Real-World Longitudinal Experience of Botulinum Toxin Therapy for Parkinson and Essential Tremor
Abstract
:1. Introduction
2. Results
2.1. Study Population
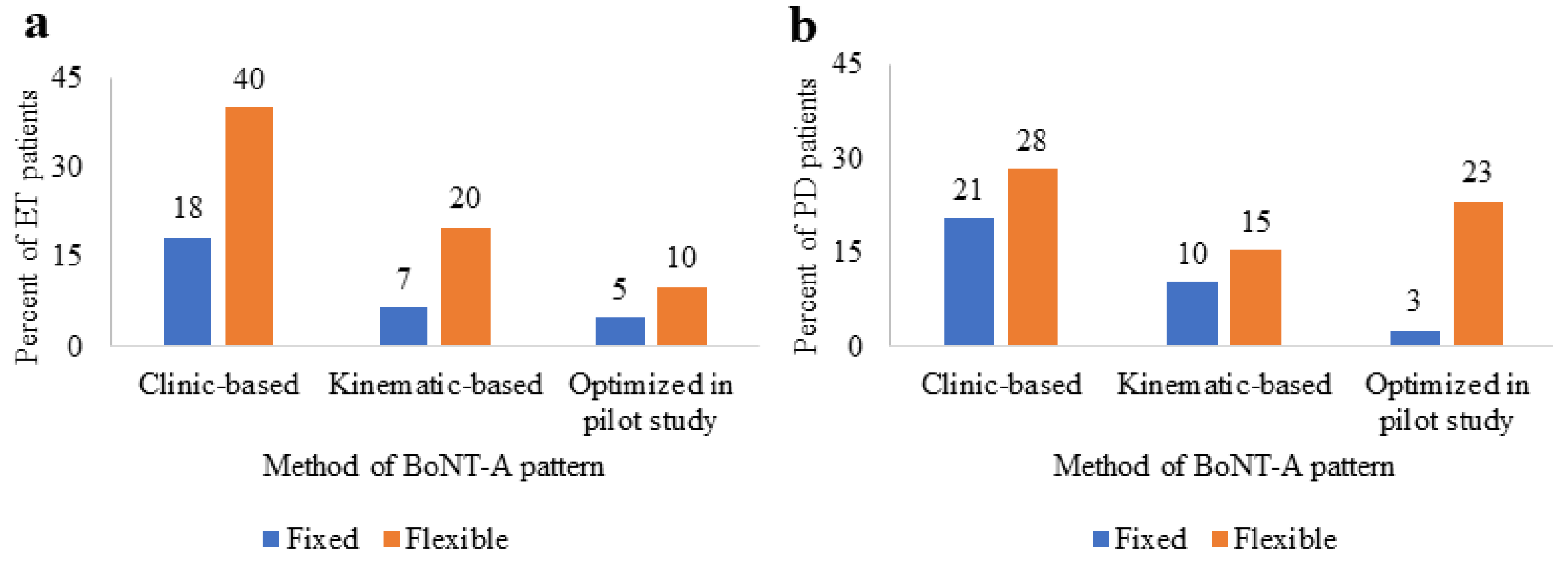
2.2. BoNT-A Therapy
2.3. BoNT-A Dosing
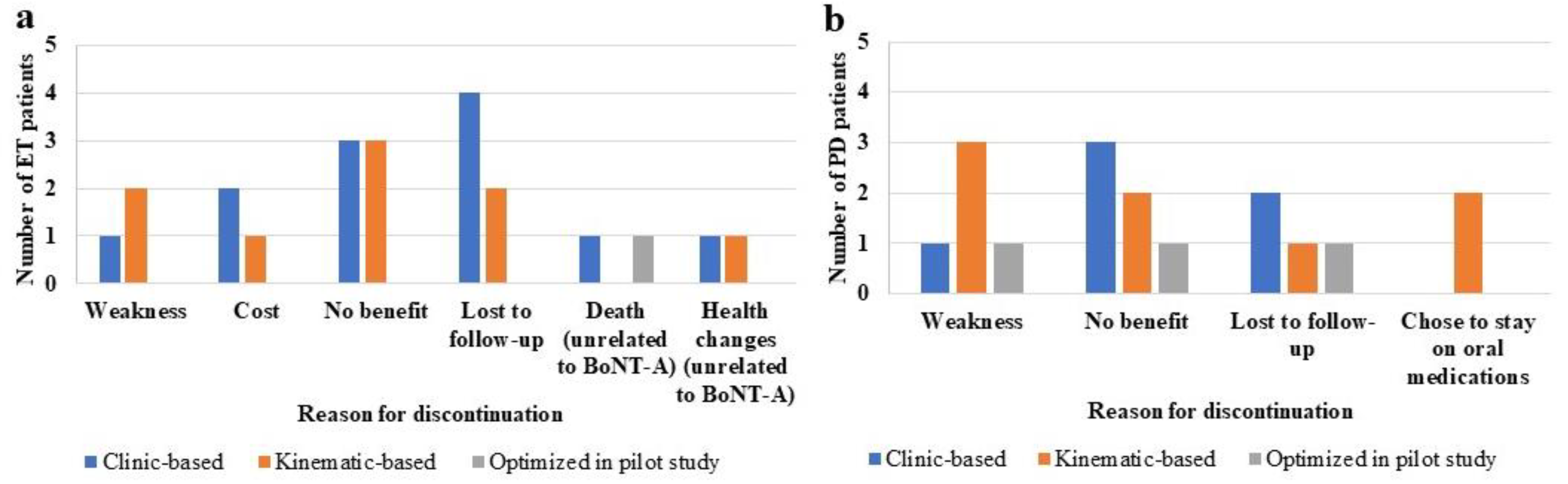
2.4. BoNT-A Therapy Discontinuation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design
5.2. Outcome Measures
5.3. BoNT-A Injections
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Louis, E.D.; Machado, D.G. Tremor-Related Quality of Life: A Comparison of Essential Tremor vs. Parkinson’s Disease Patients. Parkinsonism Relat. Disord. 2015, 21, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Heusinkveld, L.E.; Hacker, M.L.; Turchan, M.; Davis, T.L.; Charles, D. Impact of Tremor on Patients with Early Stage Parkinson’s Disease. Front. Neurol. 2018, 3, 628. [Google Scholar] [CrossRef] [PubMed]
- Tröster, A.I.; Pahwa, R.; Fields, J.A.; Tanner, C.M.; Lyons, K.E. Quality of life in Essential Tremor Questionnaire (QUEST): Development and initial validation. Park. Relat. Disord. 2005, 11, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pandey, S. Treatment of essential tremor: Current status. Postgrad. Med. J. 2020, 96, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.O.; Pandey, S. Botulinum toxin for the treatment of tremor. J. Neurol. Sci. 2022, 435, 120203. [Google Scholar] [CrossRef]
- Fasano, A.; Deuschl, G. Therapeutic advances in tremor. Mov. Disord. 2015, 30, 1557–1565. [Google Scholar] [CrossRef]
- Katzenschlager, R.; Sampalo, C.; Costa, J.; Lees, A. Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst. Rev. 2003, 2, CD003735. [Google Scholar]
- Fasano, A.; Lozano, A.M.; Cubo, E. New neurosurgical approaches for tremor and Parkinson’s disease. Curr. Opin. Neurol. 2017, 30, 435–446. [Google Scholar] [CrossRef]
- Mittal, S.O.; Jog, M.; Lee, J.; Jabbari, B. Novel Botulinum Toxin Injection Protocols for Parkinson Tremor and Essential Tremor—The Yale Technique and Sensor-Based Kinematics Procedure for Safe and Effective Treatment. Tremor Other Hyperkinet. Mov. 2020, 10, 61. [Google Scholar] [CrossRef]
- Louis, E.D.; Rohl, B.; Rice, C. Defining the Treatment Gap: What Essential Tremor Patients Want That They Are Not Getting. Tremor Other Hyperkinet. Mov. 2015, 5, 331. [Google Scholar] [CrossRef]
- Bloem, B.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 12, 2284–2303. [Google Scholar] [CrossRef]
- Samotus, O.; Lee, J.; Jog, M. Personalized Bilateral Upper Limb Essential Tremor Therapy with Botulinum Toxin Using Kinematics. Toxins 2019, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Lee, J.; Jog, M. Standardized algorithm for muscle selection and dosing of botulinum toxin for Parkinson tremor using kinematic analysis. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420954083. [Google Scholar] [CrossRef]
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum Toxin in Parkinson Disease Tremor: A Randomized, Double-Blind, Placebo-Controlled Study with a Customized Injection Approach. Mayo Clin. Proc. 2017, 92, 1359–1367. [Google Scholar] [CrossRef]
- Mittal, S.O.; Machado, D.; Richardson, D.; Dubey, D.; Jabbari, B. Botulinum toxin in essential hand tremor—A randomized double-blind placebo-controlled study with customized injection approach. Park. Relat. Disord. 2018, 56, 65–69. [Google Scholar] [CrossRef]
- Zakin, E.; Simpson, D. Botulinum toxin in management of limb tremor. Toxins 2017, 9, 365. [Google Scholar] [CrossRef]
- Mittal, S.O.; Lenka, A.; Jankovic, J. Botulinum toxin for the treatment of tremor. Park. Relat. Disord. 2019, 63, 31–41. [Google Scholar] [CrossRef]
- Samotus, O.; Lee, J.; Jog, M. Developing a Consistent, Reproducible Botulinum Toxin Type A Dosing Method for Upper Limb Tremor by Kinematic Analysis. Toxins 2021, 13, 264. [Google Scholar] [CrossRef]
- Kamel, J.T.; Cordivari, C.; Catania, S. Treatment of Upper Limb Tremor with Botulinum Toxin: An Individualized Approach. Mov. Disord. Clin. Pract. 2019, 6, 652–655. [Google Scholar] [CrossRef]
- Niemann, N.; Jankovic, J. Botulinum Toxin for the Treatment of Hand Tremor. Toxins 2018, 10, 299. [Google Scholar] [CrossRef]
- Samotus, O.; Lee, J.; Jog, M. Long-term tremor therapy for Parkinson and essential tremor with sensor-guided botulinum toxin type A injections. PLoS ONE 2017, 6, e0178670. [Google Scholar] [CrossRef] [PubMed]
- Lotia, M.; Jankovic, J. Botulinum toxin for the treatment of tremor and tics. Semin. Neurol. 2016, 36, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Samotus, O.; Kumar, N.; Rizek, P.; Jog, M. Botulinum Toxin Type A Injections as Monotherapy for Upper Limb Essential Tremor Using Kinematics. CJNS 2017, 45, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Samotus, O.; Lee, J.; Jog, M. Effective Management of Upper Limb Parkinsonian Tremor by IncobotulinumtoxinA Injections Using Sensor-based Biomechanical Patterns. Tremor Other Hyperkinet. Mov. 2015, 5, 348. [Google Scholar] [CrossRef]
- Samotus, O.; Rahimi, F.; Lee, J.; Jog, M. Functional Ability Improved in Essential Tremor by IncobotulinumtoxinA Injections Using Kinematically Determined Biomechanical Patterns—A New Future. PLoS ONE 2016, 11, e0153739. [Google Scholar] [CrossRef]
| ET | PD | |
|---|---|---|
| Number of patients enrolled (n) | 68 | 45 |
| Men:Women ratio (n) | 36:32 | 28:17 |
| Age (years) a | 72 ± 9.5 (min: 46, max: 94) | 72 ± 9.2 (min: 50, max: 89) |
| Medication history b,c | ||
| Monotherapy BoNT-A | 33 (50%) | 7 (16%) |
| BoNT-A as an adjunct | ||
| therapy (optimized on anti-tremor mediations) | 33 (50%) | 37 (82%) |
| Unilateral vs. bilateral tremor b | ||
| Left arm | 7 (10%) | 11 (24%) |
| Right arm | 24 (35%) | 27 (60%) |
| Both arms | 37 (54%) | 7 (16%) |
| ET | PD | |
|---|---|---|
| Botox® to Xeomin® formulation ratio (n) a | 8:49 | 6:35 |
| 1:1 to 1:2 BoNT-A reconstitution ratio (n) a | 16:41 | 10:31 |
| Method of BoNT-A pattern b | ||
| Clinic-based | 42 (62%) | 23 (51%) |
| Kinematic-based | 17 (25%) | 10 (22%) |
| Kinematic-based and optimized in pilot study [12,13,21] c | 9 (13%) | 12 (27%) |
| Number of injections a,d | 5.5 ± 4.2 (min: 1, max: 18) | 5.3 ± 4.1 (min: 1, max: 15) |
| Number of years receiving BoNT-A a,c | 2.1 ± 1.1 (min: 1, max: 4) | 1.9 ± 1.0 (min: 1, max: 4) |
| Injection interval cycle (months) a,c | 4.7 ± 1.6 (min: 2.4, max: 12) | 4.2 ± 1.5 (min: 2.4, max: 8) |
| Self-paying (no health insurance coverage) b | 26 (46%) | 24 (58%) |
| Mean Total Dose (U) Per Upper Limb | ET | PD |
|---|---|---|
| Clinic-based | 118.2 ± 59.8 (min = 10, max = 245) | 140.9 ± 57.0 (min = 15, max = 230) |
| Kinematic-based | 136.9 ± 50.0 (min = 45, max = 250) | 137.0 ± 51.0 (min = 25, max = 220) |
| Kinematic-based, optimized in pilot study | 96.3 ± 34.3 (min = 45, max = 155) | 134.5 ± 43.8 (min = 70, max = 245) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samotus, O.; Mahdi, Y.; Jog, M. Real-World Longitudinal Experience of Botulinum Toxin Therapy for Parkinson and Essential Tremor. Toxins 2022, 14, 557. https://doi.org/10.3390/toxins14080557
Samotus O, Mahdi Y, Jog M. Real-World Longitudinal Experience of Botulinum Toxin Therapy for Parkinson and Essential Tremor. Toxins. 2022; 14(8):557. https://doi.org/10.3390/toxins14080557
Chicago/Turabian StyleSamotus, Olivia, Yekta Mahdi, and Mandar Jog. 2022. "Real-World Longitudinal Experience of Botulinum Toxin Therapy for Parkinson and Essential Tremor" Toxins 14, no. 8: 557. https://doi.org/10.3390/toxins14080557
APA StyleSamotus, O., Mahdi, Y., & Jog, M. (2022). Real-World Longitudinal Experience of Botulinum Toxin Therapy for Parkinson and Essential Tremor. Toxins, 14(8), 557. https://doi.org/10.3390/toxins14080557






