Preparation of Monoclonal Antibody against Deoxynivalenol and Development of Immunoassays
Abstract
:1. Introduction
2. Results
2.1. Animal Immunization and Hybridoma Screening
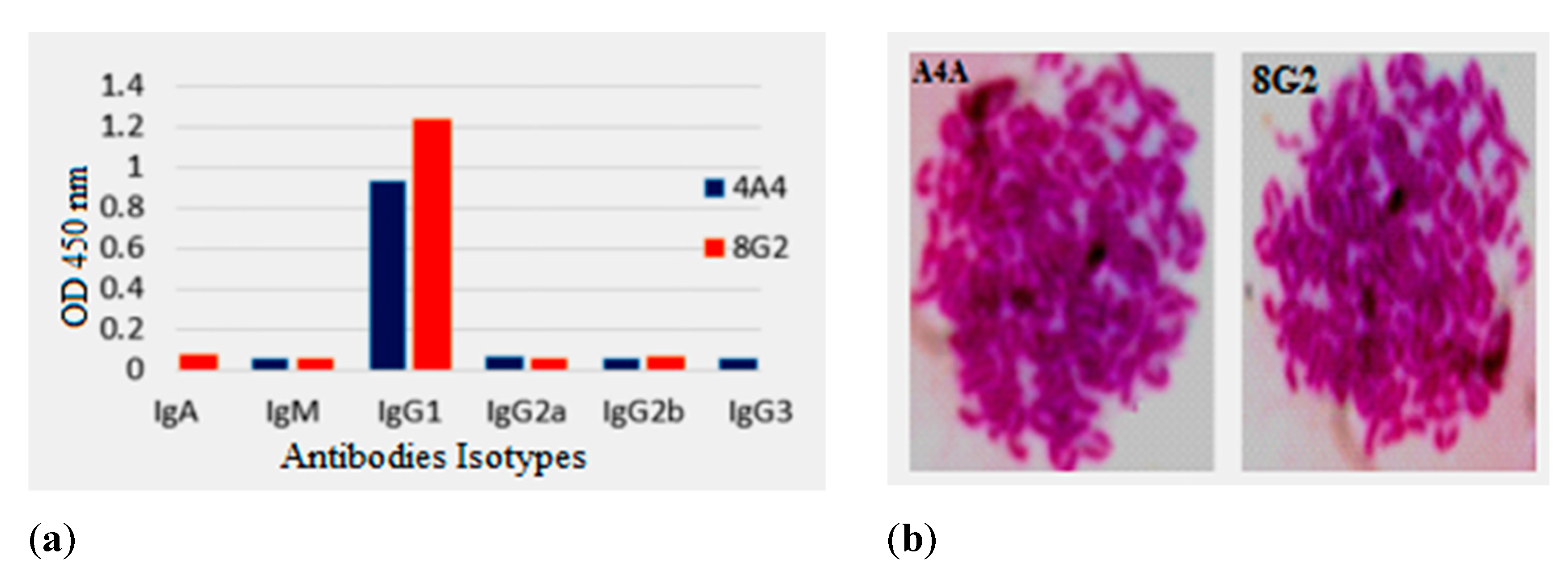
2.2. Isotypes and Chromosome Count
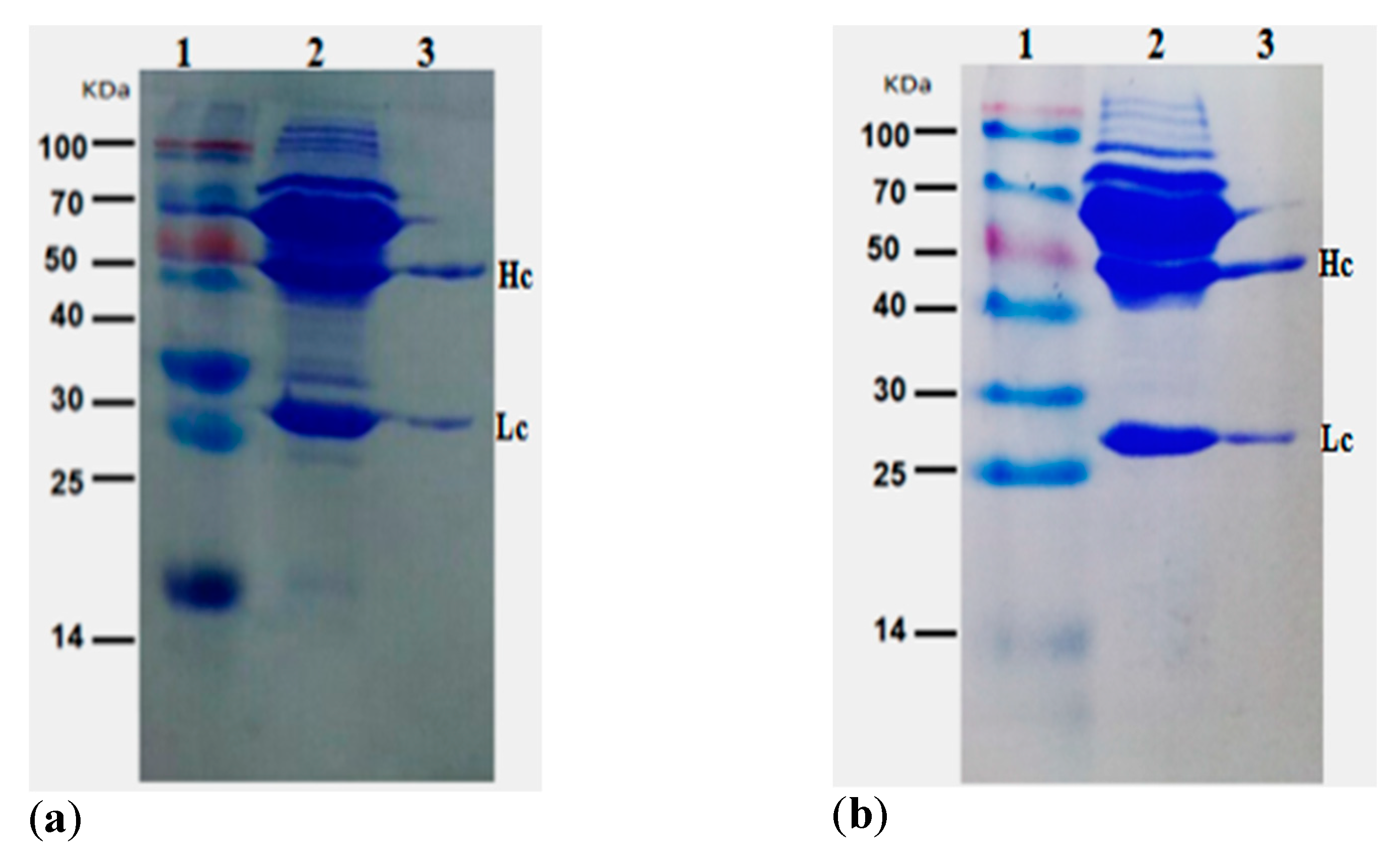
2.3. Purification of Positive mAb
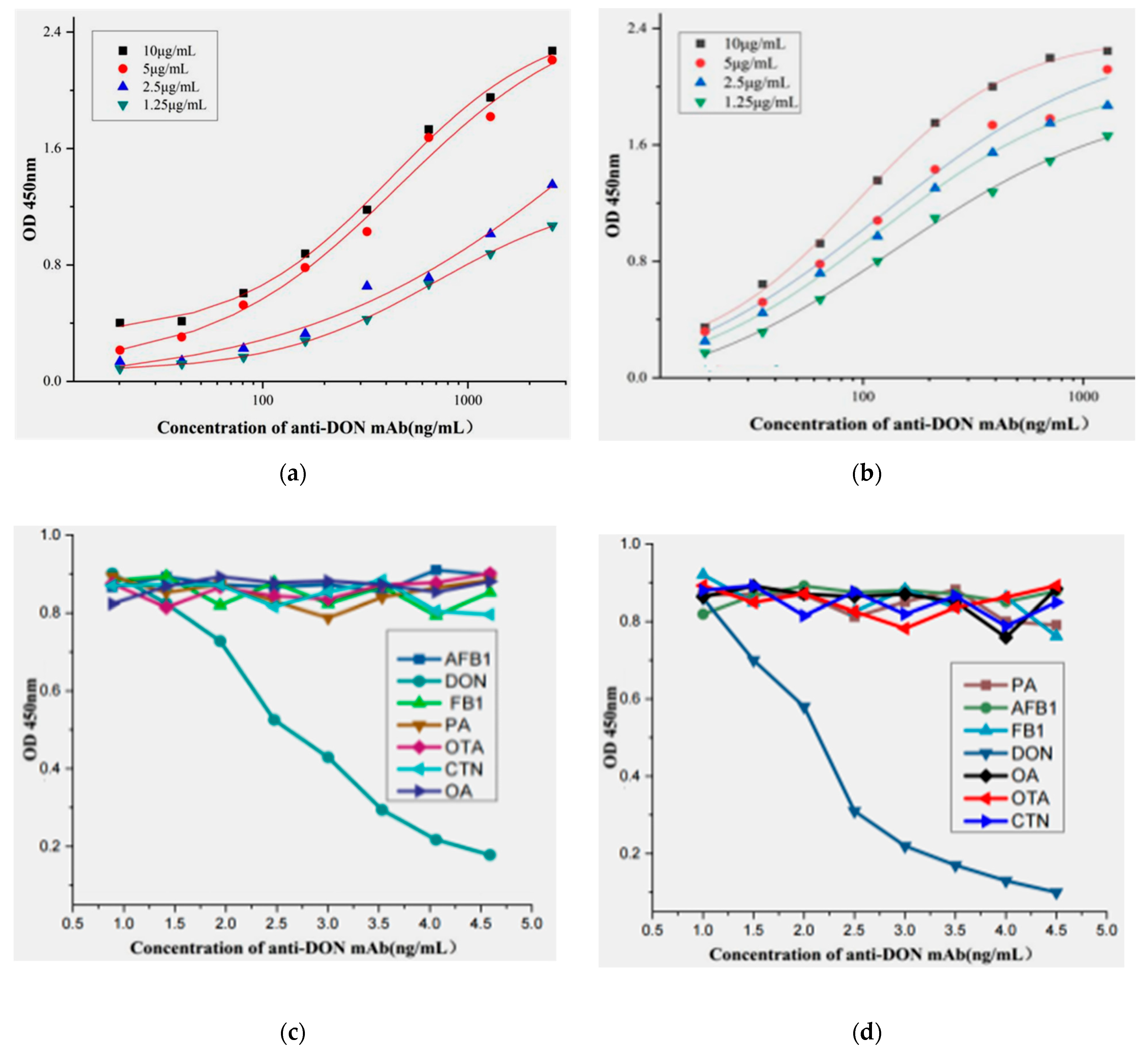
2.4. Affinity and Speciality of the Positive mAb
2.5. Establishment of Standard Curve Based on ic-ELISA
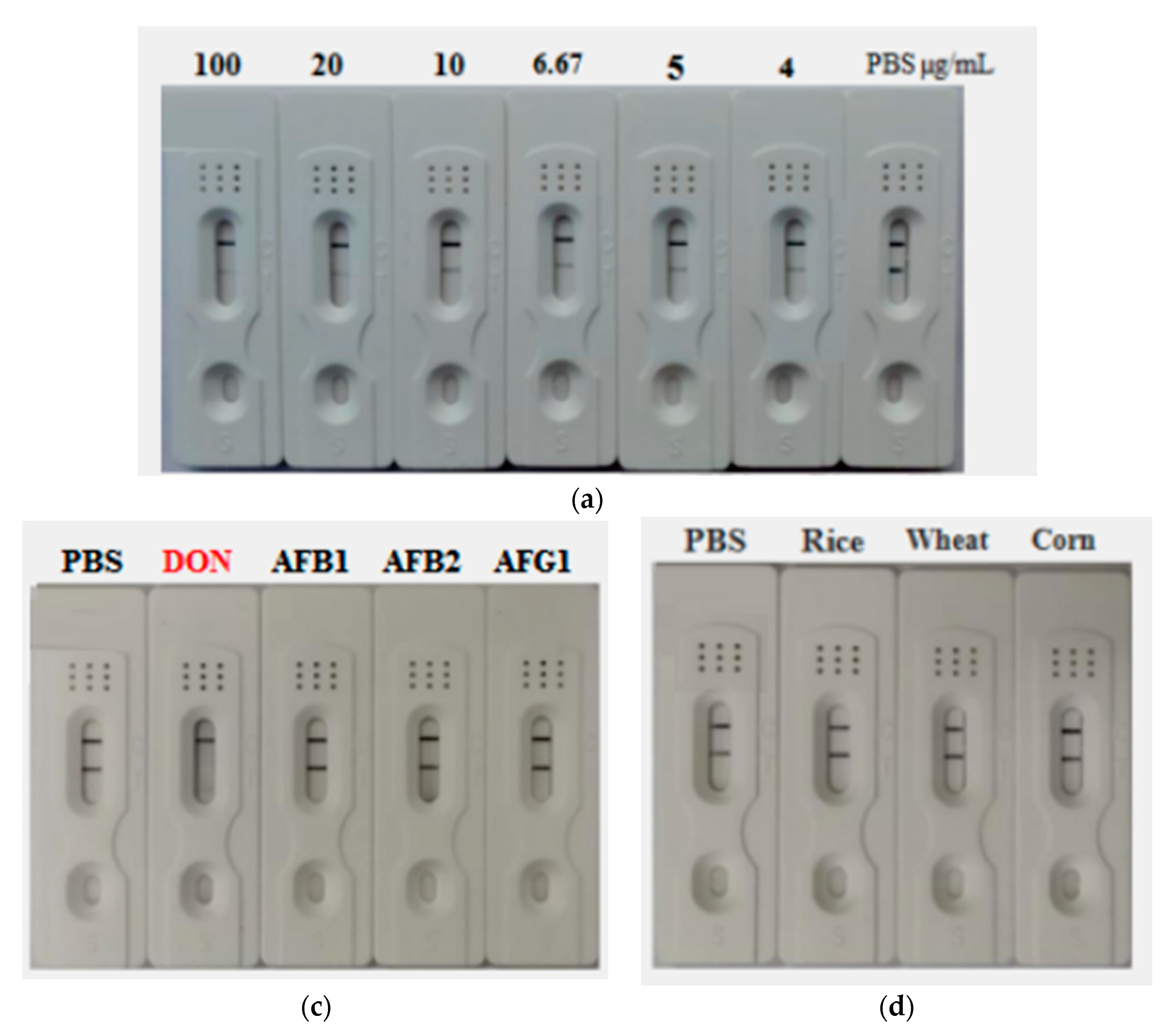
2.6. Samples Detection
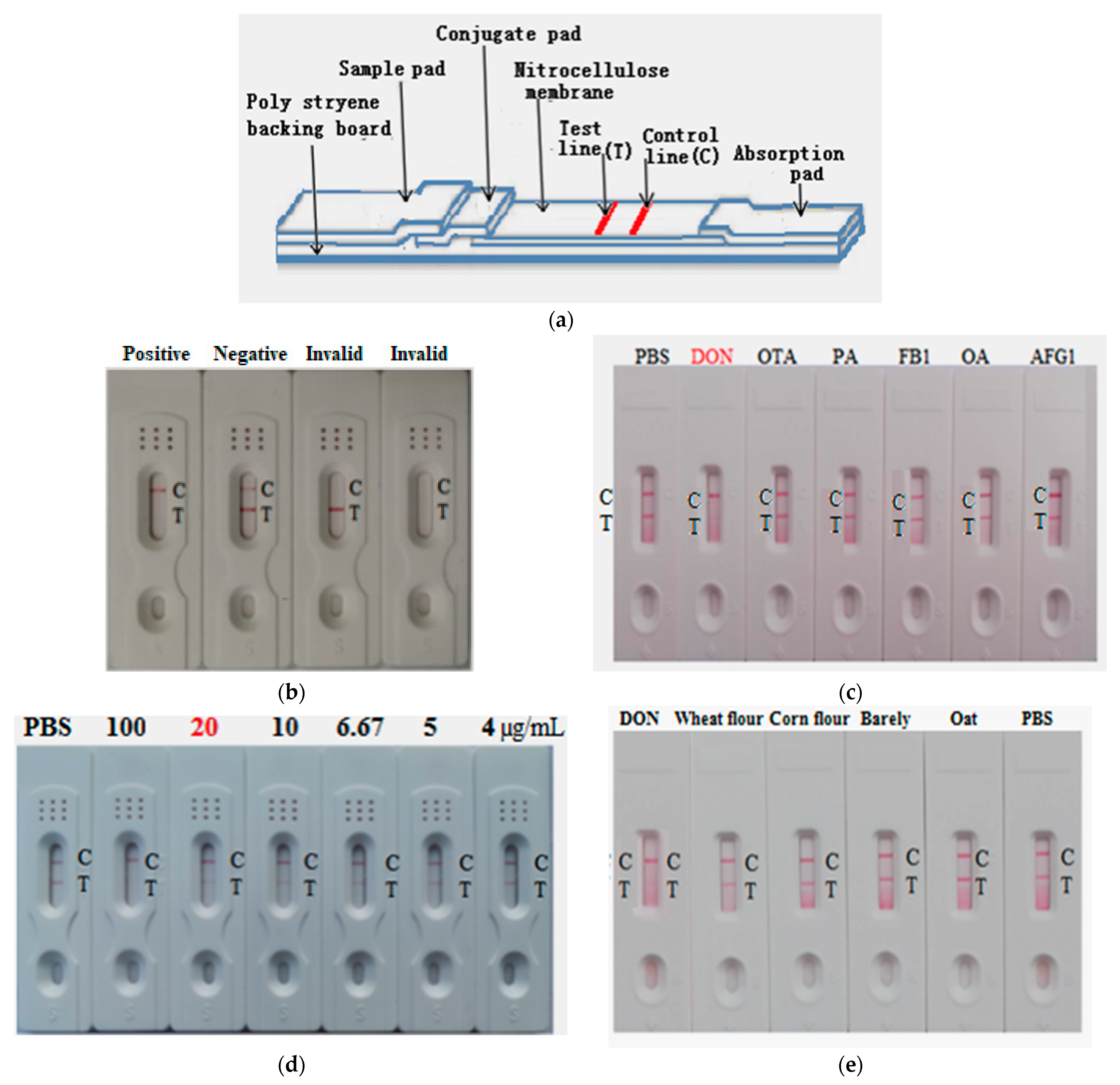
2.7. Development of AuNP-Based Strip for Determination of DON
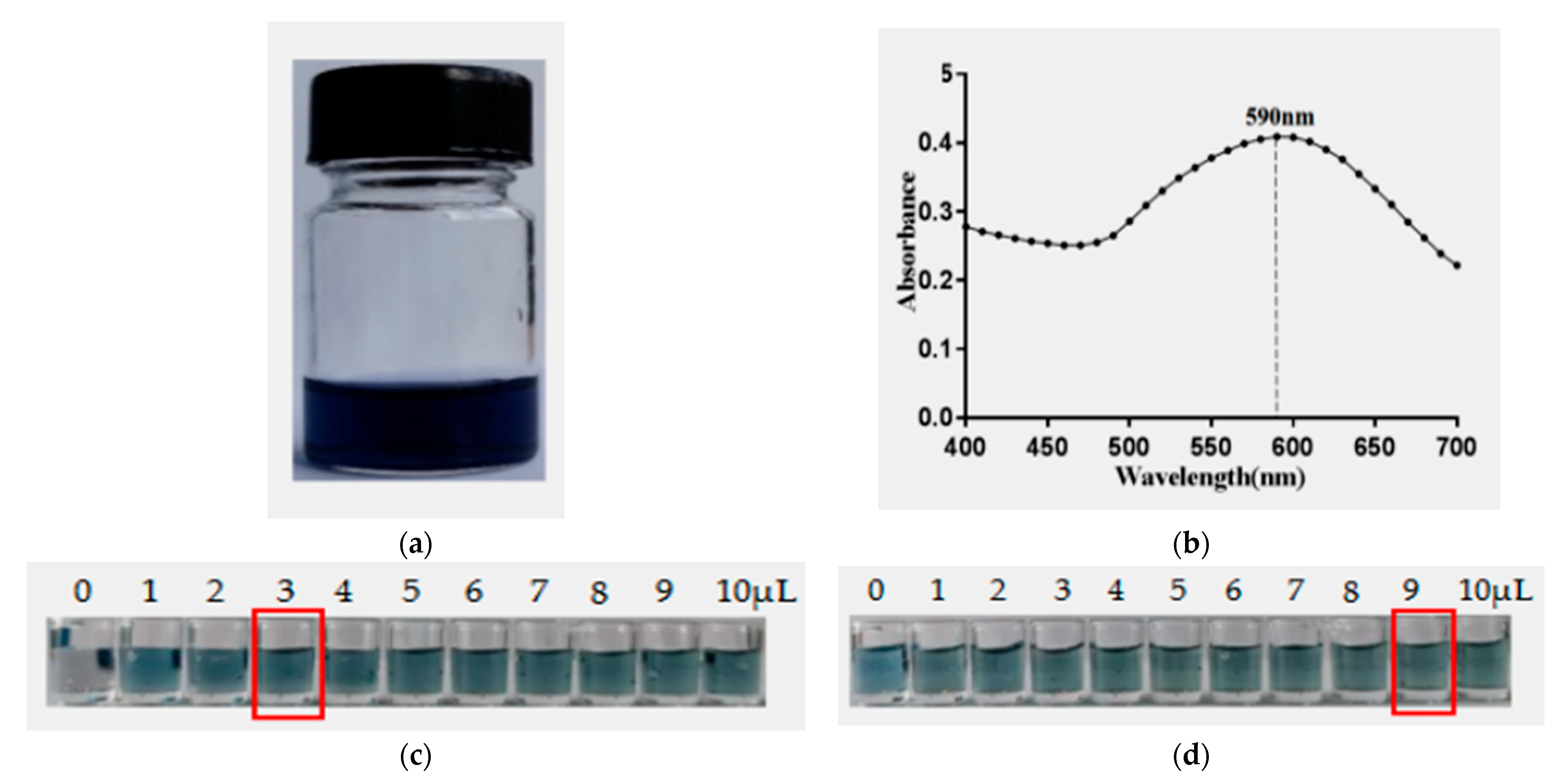
2.8. Preparation of AuNF Solution and AuNF-mAb Probe
2.9. Construction and Characterization of the AuNF Strip
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Material and Reagents
5.2. Immunization and Titer Determination
5.3. Screening and Characterization of Hybridoma Cells
5.4. Purification and Characterization of mAb from Ascites
5.5. Development of ic-ELISA and Standard Curve
5.6. Construction and Characterization of AuNP-Based Strip
5.7. Preparation of Gold Nanoflower (AuNF) and AuNF Probe
5.8. Preparation and Characterization of AuNF-Based Strip
5.9. Samples Detection
5.10. Ethical Clearance and Animal Handle
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuo, H.G.; Zhu, J.X.; Shi, L.; Zhan, C.R.; Guo, P.; Wang, Y.; Zhang, Y.; Liu, J. Development of a novel immunoaffinity column for the determination of deoxynivalenol and its acetylated derivatives in cereals. Food Anal. Methods 2018, 11, 2252–2260. [Google Scholar] [CrossRef]
- Luongo, D.; Severino, L.; Bergamo, P.; D’Arienzo, R.; Rossi, M. Trichothecenes NIV and DON modulate the maturation of murine dendritic cells. Toxicon 2010, 55, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. 2008, 25, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chen, L.; Nüssler, A.K.; Liu, L.; Yang, W. Current sights for mechanisms of deoxynivalenol-induced hepatotoxicity and prospective views for future scientific research: A mini review. J. Appl. Toxicol. 2017, 37, 518–529. [Google Scholar] [CrossRef]
- Deng, C.; Li, C.; Zhou, S.; Wang, X.; Xu, H.; Wang, D.; Gong, Y.Y.; Routledge, M.N.; Zhao, Y.; Wu, Y. Risk assessment of deoxynivalenol in high-risk area of China by human biomonitoring using an improved high throughput UPLC-MS/MS method. Sci. Rep. 2018, 8, 3901. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and quantitative analysis of mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef]
- Rupp, H.S. Determination of Deoxynivalenol in Whole Wheat Flour and Whear Bran. J. AOAC Int. 2002, 85, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Eke, Z.; Kende, A.; Torkos, K. Simultaneous detection of A and B trichothecenes by gas chromatography with flame ionization or mass selective detection. Microchem. J. 2004, 78, 211–216. [Google Scholar] [CrossRef]
- Jestoi, M.; Ritieni, A.; Rizzo, A. Analysis of the Fusarium mycotoxins fusaproliferin and trichothecenes in grains using gas chromatography− mass spectrometry. J. Agric. Food Chem. 2004, 52, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zeng, L.; Yang, H.; Zhong, Y.; Wang, J.; Ling, S.; Farhan Saeed, A.; Yuan, J.; Wang, S. Detection of okadaic acid (OA) using ELISA and colloidal gold immunoassay based on monoclonal antibody. J. Hazard. Mater. 2017, 339, 154–160. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Zhou, M. A sensitive chemiluminescence enzyme immunoassay for the determination of deoxynivalenol in wheat samples. Anal. Methods 2015, 7, 2196–2202. [Google Scholar] [CrossRef]
- Li, L.; Xia, L.; Zhao, Y.; Wang, M.; Jiang, X. Immune-affinity monolithic array with chemiluminescent detection for mycotoxins in barley. J. Sci. Food Agric. 2017, 97, 2426–2435. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, C.; Fang, S.; Zhuang, Z.; Ling, S.; Wang, S. A monoclonal antibody against F1-F0 ATP synthase beta subunit. Hybridoma 2012, 31, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.; Santos, D.; Chu, V.; Azevedo, A.; Aires-Barros, M.; Conde, J. A point-of-use microfluidic device with integrated photodetector array for immunoassay multiplexing: Detection of a panel of mycotoxins in multiple samples. Biosens. Bioelectron. 2017, 87, 823–831. [Google Scholar] [CrossRef]
- Lu, L.; Seenivasan, R.; Wang, Y.-C.; Yu, J.-H.; Gunasekaran, S. An electrochemical immunosensor for rapid and sensitive detection of mycotoxins fumonisin B1 and deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Zhang, X.; Wang, L.; Du, X.; Wei, D. Production of a soluble single-chain variable fragment antibody against okadaic acid and exploration of its specific binding. Anal. Biochem. 2016, 503, 21–27. [Google Scholar] [CrossRef]
- Rong-Hwa, S.; Shiao-Shek, T.; Der-Jiang, C.; Yao-Wen, H. Gold nanoparticle-based lateral flow assay for detection of staphylococcal enterotoxin B. Food Chem. 2010, 118, 462–466. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Aguilar, Z.P.; Xu, H.; Lai, W.; Xiong, Y. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar] [CrossRef]
- Parolo, C.; de la Escosura-Muñiz, A.; Merkoçi, A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Ren, M.; Li, Y.; Huang, Z.; Shu, M.; Yang, H.; Xiong, Y.; Xu, Y. Detection of aflatoxin B1 with immunochromatographic test strips: Enhanced signal sensitivity using gold nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hamon, C.; Novikov, S.; Scarabelli, L.; Basabe-Desmonts, L.; Liz-Marzán, L.M. Hierarchical self-assembly of gold nanoparticles into patterned plasmonic nanostructures. ACS Nano 2014, 8, 10694–10703. [Google Scholar] [CrossRef]
- Zhang, W.; Duan, H.; Chen, R.; Ma, T.; Zeng, L.; Leng, Y.; Xiong, Y. Effect of different-sized gold nanoflowers on the detection performance of immunochromatographic assay for human chorionic gonadotropin detection. Talanta 2019, 194, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Zhao, Q.; Iqbal, M.N.; Dong, M.; Li, X.; Lin, M.; Wang, R.; Lei, F.; He, C.; Wang, S. Development of immunoassay methods based on monoclonal antibody and its application in the determination of cadmium ion. J. Hazard. Mater. 2021, 411, 124992. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Shim, W.B.; Kim, J.S.; Chung, D.H. Development of a simultaneous lateral flow strip test for the rapid and simple detection of deoxynivalenol and zearalenone. J. Food Sci. 2014, 79, M2048–M2055. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Pan, F.; Liu, Z.; Wang, Z. Identification of tetrodotoxin antigens and a monoclonal antibody. Food Chem. 2009, 112, 582–586. [Google Scholar] [CrossRef]
- Ling, S.; Pang, J.; Yu, J.; Wang, R.; Liu, L.; Ma, Y.; Zhang, Y.; Jin, N.; Wang, S. Preparation and identification of monoclonal antibody against fumonisin B1 and development of detection by Ic-ELISA. Toxicon 2014, 80, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Kasture, P.; Gaud, R. Nanoflowers: The future trend of nanotechnology for multi-applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, J.; Lu, Y.; Ma, D.-Y.; Qi, M.G.; Wang, S. A competitive direct enzyme-linked immunosorbent assay for the rapid detection of deoxynivalenol: Development and application in agricultural products and feedstuff. Food Agric. Immunol. 2017, 28, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Xu, L.; Kuang, H.; Xiao, J.; Xu, C. Fluorescent microsphere immunochromatographic sensor for ultrasensitive monitoring deoxynivalenol in agricultural products. Microchem. J. 2021, 164, 106024. [Google Scholar] [CrossRef]
- Maragos, C.; Busman, M.; Plattner, R. Development of monoclonal antibodies for the fusarin mycotoxins. Food Addit. Contam. 2008, 25, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Hao, X.-Q.; Li, X.-B.; Liu, G.-W.; Zhang, Z.-G.; Yang, Z.-J.; Wang, Z.; Tang, J.; Yang, W.; Sun, J. Preparation of novel monoclonal antibodies against chelated cadmium ions. Biol. Trace Elem. Res. 2013, 152, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Fadlalla, M.H.; Ling, S.; Wang, R.; Li, X.; Yuan, J.; Xiao, S.; Wang, K.; Tang, S.; Elsir, H.; Wang, S. Development of ELISA and lateral flow immunoassays for ochratoxins (OTA and OTB) detection based on monoclonal antibody. Front. Cell. Infect. Microbiol. 2020, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.-J.; Lee, D.-H.; Kim, D.-O.; Min, W.-K.; Bong, K.-T.; Lee, G.-G.; Seo, J.-H. Production of a monoclonal antibody against ochratoxin A and its application to immunochromatographic assay. J. Agric. Food Chem. 2005, 53, 8447–8451. [Google Scholar] [CrossRef]
- Ling, S.; Chen, Q.-A.; Zhang, Y.; Wang, R.; Jin, N.; Pang, J.; Wang, S. Development of ELISA and colloidal gold immunoassay for tetrodotoxin detetcion based on monoclonal antibody. Biosens. Bioelectron. 2015, 71, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, F.; Ponzi, M.; Crescenzi, M.; Alessandroni, J.; Guadagni, F. A simple and effective method to analyze membrane proteins by SDS-PAGE and MALDI mass spectrometry. Anticancer Res. 2010, 30, 1121–1129. [Google Scholar]
- Jin, N.; Ling, S.; Yang, C.; Wang, S. Preparation and identification of monoclonal antibody against Citreoviridin and development of detection by Ic-ELISA. Toxicon 2014, 90, 226–236. [Google Scholar] [CrossRef]
- Lenc, L.; Czecholiński, G.; Wyczling, D.; Turów, T.; Kaźmierczak, A. Fusarium head blight (FHB) and Fusarium spp. on grain of spring wheat cultivars grown in Poland. J. Plant Prot. Res. 2015, 55, 266–277. [Google Scholar] [CrossRef]
- Masinde, L.A.; Sheng, W.; Xu, X.; Zhang, Y.; Yuan, M.; Kennedy, I.R.; Wang, S. Colloidal gold based immunochromatographic strip for the simple and sensitive determination of aflatoxin B1 and B2 in corn and rice. Microchim. Acta 2013, 180, 921–928. [Google Scholar] [CrossRef]
- Zhan, L.; Guo, S.-z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.; Bischof, J.C. The role of nanoparticle design in determining analytical performance of lateral flow immunoassays. Nano Lett. 2017, 17, 7207–7212. [Google Scholar] [CrossRef] [Green Version]


| Intra Assay(n = 3) * | Inter Assay(n = 3) # | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Spiked Level (ng/mL) | Measured Level (ng/mL) | Recovery (%) | SD | CV (%) | Measured Level (ng/mL) | Recovery (%) | SD | CV (%) |
| Corn | 0.5 | 0.45 | 90.00 | 5.342 | 11. 87 | 0.46 | 92 | 4.54 | 9.88 |
| 5 | 4.39 | 87.80 | 58.43 | 13.31 | 4.77 | 95.4 | 34.7 | 7.27 | |
| 100 | 96.53 | 96.53 | 330.1 | 3.42 | 96.61 | 96.61 | 308.2 | 3.19 | |
| 1000 | 951.2 | 91.52 | 3367.248 | 3.54 | 928.4 | 92.84 | 13.76 | 11.02 | |
| Average | 91.46 | 94.21 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, H.E.; Xu, A.; Xu, Y.; Fadlalla, M.H.; Wang, S. Preparation of Monoclonal Antibody against Deoxynivalenol and Development of Immunoassays. Toxins 2022, 14, 533. https://doi.org/10.3390/toxins14080533
Mokhtar HE, Xu A, Xu Y, Fadlalla MH, Wang S. Preparation of Monoclonal Antibody against Deoxynivalenol and Development of Immunoassays. Toxins. 2022; 14(8):533. https://doi.org/10.3390/toxins14080533
Chicago/Turabian StyleMokhtar, Hoyda Elsir, Aidi Xu, Yang Xu, Mohamed Hassan Fadlalla, and Shihua Wang. 2022. "Preparation of Monoclonal Antibody against Deoxynivalenol and Development of Immunoassays" Toxins 14, no. 8: 533. https://doi.org/10.3390/toxins14080533
APA StyleMokhtar, H. E., Xu, A., Xu, Y., Fadlalla, M. H., & Wang, S. (2022). Preparation of Monoclonal Antibody against Deoxynivalenol and Development of Immunoassays. Toxins, 14(8), 533. https://doi.org/10.3390/toxins14080533





