Clinical Characteristics of Ciguatera Poisoning in Martinique, French West Indies—A Case Series
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Incidence of CP in the World
3.2. Clinical Characteristics of CP
3.3. Fish Involved in CP
3.4. Study Limitations
4. Conclusions
5. Materials and Methods
5.1. Study Design and Data Collection
5.2. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Region | Period | Incidence (/10,000 Patient-Years) | Reference |
|---|---|---|---|
| Martinique | 1996–2006 | 0.20 | [5] |
| Reunion (Indian Ocean) | 2000–2010 | 0.20 | [6] |
| Guadeloupe | 1996–2006 | 0.30 | [5] |
| Guadeloupe | 2013–2016 | 1.47 | [7] |
| Florida | 2000–2011 | 5.6 | [8] |
| French Polynesia (Pacific Ocean) | 2016 | 18.0 | [9] |
| Virgin Islands | 1996–2006 | 19.9 | [5] |
| Antigua | 1996–2006 | 34.4 | [5] |
| Montserrat | 1996–2006 | 58.6 | [5] |
| Pacific Island Countries and Territories | 1998–2008 | 194 | [10] |
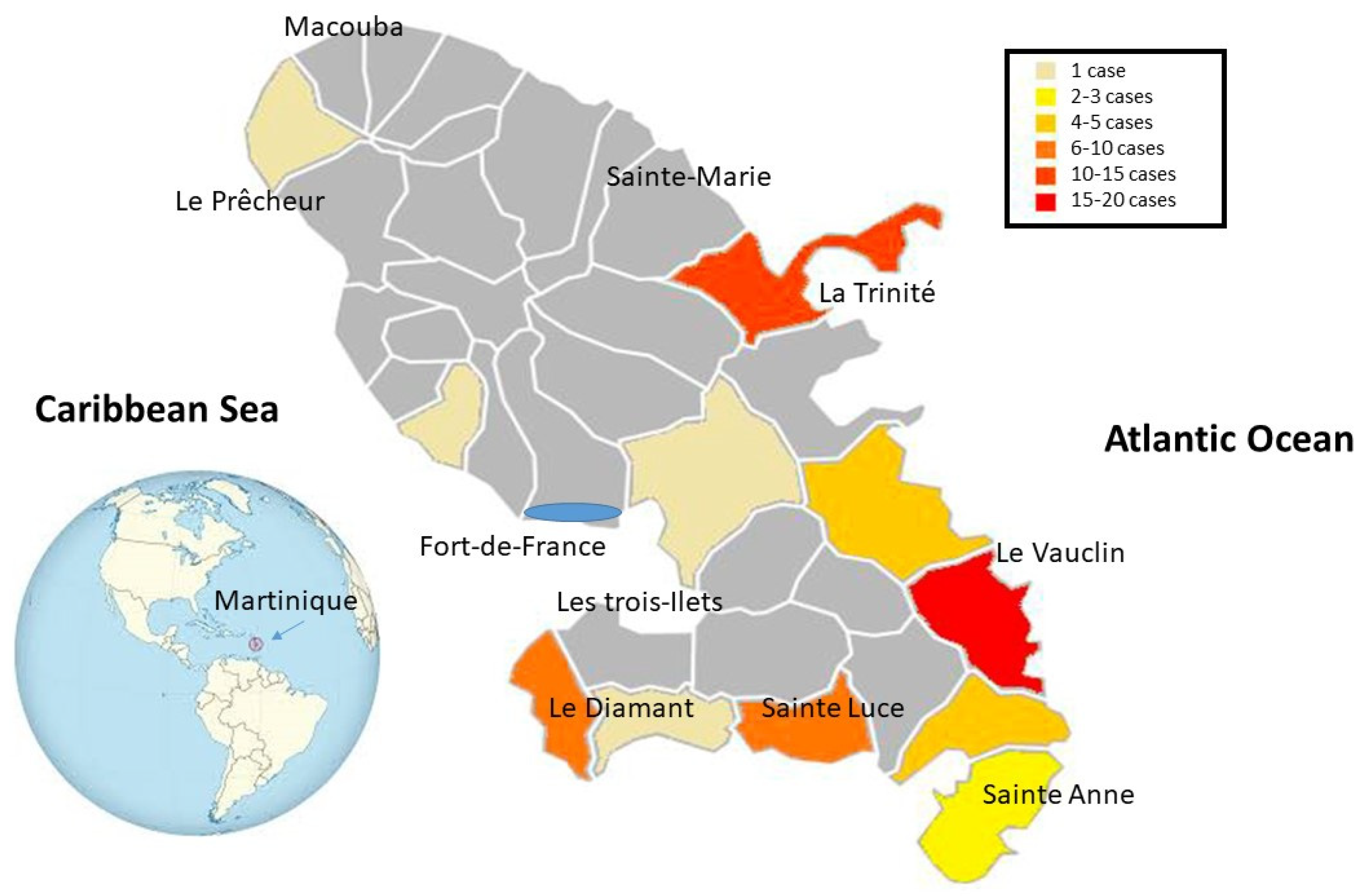
Appendix B
References
- Marcus, E.N. Ciguatera Fish poisoning. In UpToDate; Traub, S.J., Danzl, D.F., Sample, J.A., Ganetsky, M., Eds.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2022; Available online: https://www.uptodate.com/contents/ciguatera-fish-poisoning (accessed on 27 April 2022).
- Whittle, K.; Gallacher, S. Marine toxins. Br. Med. Bull. 2000, 56, 236–253. [Google Scholar] [CrossRef]
- Kibler, R.; Tester, P.A.; Kunkel, K.E.; Moore, S.K.; Litaker, R.W. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Model. 2015, 316, 194–210. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera-causing dinoflagellates in the genera Gambierdiscus and Fukuyoa: Distribution, ecophysiology, and toxicology. In Dinoflagellates; Subba Rao, D.V., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2020; Available online: https://www.novapublishers.com/wp-content/uploads/2020/08/978-1-53617-888-3.pdf (accessed on 27 April 2022).
- Dickey, R.W.; Plakas, S.M. Ciguatera: A public health perspective. Toxicon 2010, 56, 123–136. [Google Scholar] [CrossRef]
- Tester, P.A.; Feldman, R.L.; Nau, A.W.; Kibler, S.R.; Wayne Litaker, R. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon 2010, 56, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Boucaud-Maitre, D.; Vernoux, J.P.; Pelczar, S.; Daudens-Vaysse, E.; Aubert, L.; Boa, S.; Ferracci, S.; Garnier, R. Incidence and clinical characteristics of ciguatera fish poisoning in Guadeloupe (French West Indies) between 2013 and 2016: A retrospective cases-series. Sci. Rep. 2018, 8, 3095. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Reich, A.; Morris, J.G., Jr. Epidemiology of Ciguatera in Florida. Am. J. Trop. Med. Hyg. 2015, 93, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Gatti, C.; Giard, M. Situation de la Ciguatera en Polynésie Française. 2018. Available online: https://www.service-public.pf/dsp/wp-content/uploads/sites/12/2019/04/Bilan-ciguatera-2018.pdf (accessed on 27 April 2022).
- Skinner, M.P.; Brewer, T.D.; Johnstone, R.; Fleming, L.E.; Lewis, R.J. Ciguatera fish poisoning in the Pacific islands (1998 to 2008). PLoS Negl. Trop. Dis. 2011, 5, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y. Characteristic features and contributory factors in fatal ciguatera fish poisoning, implications for prevention and public education. Am. J. Trop. Med. Hyg. 2016, 94, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fleming, L.E.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.Y.; Backer, L.; Ayyar, R.; Weisman, R.; et al. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–579. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Raposo-Garcia, S.; Louzao, M.C.; Fuwa, H.; Sasaki, M.; Vale, C.; Botana, L.M. Determination of the toxicity equivalency factors for ciguatoxins using human sodium channels. Food Chem. Toxicol. 2022, 160, 112812. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Report of the Expert Meeting on Ciguatera Poisoning: Rome Food & Agriculture Org, 19–23 November 2018. Available online: https://apps.who.int/iris/handle/10665/332640 (accessed on 27 April 2022).
- Institut National de la Statistique et des Études Économiques. Recensement de la Population en Martinique: 368,783 habitants au 1 janvier 2018. 2018. Available online: https://www.insee.fr/fr/statistiques/5005738#titre-bloc-7 (accessed on 27 April 2022).
- Centers for Disease Control and Prevention (CDC). Ciguatera fish poisoning—New York City, 2010–2011. Morb. Mortal. Wkly Rep. 2013, 62, 61–65. [Google Scholar]
- Gatti, C.; LMT-ILM. Etat de la Ciguatera en Polynésie Française. Réseau de Surveillance de la Ciguatera. 2015. Available online: http://www.ressources-marines.gov.pf/wp-content/uploads/sites/24/2018/02/ilm_bilan_ciguatera_en_polynesie_francaise_2015.pdf (accessed on 27 April 2022).
- Schlaich, C.; Hagelstein, J.G.; Burchard, G.D.; Schmiedel, S. Outbreak of ciguatera fish poisoning on a cargo ship in the port of hamburg. J. Travel Med. 2012, 19, 238–242. [Google Scholar] [CrossRef]
- Mattei, C.; Vetter, I.; Eisenblätter, A.; Krock, B.; Ebbecke, M.; Desel, H.; Zimmermann, K. Ciguatera fish poisoning: A first epidemic in Germany highlights an increasing risk for European countries. Toxicon 2014, 91, 76–83. [Google Scholar] [CrossRef]
- Bavastrelli, M.; Bertucci, P.; Midulla, M.; Giardini, O.; Sanguigni, S. Ciguatera fish poisoning: An emerging syndrome in Italian travelers. J. Travel Med. 2011, 8, 139–142. [Google Scholar] [CrossRef]
- De Haro, L.; Pommier, P.; Valli, M. Emergence of imported ciguatera in Europe: Report of 18 cases at the poison control centre of Marseille. Clin. Toxicol. 2003, 41, 927–930. [Google Scholar] [CrossRef]
- Kodama, A.; Hokama, Y. Variations in symptomatology of ciguatera poisoning. Toxicon 1988, 27, 593–595. [Google Scholar] [CrossRef]
- Pottier, I.; Vernoux, J.P.; Lewis, R. Ciguatera Fish Poisoning in the Caribbean Islands and western Atlantic. Rev. Environ. Contam. Toxicol. 2011, 168, 99–141. [Google Scholar]
- Cardoso, T.; Chappert, J.L.; Quénel, P. CIRE Antilles Guyane. La ciguatera en Martinique et Guadeloupe. Bull. D’alerte Surveill. Antill. Guyane CIRE 2008, 8, 1–4. Available online: https://www.sentinelles971.com/wp-content/uploads/2012/12/Basag2008_8-cigua.pdf (accessed on 27 April 2022).
- Gatti, C.; Oelher, E.; Legrand, A.M. Severe seafood poisoning in French Polynesia: A retrospective analysis of 129 medical files. Toxicon 2008, 51, 746–753. [Google Scholar] [CrossRef]
- Pearn, J. Neurology of ciguatera. J. Neurol. Neurosurg. Psychiatry 2001, 70, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Kim, D.H.; Seo, M.W.; Shin, B.S. Reversible Cerebellar Dysfunction Associated with Ciguatera Fish Poisoning. J. Emerg. Med. 2012, 43, 674–676. [Google Scholar] [CrossRef]
- Cameron, J.; Capra, M.F. The basis of the paradoxical disturbance of temperature perception in ciguatera poisoning. J. Toxicol. Clin. Toxicol. 1993, 31, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Château-Degat, M.L.; Beuter, A.; Vauterin, G.; Nguyen, N.L.; Chinain, M.; Darius, T.; Legrand, A.M.; Chansin, R.; Dewailly, E. Neurologic signs of ciguatera disease: Evidence of their persistence. Am. J. Trop. Med. Hyg. 2007, 77, 1170–1175. [Google Scholar] [CrossRef]
- Vigneau, J.-F.; Kierzek, G.; Dumas, F.; Pourriat, J.-L. Ciguatera: Recrudescence of symptomatology of a previous intoxication. Ann. Fr. Anesth. Réanim. 2008, 27, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.; Bourrat, M.B.; Pauillac, S. Prevalence, symptoms and chronicity of ciguatera in New Caledonia: Results from an adult population survey conducted in Noumea during 2005. Toxicon 2009, 56, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y. Severe bradycardia and prolonged hypotension in ciguatera. Singap. Med. J. 2013, 54, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Mullins, M.E.; Hoffman, R.S. Is mannitol the treatment of choice for patients with ciguatera fish poisoning? Clin. Toxicol. 2017, 55, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Bouchut, J. Nouveautés Dans la Ciguatera: Étude des Facteurs de Risque de Gravite Parmi une Cohorte Hospitalière Polynésienne et Conséquences sur la Prise en Charge en Structure de Soins de Santé Primaires. Médecine Humaine et Pathologie. 2014. Available online: https://dumas.ccsd.cnrs.fr/dumas-00979064/document (accessed on 27 April 2022).
- Hashimoto, Y.; Konosu, S.; Shibota, M.; Watanabe, K. Toxicity of a Turban-shell in the Pacific. Nippo Suisan Gakkaishi 1970, 36, 1163–1171. Available online: https://www.jstage.jst.go.jp/article/suisan1932/36/11/36_11_1163/_article (accessed on 27 April 2022). [CrossRef][Green Version]
- Abraham, A.; Jester, E.L.E.; Granade, H.R.; Plakas, S.M.; Dickey, R.W. Caribbean ciguatoxin profile in raw and cooked fish implicated in ciguatera. Food Chem. 2012, 131, 192–198. [Google Scholar] [CrossRef]
- Oshiro, N.; Nagasawa, H.; Kuniyoshi, K.; Kobayashi, N.; Sugita-Konishi, Y.; Asakura, H.; Yasumoto, T. Characteristic Distribution of Ciguatoxins in the Edible Parts of a Grouper, Variola louti. Toxins 2021, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, A.; De la Iglesia, P.; Darius, H.T.; Pauillac, S.; Aligizaki, K.; Fraga, S.; Chinain, M.; Diogène, J. Update on Methodologies Available for Ciguatoxin Determination: Perspectives to Confront the Onset of Ciguatera Fish Poisoning in Europe. Mar. Drugs 2010, 8, 1838–1907. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Prevalence or Value |
|---|---|
| General manifestations | 135/149 (91%) |
| Body temperature † | 37.0 °C (36.0–37.1) |
| Myalgia | 94/149 (63%) |
| Pruritus | 94/149 (63%) |
| Asthenia | 61/149 (41%) |
| Hypothermia (temperature ≤36.5 °C) * | 26/66 (39%) |
| Arthralgia | 54/149 (36%) |
| Chills | 49/149 (33%) |
| Headaches | 42/149 (28%) |
| Dizziness | 34/149 (23%) |
| Malaise | 28/149 (19%) |
| Sweating | 6/149 (4%) |
| Dyspnea | 5/149 (3%) |
| Limb edema | 5/149 (3%) |
| Gastrointestinal manifestations | 134/149 (90%) |
| Diarrhea | 119/149 (80% |
| Abdominal pain | 102/149 (69%) |
| Nausea | 88/149 (59%) |
| Vomiting | 80/149 (54%) |
| Neurological manifestations | 108/149 (72%) |
| Paresthesia | 77/149 (52%) |
| Dysgeusia | 45/149 (30%) |
| Impairment of feeling of hot and cold | 40/149 (27%) |
| Touch disorder | 37/149 (25%) |
| Dysuria | 25/149 (17%) |
| Reversal of hot and cold | 24/149 (16%) |
| Balance/coordination/language impairment | 24/149 (16%) |
| Visual disturbance | 23/149 (15%) |
| Behavioral disorder | 17/149 (11%) |
| Pain in cold | 11/149 (7%) |
| Cardiovascular manifestations | 33/149 (22%) |
| Heart rate † | 69 bpm (50–87) |
| Systolic blood pressure † | 112 mmHg (97–130) |
| Diastolic blood pressure † | 68 mmHg (59–78) |
| Bradycardia (heart rate <60 bpm) * | 24/66 (36%) |
| Hypotension (systolic blood pressure <90 mmHg) * | 10/66 (15%) |
| ECG abnormalities * | 10/66 (15%) |
| Severe cardiovascular features #,* | 8/66 (12%) |
| Palpitations | 5/149 (3%) |
| Chronic manifestations ** | 31/77 (40%) |
| Chronic pain | 20/77 (26%) |
| Chronic neurological manifestations | 15/77 (20%) |
| Chronic asthenia | 11/77 (14%) |
| Chronic pruritus | 10/77 (13%) |
| Management at the Emergency Department | Prevalence or Value |
|---|---|
| Supportive care | 42/66 (64%) |
| Fluids (0.9% NaCl) | 11/66 (17%) |
| 20% mannitol infusion | 11/66 (17%) |
| Intravenous atropine administration | 10/66 (15%) |
| Hospitalization | 24/149 (16%) |
| Length of hospital stay † | 1 days (1–2) |
| Follow-up at the clinical toxicology consultation | 39/66 (59%) |
| Incriminated Fish | Number of Incriminated Fish (N = 85) | Number of Intoxicated Patients (N = 149) |
|---|---|---|
| Carangidae (trevallies) | 50 | 87 |
| Lutjanus buccanella (snapper) | 14 | 19 |
| Scomberomorus regalis (king mackerel) | 4 | 10 |
| Gymnothorax funebris (moray eel) | 3 | 4 |
| Thunnus atlanticus (tuna) | 2 | 6 |
| Coryphaena hippurus (sea bream) | 2 | 3 |
| Syphyraena barracuda (barracuda) | 1 | 9 |
| Epinephelus morio (grouper) | 1 | 1 |
| Strobus gigas (spider conchs) | 1 | 1 |
| Salmo salar (salmon) | 1 | 1 |
| Mixed fish | 1 | 4 |
| Not described | 4 | 4 |
| Clinical Characteristics | Patients Intoxicated by Trevallies (Carangidae) (n = 87) | Patients Intoxicated by Other Fish Species (n = 58) | p-Value |
|---|---|---|---|
| Fish head or viscera ingestion (N = 144) | 20/86 (24%) | 5/58 (9%) | 0.03 |
| Acute vomiting (N = 145) | 64/87 (74%) | 23/58 (40%) | <0.0001 |
| Acute nausea (N = 145) | 61/87 (70%) | 24/58 (41%) | 0.001 |
| Acute abdominal pain (N = 145) | 68/87 (78%) | 31/58 (53%) | 0.002 |
| Acute hypotension (N = 64) | 14/35 (40%) | 5/29 (17%) | 0.05 |
| Mannitol administration (N = 63) | 8/33 (24%) | 2/30 (6%) | 0.09 |
| Persistent symptoms (N = 74) | 19/36 (50%) | 9/36 (25%) | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Résière, D.; Florentin, J.; Mehdaoui, H.; Mahi, Z.; Gueye, P.; Hommel, D.; Pujo, J.; NKontcho, F.; Portecop, P.; Nevière, R.; et al. Clinical Characteristics of Ciguatera Poisoning in Martinique, French West Indies—A Case Series. Toxins 2022, 14, 535. https://doi.org/10.3390/toxins14080535
Résière D, Florentin J, Mehdaoui H, Mahi Z, Gueye P, Hommel D, Pujo J, NKontcho F, Portecop P, Nevière R, et al. Clinical Characteristics of Ciguatera Poisoning in Martinique, French West Indies—A Case Series. Toxins. 2022; 14(8):535. https://doi.org/10.3390/toxins14080535
Chicago/Turabian StyleRésière, Dabor, Jonathan Florentin, Hossein Mehdaoui, Zakaria Mahi, Papa Gueye, Didier Hommel, Jean Pujo, Flaubert NKontcho, Patrick Portecop, Rémi Nevière, and et al. 2022. "Clinical Characteristics of Ciguatera Poisoning in Martinique, French West Indies—A Case Series" Toxins 14, no. 8: 535. https://doi.org/10.3390/toxins14080535
APA StyleRésière, D., Florentin, J., Mehdaoui, H., Mahi, Z., Gueye, P., Hommel, D., Pujo, J., NKontcho, F., Portecop, P., Nevière, R., Kallel, H., & Mégarbane, B. (2022). Clinical Characteristics of Ciguatera Poisoning in Martinique, French West Indies—A Case Series. Toxins, 14(8), 535. https://doi.org/10.3390/toxins14080535






