01 h | 0.12 ± 0.01 h | 0.49 ± 0.01 n | 51.0 ± 0.52 m | ||
| 4 h | 3.0 | 0.13 ± 0.01 i | 0.11 ± 0.01 f | 0.12 ± 0.01 a | 0.11 ± 0.01 h | 0.47 ± 0.01 m | 53.0 ± 0.01 l | |
| 6.8 | 0.13 ± 0.01 i | 0.12 ± 0.01 f | 0.11 ± 0.02 g | 0.13 ± 0.01 i | 0.49 ± 0.02 n | 51.0 ± 0.05 m | ||
| S. cerevisiae | 2 h | 3.0 | 0.08 ± 0.01 g | 0.07 ± 0.02 e | 0.08 ± 0.01 f | 0.06 ± 0.01 f | 0.29 ± 0.01 k | 71.0 ± 0.04 j |
| 6.8 | 0.08 ± 0.02 a | 0.08 ± 0.01 e | 0.06 ± 0.01 e | 0.07 ± 0.02 f,g | 0.29 ± 0.02 k | 71.0 ± 0.14 j | ||
| 4 h | 3.0 | 0.07 ± 0.01 f | 0.06 ± 0.01 d | 0.08 ± 0.02 f | 0.06 ± 0.01 f | 0.27 ± 0.01 j | 73.0 ± 0.11 i | |
| 6.8 | 0.08 ± 0.01 g | 0.07 ± 0.02 d | 0.06 ± 0.01 e | 0.08 ± 0.02 g | 0.29 ± 0.01 a | 71.0 ± 0.07 j | ||
| A. charcoal + L. rhamnosus | 2 h | 3.0 | 0.06 ± 0.01 e | 0.05 ± 0.01 c | 0.05 ± 0.01 d | 0.04 ± 0.01 e | 0.20 ± 0.01 h | 80.0 ± 0.41 g |
| 6.8 | 0.05 ± 0.01 d | 0.04 ± 0.01 c | 0.06 ± 0.01 e | 0.05 ± 0.01 e | 0.20 ± 0.02 h | 80.0 ± 0.31 g | ||
| 4 h | 3.0 | 0.05 ± 0.01 d | 0.06 ± 0.01 e | 0.05 ± 0.01 d | 0.06 ± 0.01 f | 0.22 ± 0.01 i | 78.0 ± 0.09 h | |
| 6.8 | 0.04 ± 0.01 d | 0.05 ± 0.01 d | 0.06 ± 0.01 e | 0.05 ± 0.01 e | 0.20 ± 0.01 h | 80.0 ± 0.14 g | ||
| A. charcoal + S. cerevisiae | 2 h | 3.0 | 0.03 ± 0.01 c | 0.02 ± 0.01 c | 0.03 ± 0.01 c | 0.02 ± 0.01 d | 0.10 ± 0.01 e | 89.0 ± 0.21 d |
| 6.8 | 0.03 ± 0.01 c | 0.03 ± 0.01 c | 0.02 ± 0.01 c | 0.02 ± 0.01 d | 0.10 ± 0.01 e | 89.0 ± 0.07 d | ||
| 4 h | 3.0 | 0.02 ± 0.02 b,c | 0.02 ± 0.01 c | 0.03 ± 0.03 c | 0.02 ± 0.02 d | 0.09 ± 0.02 e | 91.0 ± 0.22 c | |
| 6.8 | 0.02 ± 0.0 b,c | 0.03 ± 0.01 c | 0.02 ± 0.01 c | 0.02 ± 0.01 d | 0.09 ± 0.01 e | 91.0 ± 0.17 c | ||
| L. rhamnosus + S. cerevisiae | 2 h | 3.0 | 0.05 ± 0.01 d | 0.03 ± 0.01 c | 0.04 ± 0.01 d | 0.05 ± 0.01 e | 0.17 ± 0.01 g | 83.0 ± 0.11 e,f |
| 6.8 | 0.04 ± 0.01 d | 0.04 ± 0.01 d | 0.03 ± 0.01 c | 0.04 ± 0.01 e | 0.15 ± 0.02 f | 85.0 ± 0.06 e | ||
| 4 h | 3.0 | 0.03 ± 0.01 c | 0.05 ± 0.02 d | 0.04 ± 0.01 d | 0.03 ± 0.01 d | 0.15 ± 0.01 f | 85.0 ± 0.32 e | |
| 6.8 | 0.03 ± 0.02 c | 0.04 ± 0.01 d | 0.05 ± 0.01 d | 0.04 ± 0.02 e | 0.16 ± 0.01 f | 84.0 ± 0.41 e | ||
| A. charcoal + L. rhamnosus + S. cerevisiae | 2 h | 3.0 | 0.01 ± 0.01 b | 0.01 ± 0.01 b | 0.007 ± ±0.02 b | 0.005 ± 0.01 c | 0.032 ± 0.02 d | 96.8 ± 0.08 b |
| 6.8 | 0.01 ± 0.01 b | 0.006 ± 0.02 a | 0.004 ± 0.01 a | 0.003 ± 0.01 a | 0.023 ± 0.01 c | 97.7 ± 0.45 a,b | ||
| 4 h | 3.0 | 0.005 ± 0.01 a | 0.003 ± 0.01 a | 0.005 ± 0.01 a | 0.006 ± 0.01 b | 0.019 ± 0.01 b | 98.1 ± 0.41 a | |
| 6.8 | 0.003 ± 0.01 a | n.d. | n.d. | n.d. | 0.003 ± 0.01 a | 99.7 ± 0.13 a | ||
| Sample | Time | pH | Aflatoxins Conc. (μg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | Total AFs | Adsorption% | |||
| Chocolate (−Ve control) | 2 h | 3.0 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 |
| 6.8 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 | ||
| 4 h | 3.0 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 | |
| 6.8 | n.d. | n.d. | n.d. | n.d. | n.d. | 0 | ||
| Chocolate + AFs (+Ve control) | 2 h | 3.0 | 0.24 ± 0.02 c | 0.25 ± 0.01 c | 0.24 ± 0.01 c | 0.25 ± 0.03 c | 0.98 ± 0.01 c | 0 |
| 6.8 | 0.24 ± 0.02 c | 0.26 ± 0.01 d | 0.25 ± 0.02 c | 0.24 ± 0.06 c | 0.99 ± 0.02 c | 0 | ||
| 4 h | 3.0 | 0.25 ± 0.01 c | 0.24 ± 0.02 c | 0.25 ± 0.01 c | 0.24 ± 0.03 c | 0.98 ± 0.01 c | 0 | |
| 6.8 | 0.25 ± 0.03 c | 0.25 ± 0.01 c | 0.24 ± 0.03 c | 0.25 ± 0.01 c | 0.99 ± 0.01 c | 0 | ||
| Chocolate + AF + tri-mix (A. charcoal + L. rhamnosus + S. cerevisiae) | 2 h | 3.0 | 0.021 ± 0.02 b | 0.016 ± 0.01 b | 0.005 ± 0.01 a | 0.004 ± 0.02 b | 0.046 ± 0.02 a | 95.40 ± 0.11 c |
| 6.8 | 0.020 ± 0.01 b | 0.015 ± 0.01 b | 0.06 ± 0.02 b | 0.003 ± 0.02 a | 0.098 ± 0.01 b | 90.20 ± 0.24 c | ||
| 4 h | 3.0 | 0.017 ± 0.02 a,b | 0.015 ± 0.01 b | 0.005 ± 0.01 a | 0.002 ± 0.03 a | 0.039 ± 0.01 a | 96.10 ± 0.47 a | |
| 6.8 | 0.014 ± 0.0 a | 0.013 ± 0.01 a | 0.004 ± 0.01 a | 0.001 ± 0.01 a | 0.032 ± 0.01 a | 96.80 ± 0.15 a | ||
| Sample | Concentration (µg/mL) | Viability% | Inhibition% |
|---|---|---|---|
| Charcoal | 390 | 18 | 82 |
| 195 | 26 | 74 | |
| 97.5 | 29 | 71 | |
| 48.75 | 47 | 53 | |
| 24.37 | 58 | 42 | |
| 12.18 | 72 | 28 | |
| 6.09 | 94 | 6 |
| Treatment/Group | Sensorial Properties Mean ± SD | |||||
|---|---|---|---|---|---|---|
| Color | Odor | Taste | Texture | Appearance | Overall Acceptance | |
| Control | 8.4 ± 0.96 a | 8.5 ± 0.84 a | 8.6 ± 0.84 a | 8.6 ± 0.84 a | 7.9 ± 1.37 a | 8.6 ± 0.84 a |
| A. charcoal + L. rhamnosus + S. cerevisiae | 8.4 ± 0.94 a | 8.1 ± 0.99 a | 8.0 ± 1.05 b | 8.0 ± 1.05 b | 7.8 ± 1.31 a | 8.3 ± 0.78 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamad, G.M.; Amer, A.; El-Nogoumy, B.; Ibrahim, M.; Hassan, S.; Siddiqui, S.A.; EL-Gazzar, A.M.; Khalifa, E.; Omar, S.A.; Abd-Elmohsen Abou-Alella, S.; et al. Evaluation of the Effectiveness of Charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as Aflatoxin Adsorbents in Chocolate. Toxins 2023, 15, 21. https://doi.org/10.3390/toxins15010021
Hamad GM, Amer A, El-Nogoumy B, Ibrahim M, Hassan S, Siddiqui SA, EL-Gazzar AM, Khalifa E, Omar SA, Abd-Elmohsen Abou-Alella S, et al. Evaluation of the Effectiveness of Charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as Aflatoxin Adsorbents in Chocolate. Toxins. 2023; 15(1):21. https://doi.org/10.3390/toxins15010021
Chicago/Turabian StyleHamad, Gamal M., Amr Amer, Baher El-Nogoumy, Mohamed Ibrahim, Sabria Hassan, Shahida Anusha Siddiqui, Ahmed M. EL-Gazzar, Eman Khalifa, Sabrien A. Omar, Sarah Abd-Elmohsen Abou-Alella, and et al. 2023. "Evaluation of the Effectiveness of Charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as Aflatoxin Adsorbents in Chocolate" Toxins 15, no. 1: 21. https://doi.org/10.3390/toxins15010021
APA StyleHamad, G. M., Amer, A., El-Nogoumy, B., Ibrahim, M., Hassan, S., Siddiqui, S. A., EL-Gazzar, A. M., Khalifa, E., Omar, S. A., Abd-Elmohsen Abou-Alella, S., Ibrahim, S. A., Esatbeyoglu, T., & Mehany, T. (2023). Evaluation of the Effectiveness of Charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as Aflatoxin Adsorbents in Chocolate. Toxins, 15(1), 21. https://doi.org/10.3390/toxins15010021