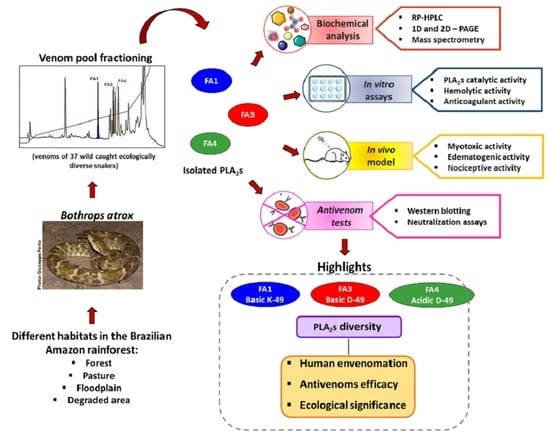
Diversity of Phospholipases A2 from Bothrops atrox Snake Venom: Adaptive Advantages for Snakes Compromising Treatments for Snakebite Patients
Abstract
1. Introduction
2. Results
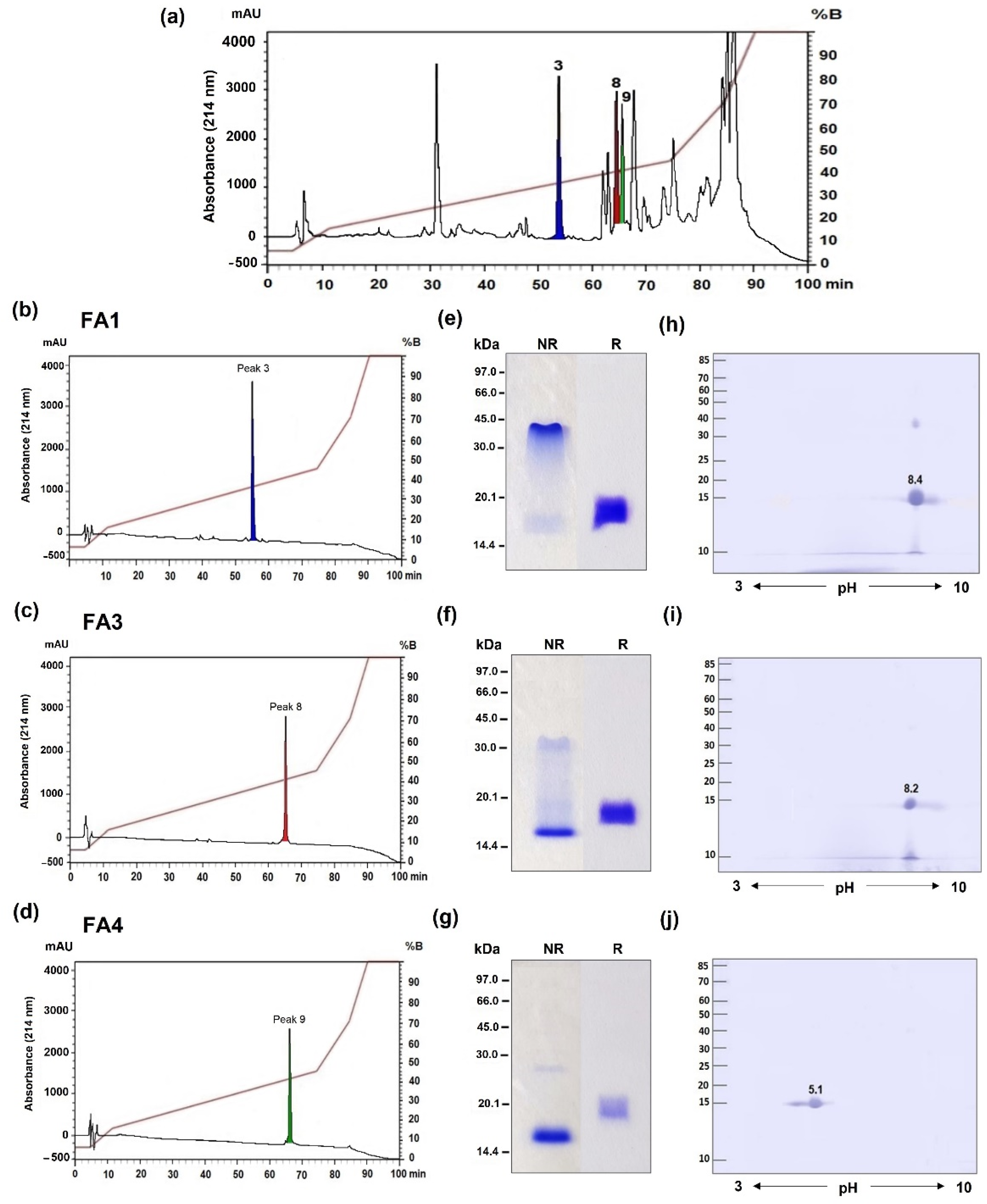
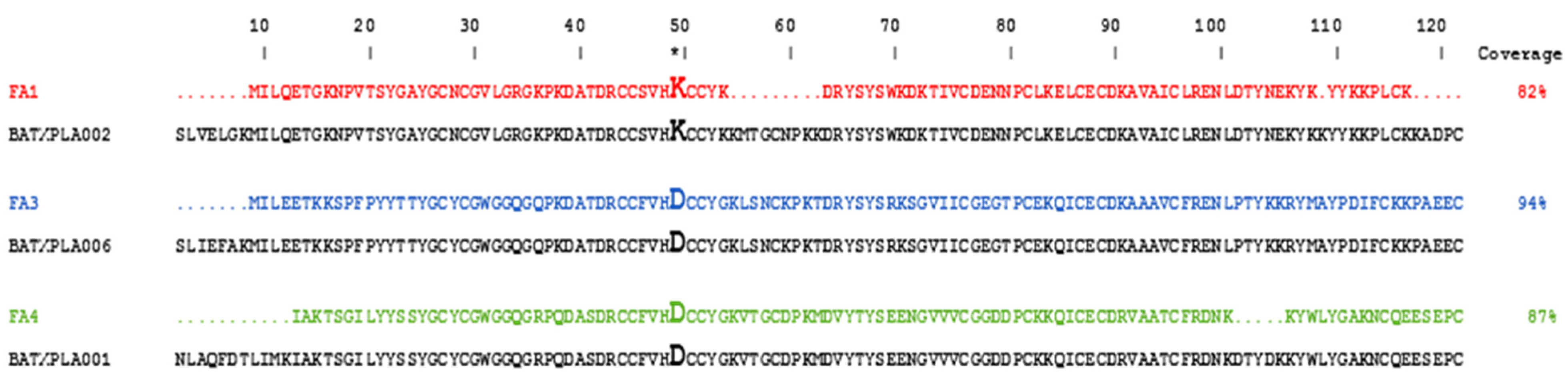
2.1. Identification of Three Distinct PLA2 Isoforms in B. atrox Venom
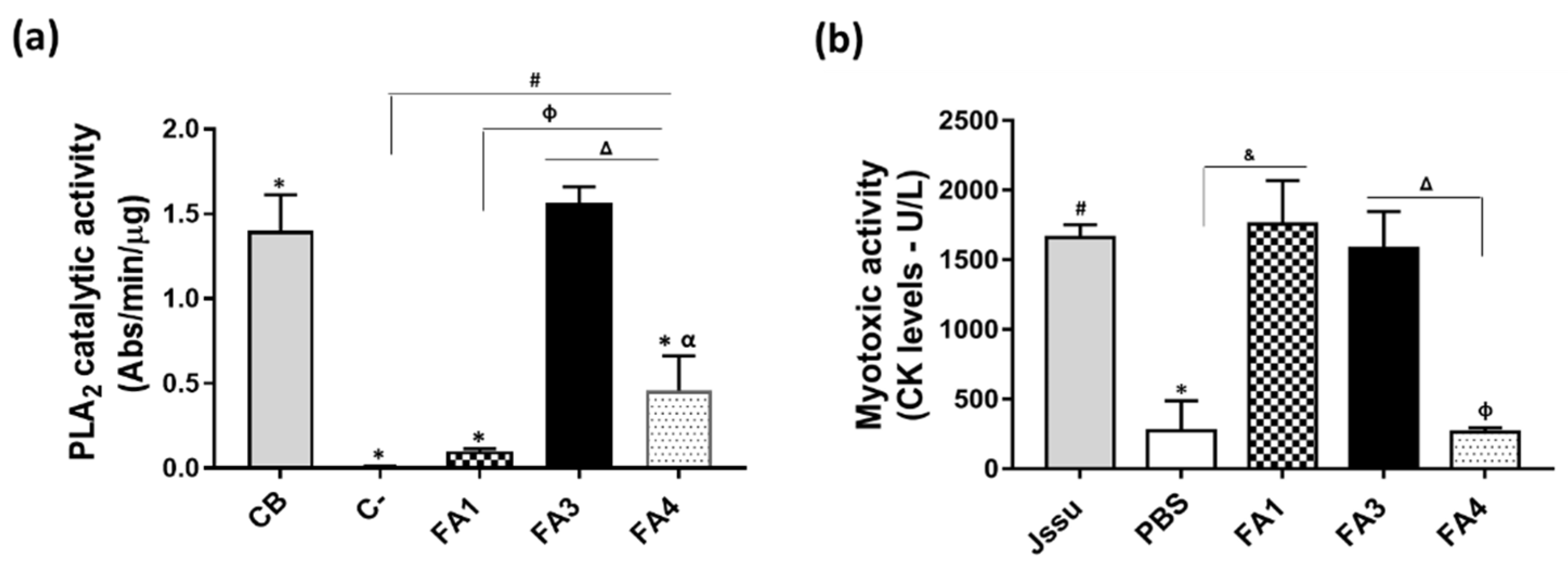
2.2. Functional Differences in Three PLA2 Fractions Obtained from B. atrox Venom
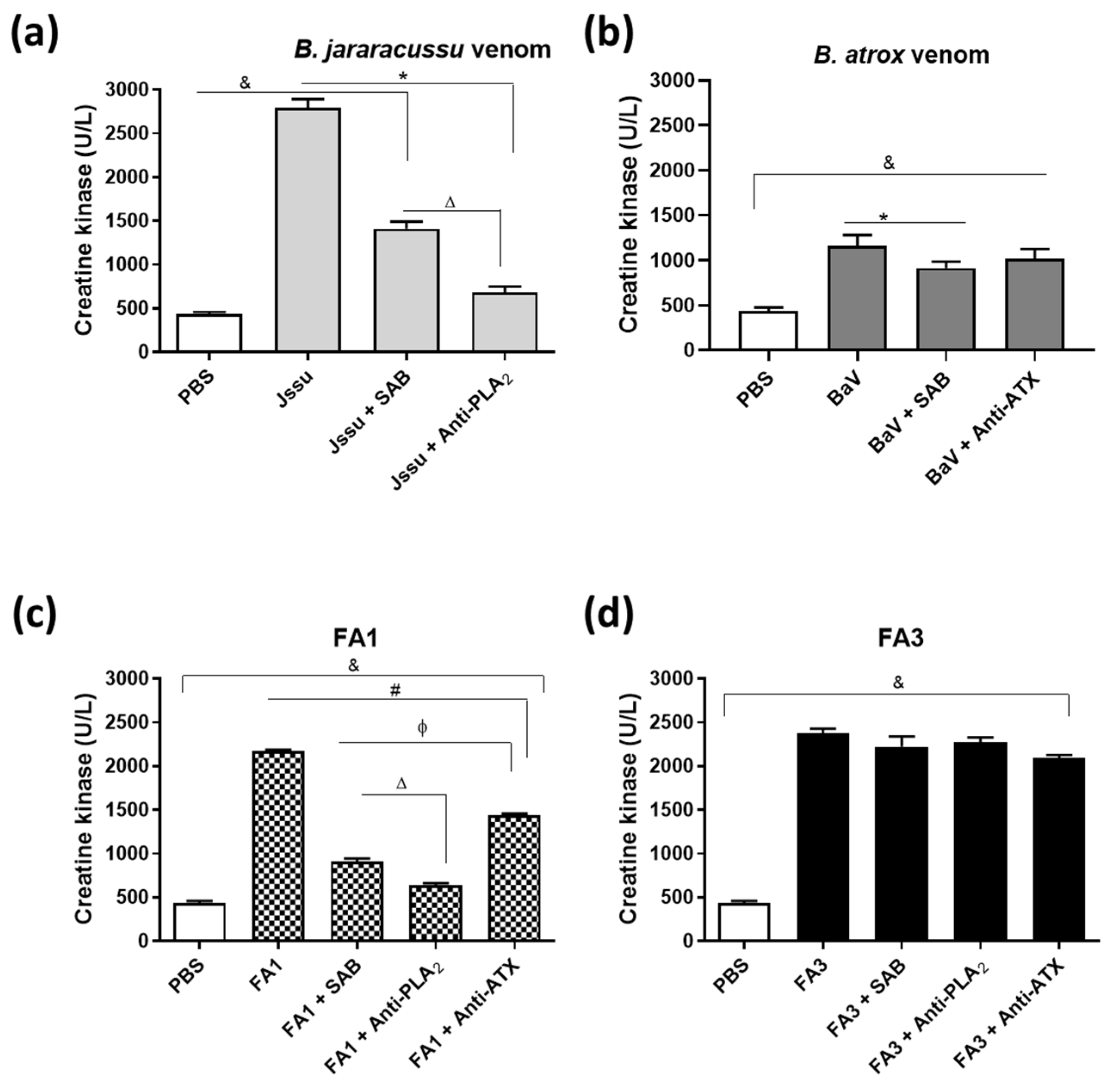
2.3. Differences in Recognition and Neutralization by Antivenoms of PLA2s from B. atrox Venom
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethical Statement for the Use of Experimental Animals and Human Samples
5.2. Venoms and Antivenoms
5.3. Isolation of the PLA2s Using Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC)
5.4. Gel Electrophoresis
5.5. Identification of the PLA2s via Mass Spectrometry
5.6. Functional Characterization of the PLA2s from B. atrox Venom
5.6.1. PLA2s Catalytic Activity
5.6.2. Hemolytic (Direct and Indirect) Activity
5.6.3. Evaluation of the PLA2s Anticoagulant Activity on Human and Animal Plasmas
5.6.4. Myotoxic Activity
5.6.5. Edematogenic Activity
5.6.6. Assessment of PLA2s-Induced Nociceptive Activity
5.7. Reactivity with Antivenoms
5.8. Neutralization of the Myotoxic Activity
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Amazonas, D.R.; Portes-Junior, J.A.; Nishiyama, M.Y., Jr.; Nicolau, C.A.; Chalkidis, H.M.; Mourão, R.H.V.; Grazziotin, F.G.; Rokyta, D.R.; Gibbs, H.L.; Valente, R.H.; et al. Molecular mechanisms underlying intraspecific variation in snake venom. J. Proteom. 2018, 181, 60–72. [Google Scholar] [CrossRef]
- Zancolli, G.; Calvete, J.J.; Cardwell, M.D.; Greene, H.W.; Hayes, W.K.; Hegarty, M.J.; Herrmann, H.W.; Holycross, A.T.; Lannutti, D.I.; Mulley, J.F.; et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. Biol. Sci. 2019, 286, 20182735. [Google Scholar] [CrossRef]
- Sousa, L.F.; Portes-Junior, J.A.; Nicolau, C.A.; Bernardoni, J.L.; Nishiyama, M.Y.; Amazonas, D.R.; Freitas-de-Sousa, L.A.; Mourão, R.H.; Chalkidis, H.M.; Valente, R.H.; et al. Functional proteomic analyses of Bothrops atrox venom reveals phenotypes associated with habitat variation in the Amazon. J. Proteom. 2017, 159, 32–46. [Google Scholar] [CrossRef]
- Margres, M.J.; Bigelow, A.T.; Lemmon, E.M.; Lemmon, A.R.; Rokyta, D.R. Selection To Increase Expression, Not Sequence Diversity, Precedes Gene Family Origin and Expansion in Rattlesnake Venom. Genetics 2017, 206, 1569–1580. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; van der Weerd, L.; Kochva, E.; Renjifo, C. Evolution and diversification of the Toxicofera reptile venom system. J. Proteom. 2009, 72, 127–136. [Google Scholar] [CrossRef]
- Freitas-de-Sousa, L.A.; Nachtigall, P.G.; Portes-Junior, J.A.; Holding, M.L.; Nystrom, G.S.; Ellsworth, S.A.; Guimarães, N.C.; Tioyama, E.; Ortiz, F.; Silva, B.R.; et al. Size Matters: An Evaluation of the Molecular Basis of Ontogenetic Modifications in the Composition of. Toxins 2020, 12, 791. [Google Scholar] [CrossRef]
- Zelanis, A.; Menezes, M.C.; Kitano, E.S.; Liberato, T.; Tashima, A.K.; Pinto, A.F.; Sherman, N.E.; Ho, P.L.; Fox, J.W.; Serrano, S.M. Proteomic identification of gender molecular markers in Bothrops jararaca venom. J. Proteom. 2016, 139, 26–37. [Google Scholar] [CrossRef]
- Calvete, J.J.; Sanz, L.; Perez, A.; Borges, A.; Vargas, A.M.; Lomonte, B.; Angulo, Y.; Maria Gutierrez, J.; Chalkidis, H.M.; Mourao, R.H.V.; et al. Snake population venomics and antivenomics of Bothrops atrox: Paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J. Proteom. 2011, 74, 510–527. [Google Scholar] [CrossRef]
- Moretto Del-Rei, T.H.; Sousa, L.F.; Rocha, M.M.T.; Freitas-de-Sousa, L.A.; Travaglia-Cardoso, S.R.; Grego, K.; Sant’Anna, S.S.; Chalkidis, H.M.; Moura-da-Silva, A.M. Functional variability of Bothrops atrox venoms from three distinct areas across the Brazilian Amazon and consequences for human envenomings. Toxicon 2019, 164, 61–70. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Gubensek, F.; Sket, D.; Turk, V.; Lebez, D. Fractionation of Vipera ammodytes venom and seasonal variation of its composition. Toxicon 1974, 12, 167–171. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Kini, R.M.; Chan, Y.M. Accelerated evolution and molecular surface of venom phospholipase A2 enzymes. J. Mol. Evol. 1999, 48, 125–132. [Google Scholar] [CrossRef]
- Brust, A.; Sunagar, K.; Undheim, E.A.; Vetter, I.; Yang, D.C.; Casewell, N.R.; Jackson, T.N.; Koludarov, I.; Alewood, P.F.; Hodgson, W.C.; et al. Differential evolution and neofunctionalization of snake venom metalloprotease domains. Mol. Cell Proteom. 2013, 12, 651–663. [Google Scholar] [CrossRef]
- Lynch, V.J. Inventing an arsenal: Adaptive evolution and neofunctionalization of snake venom phospholipase A2 genes. BMC Evol. Biol. 2007, 7, 2. [Google Scholar] [CrossRef][Green Version]
- Lomonte, B.; Rangel, J. Snake venom Lys49 myotoxins: From phospholipases A(2) to non-enzymatic membrane disruptors. Toxicon 2012, 60, 520–530. [Google Scholar] [CrossRef]
- Fortes-Dias, C.L.; Santos, R.M.; Magro, A.J.; Fontes, M.R.; Chávez-Olórtegui, C.; Granier, C. Identification of continuous interaction sites in PLA(2)-based protein complexes by peptide arrays. Biochimie 2009, 91, 1482–1492. [Google Scholar] [CrossRef]
- de Oliveira, A.H.; Giglio, J.R.; Andrião-Escarso, S.H.; Ito, A.S.; Ward, R.J. A pH-induced dissociation of the dimeric form of a lysine 49-phospholipase A2 abolishes Ca2+-independent membrane damaging activity. Biochemistry 2001, 40, 6912–6920. [Google Scholar] [CrossRef]
- Angulo, Y.; Gutiérrez, J.M.; Soares, A.M.; Cho, W.; Lomonte, B. Myotoxic and cytolytic activities of dimeric Lys49 phospholipase A2 homologues are reduced, but not abolished, by a pH-induced dissociation. Toxicon 2005, 46, 291–296. [Google Scholar] [CrossRef]
- Šribar, J.; Oberčkal, J.; Križaj, I. Understanding the molecular mechanism underlying the presynaptic toxicity of secreted phospholipases A(2): An update. Toxicon 2014, 89, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Angulo, Y.; Sasa, M.; Gutiérrez, J.M. The phospholipase A2 homologues of snake venoms: Biological activities and their possible adaptive roles. Protein Pept. Lett. 2009, 16, 860–876. [Google Scholar] [CrossRef] [PubMed]
- Zuliani, J.P.; Fernandes, C.M.; Zamuner, S.R.; Gutiérrez, J.M.; Teixeira, C.F. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: Role of catalytic activity. Toxicon 2005, 45, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Ownby, C.L. Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon 2003, 42, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Chioato, L.; Ward, R.J. Mapping structural determinants of biological activities in snake venom phospholipases A2 by sequence analysis and site directed mutagenesis. Toxicon 2003, 42, 869–883. [Google Scholar] [CrossRef]
- Lomonte, B.; Angulo, Y.; Calderón, L. An overview of lysine-49 phospholipase A2 myotoxins from crotalid snake venoms and their structural determinants of myotoxic action. Toxicon 2003, 42, 885–901. [Google Scholar] [CrossRef]
- Soares, A.M.; Andrião-Escarso, S.H.; Bortoleto, R.K.; Rodrigues-Simioni, L.; Arni, R.K.; Ward, R.J.; Gutiérrez, J.M.; Giglio, J.R. Dissociation of enzymatic and pharmacological properties of piratoxins-I and -III, two myotoxic phospholipases A2 from Bothrops pirajai snake venom. Arch. Biochem. Biophys. 2001, 387, 188–196. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef]
- Denegri, M.E.G.; Acosta, O.C.; Huancahuire-Vega, S.; Martins-de-Souza, D.; Marangoni, S.; Maruñak, S.L.; Teibler, G.P.; Leiva, L.C.; Ponce-Soto, L.A. Isolation and functional characterization of a new acidic PLA(2) Ba SpII RP4 of the Bothrops alternatus snake venom from Argentina. Toxicon 2010, 56, 64–74. [Google Scholar] [CrossRef]
- Moreira, V.; Dos-Santos, M.C.; Nascimento, N.G.; Borges da Silva, H.; Fernandes, C.M.; D’Império Lima, M.R.; Teixeira, C. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon 2012, 60, 12–20. [Google Scholar] [CrossRef]
- Kini, R.M. Anticoagulant proteins from snake venoms: Structure, function and mechanism. Biochem. J. 2006, 397, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Jacob-Ferreira, A.L.; Bernardes, C.P.; Cintra, A.C.; Sampaio, S.V. Purification procedure for the isolation of a P-I metalloprotease and an acidic phospholipase A2 from Bothrops atrox snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 28. [Google Scholar] [CrossRef]
- Núñez, V.; Arce, V.; Gutiérrez, J.M.; Lomonte, B. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops atrox. Toxicon 2004, 44, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kanashiro, M.M.; de Cássia, M.; Escocard, R.; Petretski, J.H.; Prates, M.V.; Alves, E.W.; Machado, O.L.; da Silva, W.D.; Kipnis, T.L. Biochemical and biological properties of phospholipases A(2) from Bothrops atrox snake venom. Biochem. Pharmacol. 2002, 64, 1179–1186. [Google Scholar] [CrossRef]
- Furtado, J.L.; Oliveira, G.A.; Pontes, A.S.; Setúbal, S.a.S.; Xavier, C.V.; Lacouth-Silva, F.; Lima, B.F.; Zaqueo, K.D.; Kayano, A.M.; Calderon, L.A.; et al. Activation of J77A.1 macrophages by three phospholipases A2 isolated from Bothrops atrox snake venom. Biomed. Res. Int. 2014, 2014, 683123. [Google Scholar] [CrossRef] [PubMed]
- Proleón, A.; Torrejón, D.; Urra, F.A.; Lazo, F.; López-Torres, C.; Fuentes-Retamal, S.; Quispe, E.; Bautista, L.; Agurto, A.; Gavilan, R.G.; et al. Functional, immunological characterization, and anticancer activity of BaMtx: A new Lys49-PLA. Int. J. Biol. Macromol. 2022, 206, 990–1002. [Google Scholar] [CrossRef]
- Wen, F.H.; Monteiro, W.M.; Moura da Silva, A.M.; Tambourgi, D.V.; da Silva, I.M.; Sampaio, V.S.; dos Santos, M.C.; Sachett, J.; Ferreira, L.C.L.; Kalil, J.; et al. Snakebites and Scorpion Stings in the Brazilian Amazon: Identifying Research Priorities for a Largely Neglected Problem. PLoS Negl. Trop. Dis. 2015, 9, e0003701. [Google Scholar] [CrossRef]
- Martins, M.; Gordo, M. Bothrops atrox (common lancehead). Diet Herpetol. Rev. 1993, 24, 2. [Google Scholar]
- Pardal, P.P.O.; Souza, S.M.; Monteiro, M.R.C.C.; Fan, H.W.; Cardoso, J.L.C.; Franca, F.O.S.; Tomy, S.C.; Sano-Martins, I.S.; Sousa-e-Silva, M.C.C.; Colombini, M.; et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 28–42. [Google Scholar] [CrossRef]
- Monteiro, W.M.; Contreras-Bernal, J.C.; Bisneto, P.F.; Sachett, J.; Mendonça da Silva, I.; Lacerda, M.; Guimarães da Costa, A.; Val, F.; Brasileiro, L.; Sartim, M.A.; et al. Bothrops atrox, the most important snake involved in human envenomings in the amazon: How venomics contributes to the knowledge of snake biology and clinical toxinology. Toxicon X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Sousa, L.F.; Holding, M.L.; Del-Rei, T.H.M.; Rocha, M.M.T.; Mourão, R.H.V.; Chalkidis, H.M.; Prezoto, B.; Gibbs, H.L.; Moura-da-Silva, A.M. Individual Variability in Bothrops atrox Snakes Collected from Different Habitats in the Brazilian Amazon: New Findings on Venom Composition and Functionality. Toxins 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.; Leiguez, E.; Janovits, P.M.; Maia-Marques, R.; Fernandes, C.M.; Teixeira, C. Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation. Toxins 2021, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Delatorre, P.; Rocha, B.A.; Santi-Gadelha, T.; Gadelha, C.A.; Toyama, M.H.; Cavada, B.S. Crystal structure of Bn IV in complex with myristic acid: A Lys49 myotoxic phospholipase A₂ from Bothrops neuwiedi venom. Biochimie 2011, 93, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Díaz, C.; Gutiérrez, J.M.; Lomonte, B.; Gené, J.A. The effect of myotoxins isolated from Bothrops snake venoms on multilamellar liposomes: Relationship to phospholipase A2, anticoagulant and myotoxic activities. Biochim. Biophys. Acta 1991, 1070, 455–460. [Google Scholar] [CrossRef]
- Landucci, E.C.; de Castro, R.C.; Toyama, M.; Giglio, J.R.; Marangoni, S.; De Nucci, G.; Antunes, E. Inflammatory oedema induced by the lys-49 phospholipase A(2) homologue piratoxin-i in the rat and rabbit. Effect of polyanions and p-bromophenacyl bromide. Biochem. Pharmacol. 2000, 59, 1289–1294. [Google Scholar] [CrossRef]
- Teixeira, C.F.; Landucci, E.C.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases A2. Toxicon 2003, 42, 947–962. [Google Scholar] [CrossRef]
- Zychar, B.C.; Clissa, P.B.; Carvalho, E.; Baldo, C.; Gonçalves, L.R.C. Leukocyte recruitment induced by snake venom metalloproteinases: Role of the catalytic domain. Biochem. Biophys. Res. Commun. 2020, 521, 402–407. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Butera, D.; Tanjoni, I. Importance of snake venom metalloproteinases in cell biology: Effects on platelets, inflammatory and endothelial cells. Curr. Pharm. Des. 2007, 13, 2893–2905. [Google Scholar] [CrossRef]
- Gutierrez, J.M.; Rucavado, A.; Escalante, T.; Diaz, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef]
- Bustillo, S.; Fernández, J.; Chaves-Araya, S.; Angulo, Y.; Leiva, L.C.; Lomonte, B. Isolation of two basic phospholipases A. Toxicon 2019, 168, 113–121. [Google Scholar] [CrossRef]
- França, F.O.S.; Malaque, C. Acidente botrópico. In Animais Peçonhentos Do Brasil: Biologia, Clínica E Terapêutica Dos Acidentes; Cardoso, J.L., França, F.O.S., Wen, F.H., Malaque, C., Haddad, V., Jr., Eds.; Savier: São Paulo, Brazil, 2009; pp. 81–95. [Google Scholar]
- Sajevic, T.; Leonardi, A.; Križaj, I. Haemostatically active proteins in snake venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, M.R.; de Sousa, B.B.; da Cunha Pereira, D.F.; Mamede, C.C.N.; Matias, M.S.; de Morais, N.C.G.; de Oliveira Costa, J.; de Oliveira, F. The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon 2017, 133, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant proteins from snake venoms. Haemostasis 2001, 31, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Mounier, C.M.; Bon, C.; Kini, R.M. Anticoagulant venom and mammalian secreted phospholipases A(2): Protein- versus phospholipid-dependent mechanism of action. Haemostasis 2001, 31, 279–287. [Google Scholar] [CrossRef]
- Faure, G.; Saul, F. Structural and Functional Characterization of Anticoagulant, FXa-binding Viperidae Snake Venom Phospholipases A2. Acta Chim. Slov. 2011, 58, 671–677. [Google Scholar]
- Doolittle, R.F. Step-by-step evolution of vertebrate blood coagulation. Cold Spring Harb. Symp. Quant. Biol. 2009, 74, 35–40. [Google Scholar] [CrossRef]
- Tentoni, J.; Polini, N.N.; Casanave, E.B. Comparative vertebrate fibrinolysis. Comp. Clin. Pathol. 2010, 19, 225–234. [Google Scholar] [CrossRef]
- Chijiwa, T.; Yamaguchi, Y.; Ogawa, T.; Deshimaru, M.; Nobuhisa, I.; Nakashima, K.; Oda-Ueda, N.; Fukumaki, Y.; Hattori, S.; Ohno, M. Interisland evolution of Trimeresurus flavoviridis venom phospholipase A(2) isozymes. J. Mol. Evol. 2003, 56, 286–293. [Google Scholar] [CrossRef]
- Sousa, L.F.; Bernardoni, J.L.; Zdenek, C.N.; Dobson, J.; Coimbra, F.; Gillett, A.; Lopes-Ferreira, M.; Moura-da-Silva, A.M.; Fry, B.G. Differential coagulotoxicity of metalloprotease isoforms from Bothrops neuwiedi snake venom and consequent variations in antivenom efficacy. Toxicol. Lett. 2020, 333, 211–221. [Google Scholar] [CrossRef]
- Bernardoni, J.L.; Sousa, L.F.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.T.; Zingali, R.B.; Moura-da-Silva, A.M. Functional Variability of Snake Venom Metalloproteinases: Adaptive Advantages in Targeting Different Prey and Implications for Human Envenomation. PLoS ONE 2014, 9, e109651. [Google Scholar] [CrossRef]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. USA 2021, 118, e2015579118. [Google Scholar] [CrossRef]
- Malhotra, A.; Creer, S.; Harris, J.B.; Thorpe, R.S. The importance of being genomic: Non-coding and coding sequences suggest different models of toxin multi-gene family evolution. Toxicon 2015, 107, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Suranse, V.; Jackson, T.N.W.; Sunagar, K. Contextual Constraints: Dynamic Evolution of Snake Venom Phospholipase A. Toxins 2022, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Contreras-Bernal, J.C.; Cirilo Gimenes, S.N.; Freitas-de-Sousa, L.A.; Portes-Junior, J.A.; da Silva Peixoto, P.; Kei Iwai, L.; Mourão de Moura, V.; Ferreira Bisneto, P.; Lacerda, M.; et al. The relationship between clinics and the venom of the causative Amazon pit viper (Bothrops atrox). PLoS Negl. Trop. Dis. 2020, 14, e0008299. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) Venoms from Brazil: Differential Biochemistry and Antivenom Efficacy Resulting from Prey-Driven Venom Variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef]
- Sousa, L.F.; Nicolau, C.A.; Peixoto, P.S.; Bernardoni, J.L.; Oliveira, S.S.; Portes-Junior, J.A.; Mourao, R.H.V.; Lima-dos-Santos, I.; Sano-Martins, I.S.; Chalkidis, H.M.; et al. Comparison of Phylogeny, Venom Composition and Neutralization by Antivenom in Diverse Species of Bothrops Complex. PLoS Negl. Trop. Dis. 2013, 7, e2442. [Google Scholar] [CrossRef]
- Espino-Solis, G.P.; Riaño-Umbarila, L.; Becerril, B.; Possani, L.D. Antidotes against venomous animals: State of the art and prospectives. J. Proteom. 2009, 72, 183–199. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Lomonte, B.; Angulo, Y. Antivenoms for snakebite envenomings. Inflamm. Allergy Drug Targets 2011, 10, 369–380. [Google Scholar] [CrossRef]
- Calvete, J.J.; Cid, P.; Sanz, L.; Segura, A.; Villalta, M.; Herrera, M.; León, G.; Harrison, R.; Durfa, N.; Nasidi, A.; et al. Antivenomic assessment of the immunological reactivity of EchiTAb-Plus-ICP, an antivenom for the treatment of snakebite envenoming in sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2010, 82, 1194–1201. [Google Scholar] [CrossRef]
- Zamunér, S.R.; da Cruz-Höfling, M.A.; Corrado, A.P.; Hyslop, S.; Rodrigues-Simioni, L. Comparison of the neurotoxic and myotoxic effects of Brazilian Bothrops venoms and their neutralization by commercial antivenom. Toxicon 2004, 44, 259–271. [Google Scholar] [CrossRef]
- Prezoto, B.C.; Tanaka-Azevedo, A.M.; Marcelino, J.R.; Tashima, A.K.; Nishiduka, E.S.; Kapronezai, J.; Mota, J.O.; Rocha, M.M.T.; Serino-Silva, C.; Oguiura, N. A functional and thromboelastometric-based micromethod for assessing crotoxin anticoagulant activity and antiserum relative potency against Crotalus durissus terrificus venom. Toxicon 2018, 148, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M.; Fernandes, I.; Cardoso, D.F.; Moura-da-Silva, A.M. Lachesis muta muta venom: Immunological differences compared with Bothrops atrox venom and importance of specific antivenom therapy. Toxicon 2001, 39, 711–719. [Google Scholar] [CrossRef]
- Moura-da-Silva, A.M.; Cardoso, D.F.; Tanizaki, M.M.; Mota, I. Neutralization of Myotoxic Activity of Bothrops Venoms by Antisera to Purified Myotoxins and to Crude Venoms. Toxicon 1991, 29, 1471–1480. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Knittel, P.S.; Long, P.F.; Brammall, L.; Marques, A.C.; Almeida, M.T.; Padilla, G.; Moura-da-Silva, A.M. Characterising the enzymatic profile of crude tentacle extracts from the South Atlantic jellyfish Olindias sambaquiensis (Cnidaria: Hydrozoa). Toxicon 2016, 119, 1–7. [Google Scholar] [CrossRef]
- Magalhães, G.S.; Caporrino, M.C.; Della-Casa, M.S.; Kimura, L.F.; Prezotto-Neto, J.P.; Fukuda, D.A.; Portes-Junior, J.A.; Neves-Ferreira, A.G.; Santoro, M.L.; Barbaro, K.C. Cloning, expression and characterization of a phospholipase D from Loxosceles gaucho venom gland. Biochimie 2013, 95, 1773–1783. [Google Scholar] [CrossRef]
- Kimura, L.F.; Prezotto-Neto, J.P.; Antoniazzi, M.M.; Jared, S.G.; Santoro, M.L.; Barbaro, K.C. Characterization of inflammatory response induced by Potamotrygon motoro stingray venom in mice. Exp. Biol. Med. 2014, 239, 601–609. [Google Scholar] [CrossRef]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]



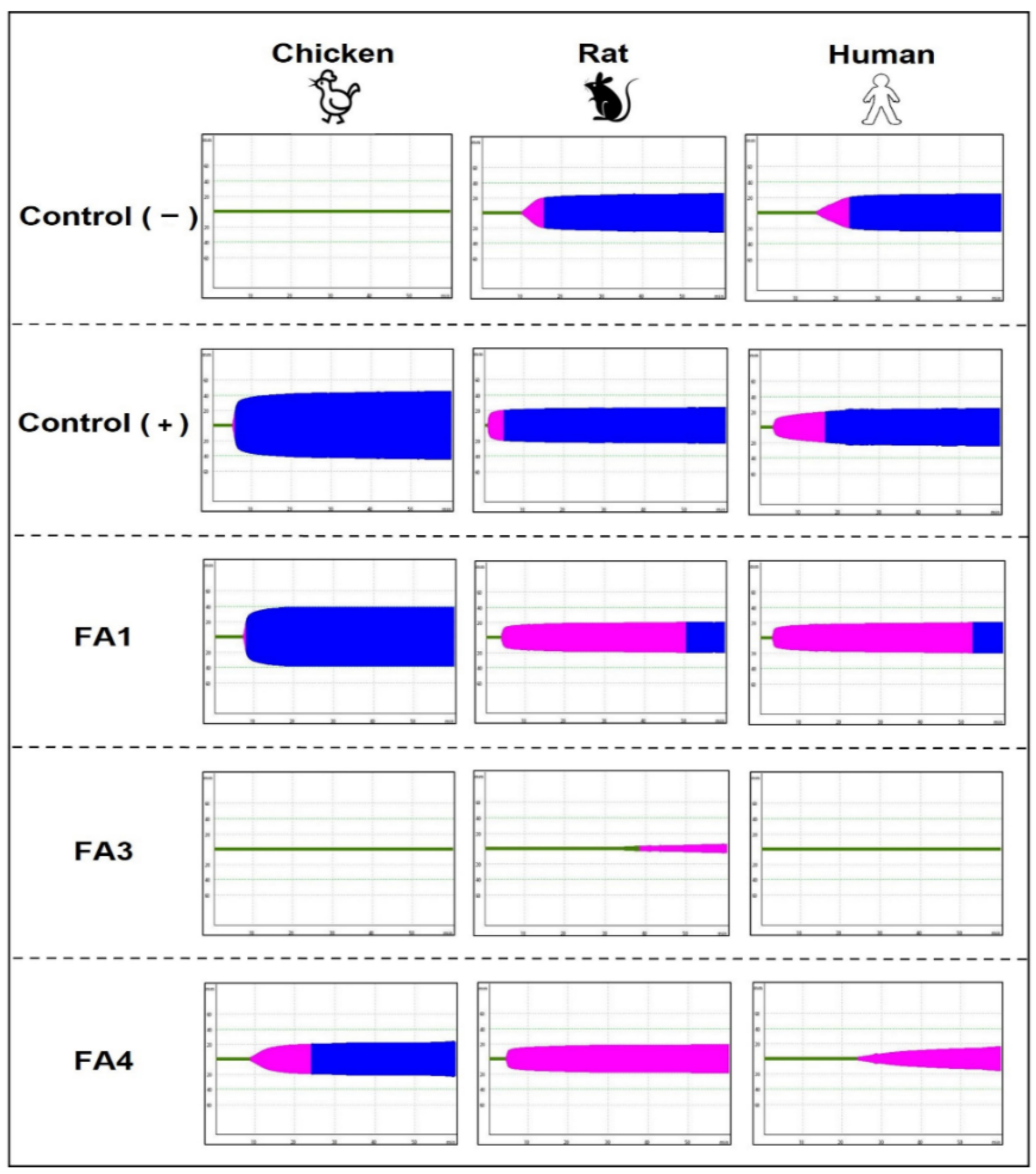

| Human Plasma | Rat Plasma | Chicken Plasma | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CT (s) | CFT (s) | MCF (mm) | CT (s) | CFT (s) | MCF (mm) | CT (s) | CFT (s) | MCF (mm) | |
| Control (−) | 857 ± 1.8 | 1349 ± 2.6 | 24.7 ± 5.3 | 609.6 ± 2.1 | 891 ± 2.5 | 26.1 ± 1.8 | unclotted * | ND | ND |
| Control (+) | 193.3 ± 1.4 | 861 ± 4.5 | 23.5 ± 1.3 | 134 ± 2.9 | 312.6 ± 3.1 | 22.3 ± 0.3 | 371 ± 1.5 | 431 ± 2.5 | 45.7 ± 0.5 |
| FA1 | 260.3 ± 6.4 | 3143.3 ± 4 | 19.7 ± 0.9 | 273.6 ± 3.1 | 3061 ± 3.2 | 19.1 ± 0.5 | 458 ± 1.4 | 963 ± 3.9 | 38.3 ± 1.5 |
| FA3 | unclotted * | ND | 19.7 ± 0.9 | 2248.5 ± 5.1 | ND | 11 ± 0.6 | unclotted * | ND | ND |
| FA4 | 1549 ± 16.6 | ND | 17 ± 0.8 | 311 ± 3.4 | ND | 18.3 ± 0.4 | 597 ± 1.8 | 1349 ± 2.6 | 22.7 ± 5.3 |
| Fraction | pI | Residue at Position 49 | Catalytic | Myotoxic | Edematogenic | Nociceptive | Anticoagulant | Antivenom Reactivity |
|---|---|---|---|---|---|---|---|---|
| FA1 | 8.4 | K | ||||||
| FA3 | 8.2 | D | ||||||
| FA4 | 5.1 | D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, L.F.; Freitas, A.P.; Cardoso, B.L.; Del-Rei, T.H.M.; Mendes, V.A.; Oréfice, D.P.; Rocha, M.M.T.; Prezoto, B.C.; Moura-da-Silva, A.M. Diversity of Phospholipases A2 from Bothrops atrox Snake Venom: Adaptive Advantages for Snakes Compromising Treatments for Snakebite Patients. Toxins 2022, 14, 543. https://doi.org/10.3390/toxins14080543
Sousa LF, Freitas AP, Cardoso BL, Del-Rei THM, Mendes VA, Oréfice DP, Rocha MMT, Prezoto BC, Moura-da-Silva AM. Diversity of Phospholipases A2 from Bothrops atrox Snake Venom: Adaptive Advantages for Snakes Compromising Treatments for Snakebite Patients. Toxins. 2022; 14(8):543. https://doi.org/10.3390/toxins14080543
Chicago/Turabian StyleSousa, Leijiane F., Amanda P. Freitas, Bruna L. Cardoso, Tiago H. M. Del-Rei, Vanessa A. Mendes, Daniele P. Oréfice, Marisa M. T. Rocha, Benedito C. Prezoto, and Ana M. Moura-da-Silva. 2022. "Diversity of Phospholipases A2 from Bothrops atrox Snake Venom: Adaptive Advantages for Snakes Compromising Treatments for Snakebite Patients" Toxins 14, no. 8: 543. https://doi.org/10.3390/toxins14080543
APA StyleSousa, L. F., Freitas, A. P., Cardoso, B. L., Del-Rei, T. H. M., Mendes, V. A., Oréfice, D. P., Rocha, M. M. T., Prezoto, B. C., & Moura-da-Silva, A. M. (2022). Diversity of Phospholipases A2 from Bothrops atrox Snake Venom: Adaptive Advantages for Snakes Compromising Treatments for Snakebite Patients. Toxins, 14(8), 543. https://doi.org/10.3390/toxins14080543