Cocktails of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Diets of Dairy Cows in Austria: Inferences from Diet Composition and Geo-Climatic Factors
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Diets
2.1.1. Type of Rations and Main Dietary Components
2.1.2. Chemical Composition and Particle Size Distribution of Basal Rations
2.1.3. Hygienic Status of the Main Dietary Ingredients
2.1.4. Geo-Climatic Factors
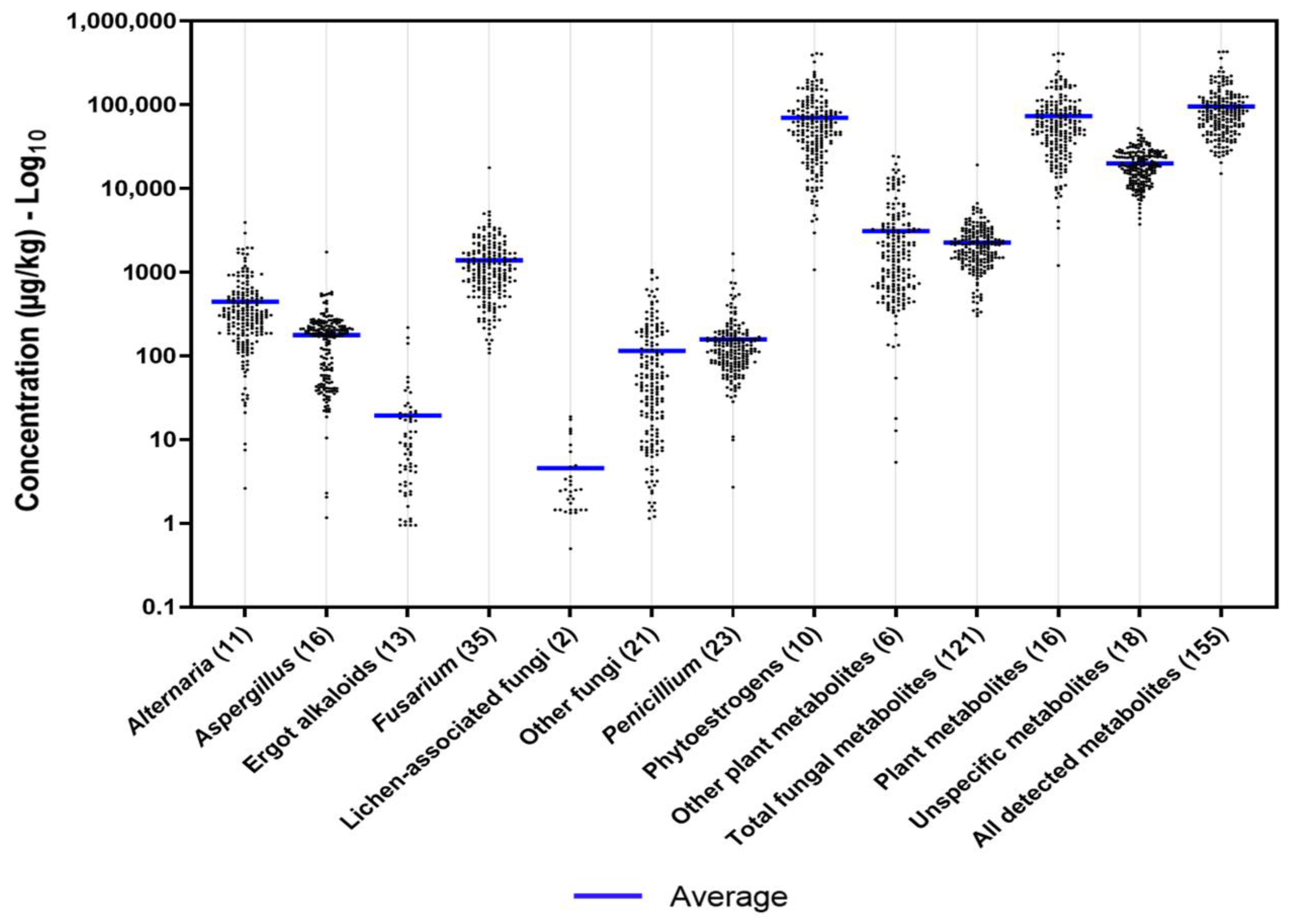
2.2. Occurrence and Concentrations of the Detected Metabolites
2.2.1. Groups of Metabolites
2.2.2. Mycotoxins Included in the EU Legislation and Related Compounds
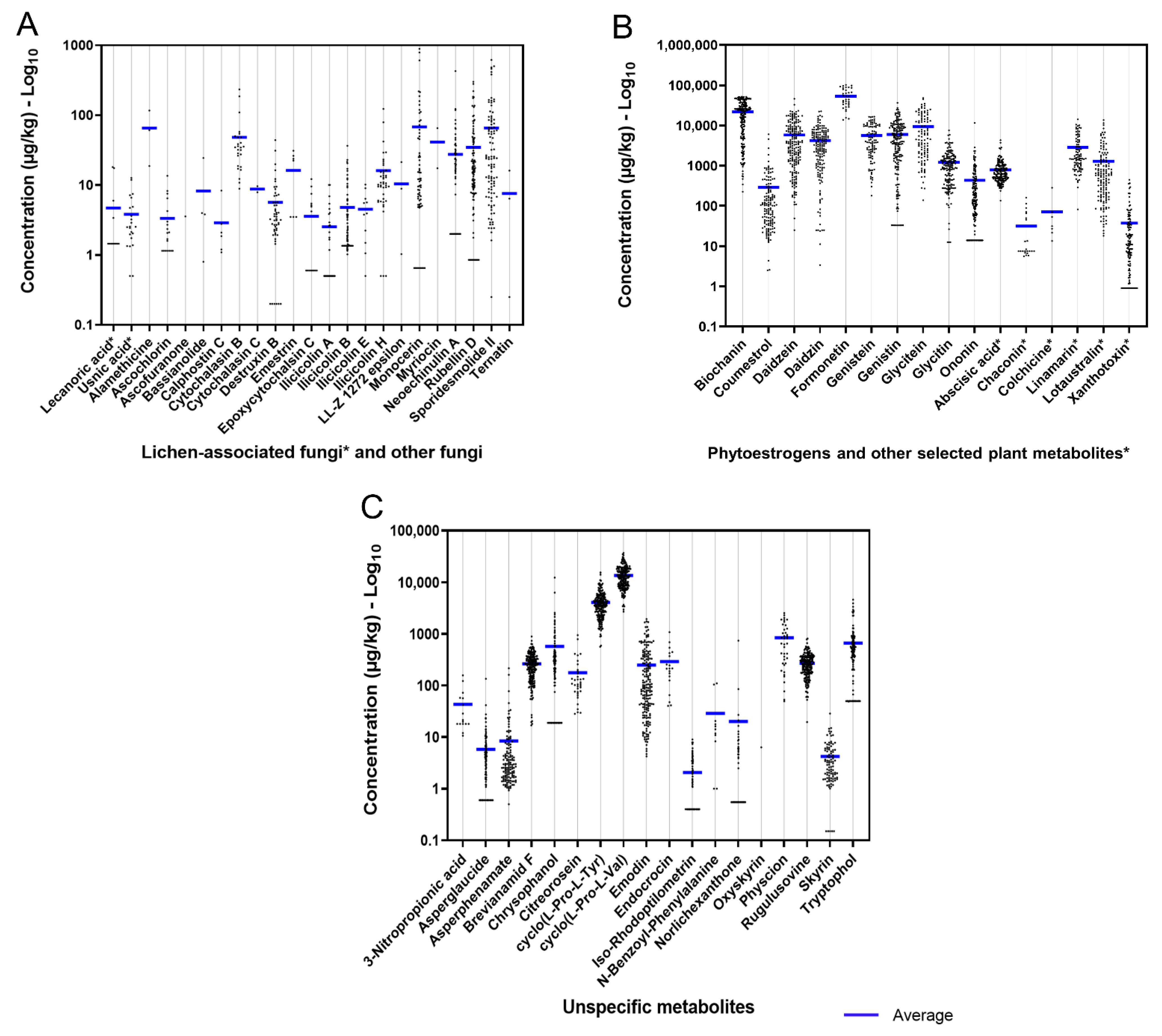
2.2.3. Emerging Mycotoxins
2.2.4. Other Mycotoxins and Metabolites from Fusarium, Alternaria, Aspergillus, and Penicillium
2.2.5. Metabolites from Lichen-Associated Fungi and Other Fungi Genera
2.2.6. Plant Secondary Metabolites (Phytoestrogens and Other Plant Metabolites)
2.2.7. Unspecific Metabolites (Derived from Multi-Kingdom Producers)
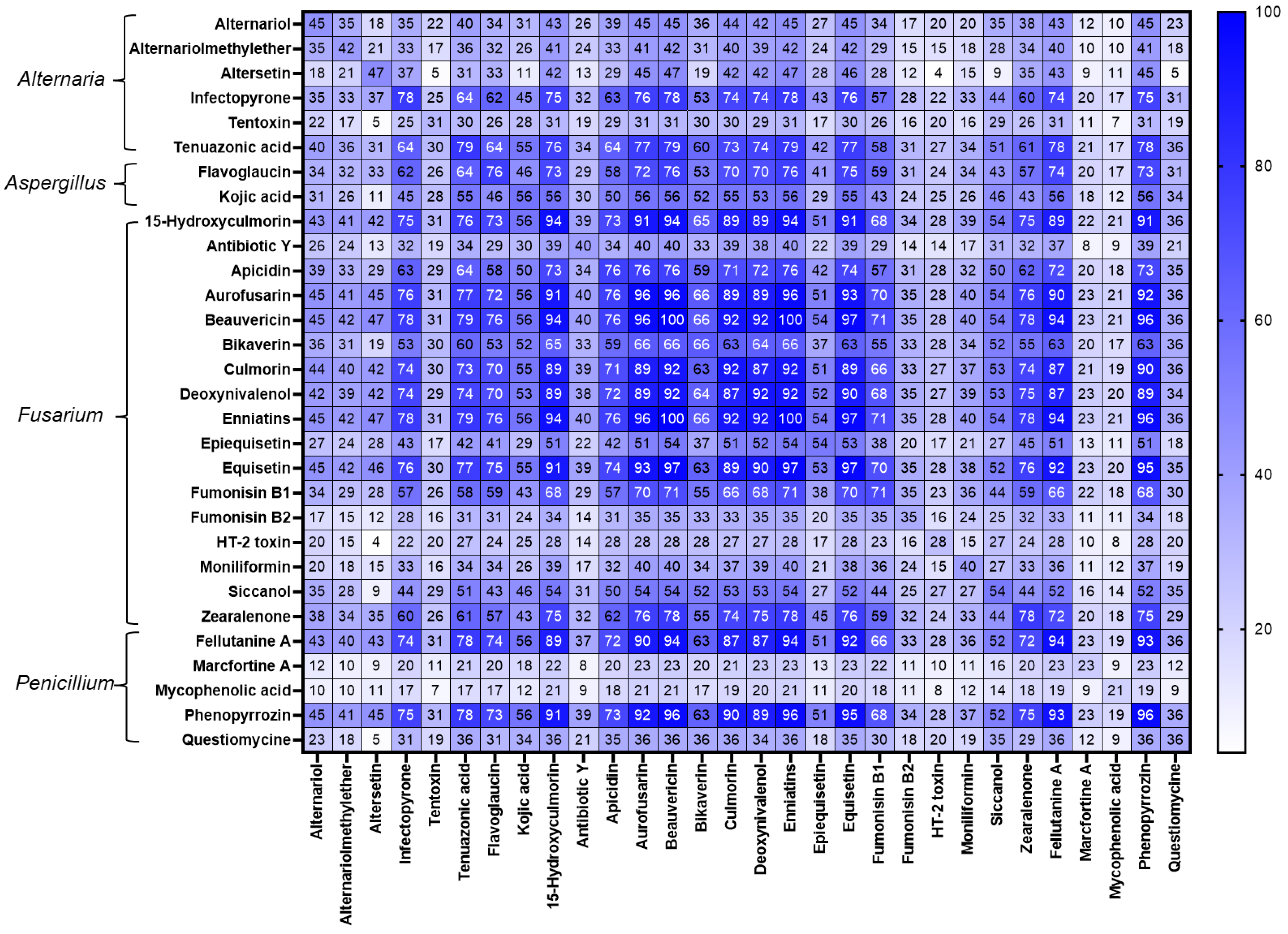
2.3. Co-Occurrence of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites
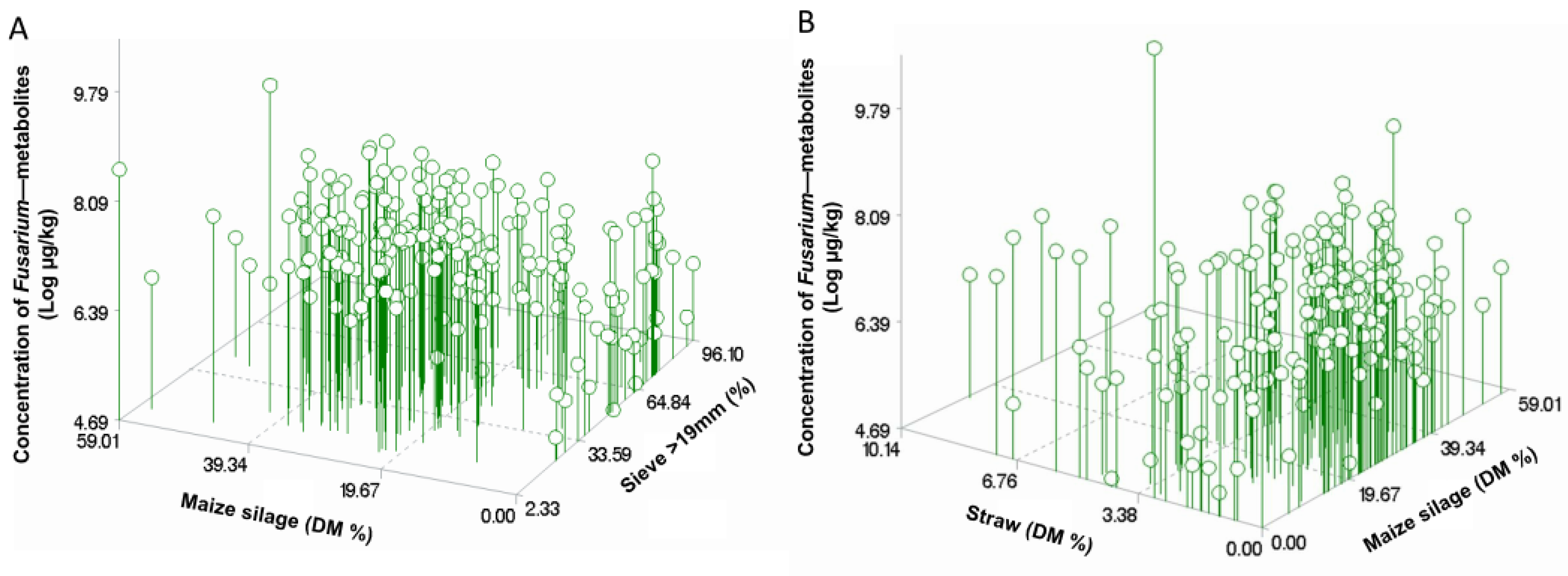
2.4. Dietary Composition and Geo-Climatic Factors in Relation to the Concentration of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sampling and Sample Preparation
5.2. Data Collection
5.3. Chemical Proximate Analysis and Particle Size Distribution of the Rations
5.4. Sample Extraction and Multi-Metabolite Analysis (LC-ESI-MS/MS)
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BMLRT (Bundesministerium für Landwirtschaft, Regionen und Tourismus). Grüner Bericht 2021. Die Situation der österreichischen Land- und Forstwirtschaft. BMLRT, Vienna. Available online: https://gruenerbericht.at/cm4/jdownload/send/2-gr-bericht-terreich/2393-gb2021 (accessed on 1 June 2022).
- FAO; IDF; IFCN. World Mapping of Animal Feeding Systems in the Dairy Sector; FAO: Rome, Italy; IDF: Brussels, Belgium; IFCN: Rome, Italy, 2014; pp. 1–36. [Google Scholar]
- Webster, J. Understanding the Dairy Cow; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 99–100. [Google Scholar]
- Mayne, C.; Gordon, F. The effect of type of concentrate and level of concentrate feeding on milk production. Anim. Sci. 1984, 39, 65–76. [Google Scholar] [CrossRef]
- Sairanen, A.; Khalili, H.; Virkajärvi, P. Concentrate supplementation responses of the pasture-fed dairy cow. Livest. Sci. 2006, 104, 292–302. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on Mycotoxin Issues in Ruminants: Occurrence in Forages, Effects of Mycotoxin Ingestion on Health Status and Animal Performance and Practical Strategies to Counteract Their Negative Effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed]
- Santos Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef]
- Fletcher, M.T.; Netzel, G. Food Safety and Natural Toxins. Toxins 2020, 12, 236. [Google Scholar] [CrossRef]
- Reed, K.F.M. Fertility of herbivores consuming phytoestrogen-containing Medicago and Trifolium species. Agriculture 2016, 6, 35. [Google Scholar] [CrossRef]
- FAO; WHO. Hazards Associated with Animal Feed. Report of the Joint FAO/WHO Expert Meeting—12–15 May 2015, FAO Headquarters, Rome, Italy. FAO Animal Production and Health Report No. 13. Rome, Italy. 2019. Available online: https://www.fao.org/3/ca6825en/CA6825EN.pdf (accessed on 8 June 2022).
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. Mycotoxins in forages. In The Mycotoxin Blue Book; Nottingham University Press: Nottingham, UK, 2005; pp. 249–268. [Google Scholar]
- CAST. Mycotoxins: Risks in Plant, Animal and Human Systems, Report No. 139; Council for Agricultural Science and Technology: Ames, IA, USA, 2003. [Google Scholar]
- European Commission. Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off. J. Eur. Union. 2006, 229, 7–9. [Google Scholar]
- European Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Luxemb. Off. J. Eur. Union. 2002, 140, 10–22. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products (2013/165/EU). Off. J. Eur. Union. 2013, 91, 12–15. [Google Scholar]
- European Commission. Commission recommendation 2012/154/EU of 15 March 2012 on the monitoring of the presence of ergot alkaloids in feed and food. Off. J. Eur. Union. 2012, 77, 20–21. [Google Scholar]
- EFSA. Scientific Opinion on the risk for public and animal health related to the presence of sterigmatocystin in food and feed. EFSA J. 2013, 11, 3254. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the risks to human and animal health related to the presence of beauvericin and enniatins in food and feed. EFSA J. 2014, 12, 3802. [Google Scholar]
- Jestoi, M. Emerging Fusarium-mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin—A review. Crit. Rev. Food. Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Dvořáčková, M.; Kašparovský, T. Feedborne Mycotoxins Beauvericin and Enniatins and Livestock Animals. Toxins 2021, 13, 32. [Google Scholar] [CrossRef]
- Panasiuk, L.; Jedziniak, P.; Pietruszka, K.; Piatkowska, M.; Bocian, L. Frequency and levels of regulated and emerging mycotoxins in silage in Poland. Mycotoxin Res. 2019, 35, 17–25. [Google Scholar] [CrossRef]
- Zachariasova, M.; Dzuman, Z.; Veprikova, Z.; Hajkova, K.; Jiru, M.; Vaclavikova, M.; Zachariasova, A.; Pospichalova, M.; Florian, M.; Hajslova, J. Occurrence of multiple mycotoxins in european feedingstuffs, assessment of dietary intake by farm animals. Anim. Feed Sci. Technol. 2014, 193, 124–140. [Google Scholar] [CrossRef]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B. Mycotoxin mixtures in food and feed: Holistic, innovative, flexible risk assessment modelling approach: MYCHIF. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Speijers, G.J.A.; Speijers, M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef]
- McAllister, T.A.; Ribeiro, G.; Stanford, K.; Wang, Y. Forage-Induced Animal Disorders. In Forages, 7th ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 839–860. [Google Scholar]
- Wolawek-Potocka, I.; Bah, M.M.; Korzekwa, A.; Piskula, M.K.; Wiczkowski, W.; Depta, A.; Skarzynski, D.J. Soybean-derived phytoestrogens regulate prostaglandin secretion in endometrium during cattle estrous cycle and early pregnancy. Exp. Biol. Med. 2005, 230, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Wocławek-Potocka, I.; Korzekwa, A.; Skarzyński, D.J. Can phytoestrogens pose a danger in the reproduction of cows? Med. Weter. 2008, 64, 515–519. [Google Scholar]
- Wocławek-Potocka, I.; Mannelli, C.; Boruszewska, D.; Kowalczyk-Zieba, I.; Waśniewski, T.; Skarżyński, D.J. Diverse effects of phytoestrogens on the reproductive performance: Cow as a model. Int. J. Endocrinol. 2013, 2013, 650984. [Google Scholar] [CrossRef] [PubMed]
- Romero-R, C.M.; Castellanos, M.d.R.T.; Mendoza, R.M.; Reyes, R.A.; García, A.R. Oestrogenic syndrome in dairy cows by alfalfa comsuption with large amount of coumestrol. Vet. Mex. 1997, 28, 25–30. [Google Scholar]
- Vejdovszky, K.; Schmidt, V.; Warth, B.; Marko, D. Combinatory estrogenic effects between the isoflavone genistein and the mycotoxins zearalenone and alternariol in vitro. Mol. Nutr. Food Res. 2017, 61, 1600526. [Google Scholar] [CrossRef]
- Vejdovszky, K.; Hahn, K.; Braun, D.; Warth, B.; Marko, D. Synergistic estrogenic effects of Fusarium and Alternaria mycotoxins in vitro. Arch. Toxicol. 2017, 91, 1447–1460. [Google Scholar] [CrossRef]
- Hessenberger, S.; Botzi, K.; Degrassi, C.; Kovalsky, P.; Schwab, C.; Schatzmayr, D.; Schatzmayr, G.; Fink-Gremmels, J. Interactions between plant-derived oestrogenic substances and the mycoestrogen zearalenone in a bioassay with MCF-7 cells. Pol. J. Vet. Sci. 2017, 20, 513–520. [Google Scholar] [CrossRef]
- Martins, C.; Vidal, A.; De Boevre, M.; Assunção, R. Mycotoxins as Endocrine Disruptors–An Emerging Threat. In Encyclopedia of Mycology; Zaragoza, O., Casadevall, A., Eds.; ElSevier: St. Louis, MO, USA, 2021; Volume 2, pp. 180–192. [Google Scholar]
- Johny, A.; Fæste, C.K.; Bogevik, A.S.; Berge, G.M.; Fernandes, J.M.; Ivanova, L. Development and validation of a liquid chromatography high-resolution mass spectrometry method for the simultaneous determination of mycotoxins and phytoestrogens in plant-based fish feed and exposed fish. Toxins 2019, 11, 222. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Lanková, D.; Urbancová, K.; Krtková, V.; Hernández-Borges, J.; Rodríguez-Delgado, M.Á.; Pulkrabová, J.; Hajšlová, J. Multiclass analytical method for the determination of natural/synthetic steroid hormones, phytoestrogens, and mycoestrogens in milk and yogurt. Anal. Bioanal. Chem. 2017, 409, 4467–4477. [Google Scholar] [CrossRef]
- Grgic, D.; Varga, E.; Novak, B.; Müller, A.; Marko, D. Isoflavones in Animals: Metabolism and Effects in Livestock and Occurrence in Feed. Toxins 2021, 13, 836. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food. 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous influences on plant secondary metabolite levels. Anim. Feed Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Khiaosa-ard, R.; Nagl, V.; Faas, J.; Jenkins, T.; Sulyok, M.; Zebeli, Q. Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Austrian Pastures: Occurrences, Contamination Levels, and Implications of Geo-climatic Factors. Toxins 2021, 13, 460. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.L.; Chulze, S.; Magan, N. Impact of environmental factors and fungicides on growth and deoxinivalenol production by Fusarium graminearum isolates from Argentinian wheat. Crop Prot. 2004, 23, 117–125. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K.; Kristoffersen, A. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Bernhoft, A.; Clasen, P.-E.; Kristoffersen, A.; Torp, M. Less Fusarium infestation and mycotoxin contamination in organic than in conventional cereals. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 842–852. [Google Scholar] [CrossRef]
- Perrone, G.; Ferrara, M.; Medina, A.; Pascale, M.; Magan, N. Toxigenic fungi and mycotoxins in a climate change scenario: Ecology, genomics, distribution, prediction and prevention of the risk. Microorganisms 2020, 8, 1496. [Google Scholar] [CrossRef]
- Nichea, M.J.; Cendoya, E.; Zachetti, V.G.L.; Chiacchiera, S.M.; Sulyok, M.; Krska, R.; Torres, A.M.; Chulze, S.N.; Ramirez, M.L. Mycotoxin profile of Fusarium armeniacum isolated from natural grasses intended for cattle feed. World Mycotoxin J. 2015, 8, 451–457. [Google Scholar] [CrossRef]
- Rasmussen, R.R.; Storm, I.; Rasmussen, P.H.; Smedsgaard, J.; Nielsen, K.F. Multi-mycotoxin analysis of maize silage by LC-MS/MS. Anal. Bioana. Chem. 2010, 397, 765–776. [Google Scholar] [CrossRef]
- Storm, I.; Rasmussen, R.R.; Rasmussen, P.H. Occurrence of Pre- and Post-Harvest Mycotoxins and Other Secondary Metabolites in Danish Maize Silage. Toxins 2014, 6, 2256–2269. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, M.; Marin, S.; Sanchis, V.; Ramos, A.J. Fusarium mycotoxins in total mixed rations for dairy cows. Mycotoxin Res. 2020, 36, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Pereyra, M.L.; Chiacchiera, S.M.; Rosa, C.A.d.R.; Sager, R.L.; Dalcero, A.M.; Cavaglieri, L.R. Fungal and mycotoxin contamination in mixed feeds: Evaluating risk in cattle intensive rearing operations (feedlots). Rev. Bio Cienc. 2012, 2, 68–80. [Google Scholar]
- Yalçin, N.F.; Işik, M.K.; Tülay, A.; Halis, O.; Çoşkun, B.; Çiftçi, E. The presence of mycotoxin in total mixed rations of dairy cattle in konya and the surrounding provinces. Atatürk Üniversitesi Vet. Bil. Derg. 2016, 11, 22–31. [Google Scholar]
- Awapak, D.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Co-occurrence and toxicological relevance of secondary metabolites in dairy cow feed from Thailand. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 1013–1027. [Google Scholar] [CrossRef]
- Custodio, L.; Prados, L.F.; Yiannikouris, A.; Holder, V.; Pettigrew, J.; Kuritza, L.; de Resende, F.D.; Siqueira, G.R. Mycotoxin contamination of diets for beef cattle finishing in feedlot. R. Bras. Zootec. 2019, 48, 1–12. [Google Scholar] [CrossRef]
- Signorini, M.L.; Gaggiotti, M.; Molineri, A.; Chiericatti, C.A.; de Basilico, M.L.Z.; Basilico, J.C.; Pisani, M. Exposure assessment of mycotoxins in cow’s milk in Argentina. Food Chem. Toxicol. 2012, 50, 250–257. [Google Scholar] [CrossRef]
- Vaičiulienė, G.; Bakutis, B.; Jovaišienė, J.; Falkauskas, R.; Gerulis, G.; Kerzienė, S.; Baliukonienė, V. Prevalence of Mycotoxins and Endotoxins in Total Mixed Rations and Different Types of Ensiled Forages for Dairy Cows in Lithuania. Toxins 2021, 13, 890. [Google Scholar] [CrossRef]
- Kamphues, J.; Wolf, P.; Coenen, M.; Klaus, E.; Iben, C.; Kienzle, E.; Liesegang, A.; Männer, K.; Zebeli, Q.; Zentek, J. IV Beurteilung von den Futtermitteln. In Supplemente zur Tierernährung für Studium und Praxis; Schlütersche: Hannover, Germany, 2014; pp. 181–192. [Google Scholar]
- Szulc, J.; Okrasa, M.; Dybka-Stępień, K.; Sulyok, M.; Nowak, A.; Otlewska, A.; Szponar, B.; Majchrzycka, K. Assessment of Microbiological Indoor Air Quality in Cattle Breeding Farms. Aerosol Air Qual. Res. 2019, 20, 1353–1373. [Google Scholar] [CrossRef]
- Hajnal, E.J.; Kos, J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Mycotoxins in maize harvested in Serbia in the period 2012–2015. Part 2: Non-regulated mycotoxins and other fungal metabolites. Food Chem. 2020, 317, 126409. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging mycotoxins: Beyond traditionally determined food contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Reisinger, N.; Schurer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin Occurrence in Maize Silage-A Neglected Risk for Bovine Gut Health? Toxins 2019, 11, 577. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Ghilardelli, F.; Atzori, A.S.; Zara, S.; Novak, B.; Faas, J.; Fancello, F. Co-Occurrence of Regulated and Emerging Mycotoxins in Corn Silage: Relationships with Fermentation Quality and Bacterial Communities. Toxins 2021, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M.; Humpf, H.-U.; Marko, D.; Dänicke, S.; Mally, A.; Berthiller, F.; Klaffke, H.; Lorenz, N. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res. 2014, 30, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Nesic, K.; Ivanovic, S.; Nesic, V. Fusarial toxins: Secondary metabolites of Fusarium fungi. Rev. Environ. Contam. Toxicol. 2014, 228, 101–120. [Google Scholar] [PubMed]
- D’Mello, J.P.F.; Placinta, C.M.; Macdonald, A.M.C. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Nielsen, C.; Casteel, M.; Didier, A.; Dietrich, R.; Märtlbauer, E. Trichothecene-induced cytotoxicity on human cell lines. Mycotoxin Res. 2009, 25, 77–84. [Google Scholar] [CrossRef]
- Gallo, A.; Minuti, A.; Bani, P.; Bertuzzi, T.; Cappelli, F.P.; Doupovec, B.; Faas, J.; Schatzmayr, D.; Trevisi, E. A mycotoxin-deactivating feed additive counteracts the adverse effects of regular levels of Fusarium mycotoxins in dairy cows. J. Dairy Sci. 2020, 103, 11314–11331. [Google Scholar] [CrossRef]
- Sy-Cordero, A.A.; Pearce, C.J.; Oberlies, N.H. Revisiting the enniatins: A review of their isolation, biosynthesis, structure determination and biological activities. J. Antibiot. Res. 2012, 65, 541–549. [Google Scholar] [CrossRef]
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Giffel, M.C.T. Occurrence of Mycotoxins in Feedstuffs of Dairy Cows and Estimation of Total Dietary Intakes. J. Dairy Sci. 2008, 91, 4261–4271. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F.; Spanjer, M.C.; Scholten, J.M.; Te Giffel, M.C. Occurrence of mycotoxins in maize, grass and wheat silage for dairy cattle in the Netherlands. Food Addit. Contam. B Surveill. 2008, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.A.; Kuldau, G.A. Microbiological and molecular determination of mycobiota in fresh and ensiled maize silage. Mycologia 2007, 99, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Driehuis, F. Silage and the safety and quality of dairy foods: A review. Agric. Food Sci. 2013, 22, 16–34. [Google Scholar] [CrossRef]
- Driehuis, F.; Wilkinson, J.; Jiang, Y.; Ogunade, I.; Adesogan, A. Silage review: Animal and human health risks from silage. J. Dairy Sci. 2018, 101, 4093–4110. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Medina, Á.; Rodríguez, A.; Magan, N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Curr. Opin. Food Sci. 2015, 5, 99–104. [Google Scholar] [CrossRef]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- González-Jartín, J.M.; Rodríguez-Cañás, I.; Alfonso, A.; Sainz, M.J.; Vieytes, M.R.; Gomes, A.; Ramos, I.; Botana, L.M. Multianalyte method for the determination of regulated, emerging, and modified mycotoxins in milk: QuEChERS extraction followed by UHPLC–MS/MS analysis. Food Chem. 2021, 356, 129647. [Google Scholar] [CrossRef]
- Cary, J.W.; Ehrlich, K.C.; Bland, J.M.; Montalbano, B.G. The aflatoxin biosynthesis cluster gene, aflX, encodes an oxidoreductase involved in conversion of versicolorin A to demethylsterigmatocystin. Appl. Environ. Microbiol. 2006, 72, 1096–1101. [Google Scholar] [CrossRef]
- Hsieh, D.; Lin, M.; Yao, R. Conversion of sterigmatocystin to aflatoxin B1 by Aspergillus parasiticus. Biochem. Biophys. Res. Commun. 1973, 52, 992–997. [Google Scholar] [CrossRef]
- Mo, H.G.; Pietri, A.; MacDonald, S.J.; Anagnostopoulos, C.; Spanjere, M. Survey on sterigmatocystin in food. EFSA Supporting Publ. 2015, 12, 774E. [Google Scholar] [CrossRef]
- Veršilovskis, A.; de Saeger, S. Sterigmatocystin: Occurrence in foodstuffs and analytical methods—An overview. Mol. Nutr. Food Res. 2010, 54, 136–147. [Google Scholar] [CrossRef]
- Parrish, F.; Wiley, B.; Simmons, E.; Long, L., Jr. Production of aflatoxins and kojic acid by species of Aspergillus and Penicillium. Appl. Microbiol. 1966, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Morton, H.E.; Kocholaty, W.; Junowicz-Kocholaty, R.; Kelner, A. Toxicity and antibiotic activity of kojic acid produced by Aspergillus luteo-virescens. J.Bacteriol. 1945, 50, 579–584. [Google Scholar] [CrossRef]
- Kotani, T.; Ichimoto, I.; Tatsumi, C.; Fujita, T. Bacteriostatic activities and metal chelation of kojic acid analogs. Agric. Biol. Chem. 1976, 40, 765–770. [Google Scholar]
- Bashir, F.; Sultana, K.; Khalid, M.; Rabia, H. Kojic Acid: A Comprehensive Review. Asian J. Allied Health Sci. 2021, 6, 13–21. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific Opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407. [Google Scholar] [CrossRef]
- Aichinger, G.; Del Favero, G.; Warth, B.; Marko, D. Alternaria toxins—Still emerging? Compr. Rev. Food Sci. Food Saf. 2021, 20, 4390–4406. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Tirkey, N.N. Tenuazonic Acid: A potent mycotoxin. In Recent Trends in Human and Animal Mycology, 2nd ed.; Singh, K., Srivastava, N., Eds.; Springer Nature: Singapore, 2019; Chapter 8; pp. 203–211. [Google Scholar]
- Gil-Serna, J.; Vázquez, C.; Gonzaléz-Jaén, M.T.; Patiño, B. Mycotoxins: Toxicology. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C., Tortorello, M.L., Eds.; Elsevier: St. Louis, MO, USA, 2014; pp. 887–892. [Google Scholar]
- Schrader, T.; Cherry, W.; Soper, K.; Langlois, I.; Vijay, H. Examination of Alternaria alternata mutagenicity and effects of nitrosylation using the Ames Salmonella test. Teratog. Carcinog. Mutagen. 2001, 21, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, G.; Krüger, F.; Puntscher, H.; Preindl, K.; Warth, B.; Marko, D. Naturally occurring mixtures of Alternaria toxins: Anti-estrogenic and genotoxic effects in vitro. Arch. Toxicol. 2019, 93, 3021–3031. [Google Scholar] [CrossRef]
- Vejdovszky, K.; Warth, B.; Sulyok, M.; Marko, D. Non-synergistic cytotoxic effects of Fusarium and Alternaria toxin combinations in Caco-2 cells. Toxicol. Lett. 2016, 241, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pahlow, G.; Muck, R.E.; Driehuis, F.; Oude Elferink, S.J.; Spoelstra, S.F. Microbiology of ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy: Madison, WI, USA, 2014. [Google Scholar]
- Ogunade, I.M.; Martinez-Tuppia, C.; Queiroz, O.C.M.; Jiang, Y.; Drouin, P.; Wu, F.; Vyas, D.; Adesogan, A.T. Silage review: Mycotoxins in silage: Occurrence, effects, prevention, and mitigation. J. Dairy Sci. 2018, 101, 4034–4059. [Google Scholar] [CrossRef]
- Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J. Sci. Food Agric. 2016, 96, 2284–2302. [Google Scholar] [CrossRef]
- Malekinejad, H.; Fink-Gremmels, J. Mycotoxicoses in veterinary medicine: Aspergillosis and penicilliosis. Vet. Res. Forum. 2020, 11, 97–103. [Google Scholar]
- Penagos-Tabares, F.; Khiaosa-Ard, R.; Schmidt, M.; Pacífico, C.; Faas, J.; Jenkins, T.; Nagl, V.; Sulyok, M.; Labuda, R.; Zebeli, Q. Fungal species and mycotoxins in mouldy spots of grass and maize silages in Austria. Mycotoxin Res. 2022, 38, 117–136. [Google Scholar] [CrossRef]
- Mansfield, M.A.; Jones, A.D.; Kuldau, G.A. Contamination of fresh and ensiled maize by multiple Penicillium mycotoxins. Phytopathology 2008, 98, 330–336. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Boermans, H.J.; Swamy, H.V.L.N.; Sharma, B.S.; Karrow, N.A. Immunotoxicity of Penicillium mycotoxins on viability and proliferation of bovine macrophage cell line (BOMACs). Open Microbiol. J. 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Brennan, K.M.; Oh, S.-Y.; Yiannikouris, A.; Graugnard, D.E.; Karrow, N.A. Differential gene expression analysis of bovine macrophages after exposure to the Penicillium mycotoxins citrinin and/or ochratoxin a. Toxins 2017, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Fisher, R.E.; Swamy, H.V.L.N.; Boermans, H.J.; Yiannikouris, A.; Karrow, N.A. Silage Penicillium mycotoxins: Hidden modulators of the immune system. In Mycotoxins: Occurrence, Toxicology and Management Strategies; Rios, C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; pp. 1–40. [Google Scholar]
- Nielsen, K.F.; Sumarah, M.W.; Frisvad, J.C.; Miller, J.D. Production of metabolites from the Penicillium roqueforti complex. J. Agric. Food Chem. 2006, 54, 3756–3763. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Aghazadeh-Attari, J.; Rezabakhsh, A.; Sattari, M.; Ghasemsoltani-Momtaz, B. Neurotoxicity of mycotoxins produced in vitro by Penicillium roqueforti isolated from maize and grass silage. Hum. Exp. Toxicol. 2015, 34, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.Y.; Quinton, V.M.; Boermans, H.J.; Swamy, H.V.L.N.; Karrow, N.A. In vitro exposure of Penicillium mycotoxins with or without a modified yeast cell wall extract (mYCW) on bovine macrophages (BoMacs). Mycotoxin Res. 2015, 31, 167–175. [Google Scholar] [CrossRef]
- Fuchs, S.; Sontag, G.; Stidl, R.; Ehrlich, V.; Kundi, M.; Knasmüller, S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008, 46, 1398–1407. [Google Scholar] [CrossRef]
- Oh, S.Y.; Balch, C.G.; Cliff, R.L.; Sharma, B.S.; Boermans, H.J.; Swamy, H.; Quinton, V.M.; Karrow, N.A. Exposure to Penicillium mycotoxins alters gene expression of enzymes involved in the epigenetic regulation of bovine macrophages (BoMacs). Mycotoxin Res. 2013, 29, 235–243. [Google Scholar] [CrossRef]
- Bentley, R. Mycophenolic acid: A one hundred year odyssey from antibiotic to immunosuppressant. Chem. Rev. 2000, 100, 3801–3826. [Google Scholar] [CrossRef]
- Kopp-Holtwiesche, B.; Rehm, H. Antimicrobial action of roquefortine. J. Environ. Pathol. Toxicol. 1990, 10, 41–44. [Google Scholar]
- Noto, T.; Sawada, M.; Ando, K.; Koyama, K. Some biological properties of mycophenolic acid. J. Antibiot. Res. 1969, 22, 165–169. [Google Scholar] [CrossRef][Green Version]
- Lu, X.; Zhang, E.; Yin, S.; Fan, L.; Hu, H. Methylseleninic acid prevents patulin-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress and inactivation of p53 and MAPKs. J. Agric. Food Chem. 2017, 65, 5299–5305. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, C.; Lu, Q.; Liang, H.; Li, J.; Xu, D. Involvement of caspase in patulin-induced hepatotoxicity in vitro and in vivo. Toxicon 2022, 206, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Manni, K.; Rämö, S.; Franco, M.; Rinne, M.; Huuskonen, A. Occurrence of Mycotoxins in Grass and Whole-Crop Cereal Silages—A Farm Survey. Agriculture 2022, 12, 398. [Google Scholar] [CrossRef]
- Auerbach, H.; Oldenburg, E.; Weissbach, F. Incidence of Penicillium roqueforti and roquefortine C in silages. J. Sci. Food Agric. 1998, 76, 565–572. [Google Scholar] [CrossRef]
- Magan, N.; Lacey, J. Effect of water activity, temperature and substrate on interactions between field and storage fungi. Trans. Brit. Mycol. Soc. 1984, 82, 83–93. [Google Scholar] [CrossRef]
- Marín, S.; Sanchis, V.; Sáenz, R.; Ramos, A.; Vinas, I.; Magan, N. Ecological determinants for germination and growth of some Aspergillus and Penicillium spp. from maize grain. J. Appl. Microbiol. 1998, 84, 25–36. [Google Scholar] [CrossRef]
- Mislivec, P.; Dieter, C.; Bruce, V. Effect of temperature and relative humidity on spore germination of mycotoxic species of Aspergillus and Penicillium. Mycologia 1975, 67, 1187–1189. [Google Scholar] [CrossRef]
- Skladanka, J.; Adam, V.; Dolezal, P.; Nedelnik, J.; Kizek, R.; Linduskova, H.; Mejia, J.E.A.; Nawrath, A. How do grass species, season and ensiling influence mycotoxin content in forage? Int. J. Environ. Res. 2013, 10, 6084–6095. [Google Scholar] [CrossRef]
- Woolford, M.K. The detrimental effects of air on silage. J. Appl. Bacteriol. 1990, 68, 101–116. [Google Scholar] [CrossRef]
- Ulrich, S.; Gottschalk, C.; Biermaier, B.; Bahlinger, E.; Twarużek, M.; Asmussen, S.; Schollenberger, M.; Valenta, H.; Ebel, F.; Dänicke, S. Occurrence of type A, B and D trichothecenes, zearalenone and stachybotrylactam in straw. Arch. Anim. Nutr. 2021, 75, 105–120. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Nasr, H.; Pearson, O. Inhibition of prolactin secretion by ergot alkaloids. Eur. J. Endocrinol. 1975, 80, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Coufal-Majewski, S.; Stanford, K.; McAllister, T.; Blakley, B.; McKinnon, J.; Chaves, A.V.; Wang, Y. Impacts of cereal ergot in food animal production. Front. Vet. Sci. 2016, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.I.; Gross, M.; Cramer, B.; Humpf, H.-U.; Hamscher, G.; Usleber, E. Immunochemical analysis of paxilline and ergot alkaloid mycotoxins in grass seeds and plants. J. Agric. Food Chem. 2018, 66, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Canty, M.J.; Fogarty, U.; Sheridan, M.K.; Ensley, S.M.; Schrunk, D.E.; More, S.J. Ergot alkaloid intoxication in perennial ryegrass (Lolium perenne): An emerging animal health concern in Ireland? Ir. Vet. J. 2014, 67, 21. [Google Scholar] [CrossRef]
- Evans, T.J. Diminished reproductive performance and selected toxicants in forages and grains. Vet. Clin. N. Am. Food Anim. Pract. 2011, 27, 345–371. [Google Scholar] [CrossRef] [PubMed]
- Marczuk, J.; Zietek, J.; Zwierz, K.; Winiarczyk, S.; Lutnicki, K.; Brodzki, P.; Adaszek, L. Ergovaline poisoning in a herd of dairy cows—A case report. Med. Weter. 2019, 75, 635–639. [Google Scholar] [CrossRef]
- Miethbauer, S.; Gaube, F.; Möllmann, U.; Dahse, H.-M.; Schmidtke, M.; Gareis, M.; Pickhardt, M.; Liebermann, B. Antimicrobial, antiproliferative, cytotoxic, and tau inhibitory activity of rubellins and caeruleoramularin produced by the phytopathogenic fungus Ramularia collo-cygni. Planta Med. 2009, 75, 1523–1525. [Google Scholar] [CrossRef]
- Walters, D.R.; Havis, N.D.; Oxley, S.J. Ramularia collo-cygni: The biology of an emerging pathogen of barley. FEMS Microbiol. Lett. 2008, 279, 1–7. [Google Scholar] [CrossRef]
- Hayakawa, S.; Minato, H.; Katagiri, K. The ilicicolins, antibiotics from Cylindrocladium ilicicola. J. Antibiot. Res. 1971, 24, 653–654. [Google Scholar] [CrossRef]
- Aldridge, D.; Turner, W. Metabolites of Helminthosporium monoceras: Structures of monocerin and related benzopyrans. J. Chem. Soc. C. 1970, 18, 2598–2600. [Google Scholar] [CrossRef]
- Robeson, D.; Strobel, G. Monocerin, a phytotoxin from Exserohilum turcicum (≡ Drechslera turcica). Agric. Biol. Chem. 1982, 46, 2681–2683. [Google Scholar]
- Jouda, J.-B.; Tamokou, J.-d.-D.; Mbazoa, C.D.; Douala-Meli, C.; Sarkar, P.; Bag, P.K.; Wandji, J. Antibacterial and cytotoxic cytochalasins from the endophytic fungus Phomopsis sp. harbored in Garcinia kola (Heckel) nut. BMC complement. Altern. Med. 2016, 16, 462. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, D.; Armstrong, J.; Speake, R.; Turner, W. The cytochalasins, a new class of biologically active mould metabolites. Chem. Commun. 1967, 1, 26–27. [Google Scholar] [CrossRef]
- Oh, M.; Son, H.; Choi, G.J.; Lee, C.; Kim, J.C.; Kim, H.; Lee, Y.W. Transcription factor ART 1 mediates starch hydrolysis and mycotoxin production in Fusarium graminearum and F. verticillioides. Mol. Plant Pathol. 2016, 17, 755–768. [Google Scholar] [CrossRef]
- Rotem, J. The Genus Alternaria: Biology, Epidemiology, and Pathogenicity; APS PRESS: St. Paul, MN, USA, 1994. [Google Scholar]
- Drakopoulos, D.; Sulyok, M.; Krska, R.; Logrieco, A.F.; Vogelgsang, S. Raised concerns about the safety of barley grains and straw: A Swiss survey reveals a high diversity of mycotoxins and other fungal metabolites. Food Control 2021, 125, 107919. [Google Scholar] [CrossRef]
- Coop, I.E. Depression of lambing performance from mating on lucerne. Proc. N. Z. Soc. Anim. Prod. 1977, 37, 149–151. [Google Scholar]
- Mostrom, M.; Evans, T.J. Phytoestrogens. In Reproductive and Developmental Toxicology; Gupta, R.C., Ed.; Academic Press—Medical: Amsterdam, The Netherlands, 2011; pp. 707–722. [Google Scholar]
- Wyse, J.M.; Latif, S.; Gurusinghe, S.; Berntsen, E.D.; Weston, L.A.; Stephen, C.P. Characterization of Phytoestrogens in Medicago sativa L. and Grazing Beef Cattle. Metabolites 2021, 11, 550. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific panel on contaminations in the food chain on a request from the commission related to cyanogenic compounds as undesirable substances in animal feed. EFSA J. 2007, 434, 1–67. [Google Scholar]
- Vetter, J. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Gurnsey, M.; Jones, W.; Merrall, M.; Reid, C. Cyanide poisoning in cattle: Two unusual cases. N. Z. Vet. J. 1977, 25, 128–130. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshi, K.; Taketoshi, K.; Hideki, M.; Jiro, K.; Yoshinori, N. A comparative study on cytotoxicities and biochemical properties of anthraquinone mycotoxins emodin and skyrin from Penicillium islandicum Sopp. Toxicol. Lett. 1984, 20, 155–160. [Google Scholar] [CrossRef]
- Anderson, R.C.; Majak, W.; Rassmussen, M.A.; Callaway, T.R.; Beier, R.C.; Nisbet, D.J.; Allison, M.J. Toxicity and metabolism of the conjugates of 3-nitropropanol and 3-nitropropionic acid in forages poisonous to livestock. J. Agric. Food Chem. 2005, 53, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.; He, F.; Spencer, P.; Hammerstad, J.; Sabri, M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 1991, 18, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Maiya, S.; Grundmann, A.; Li, S.M.; Turner, G. The fumitremorgin gene cluster of Aspergillus fumigatus: Identification of a gene encoding brevianamide F synthetase. Chembiochem 2006, 7, 1062–1069. [Google Scholar] [CrossRef]
- Rahman, A.; Siddiqui, S.A.; Rahman, M.O.; Kang, S.C. Cyclo (L-Pro-L-Tyr) from Streptomyces sp. 150: Exploiting in vitro Potential in Controlling Foodborne Pathogens and Phytopathogens. Antiinfect. Agents 2020, 18, 169–177. [Google Scholar] [CrossRef]
- Zin, N.M.; Al-Shaibani, M.M.; Jalil, J.; Sukri, A.; Al-Maleki, A.R.; Sidik, N.M. Profiling of gene expression in methicillin-resistant Staphylococcus aureus in response to cyclo-(l-Val-l-Pro) and chloramphenicol isolated from Streptomyces sp., SUK 25 reveals gene downregulation in multiple biological targets. Arch. Microbiol. 2020, 202, 2083–2092. [Google Scholar] [CrossRef]
- VDLUFA. Die Chemische Untersuchung von Futtermitteln. In Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch); VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Lammers, B.; Buckmaster, D.; Heinrichs, A. A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 1996, 79, 922–928. [Google Scholar] [CrossRef]
- Kononoff, P.; Heinrichs, A.; Buckmaster, D. Modification of the Penn State forage and total mixed ration particle separator and the effects of moisture content on its measurements. J. Dairy Sci. 2003, 86, 1858–1863. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. Res. 2020, 412, 2607–2620. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Sulyok, M.; Malachová, A.; Mueller, A.; Krska, R. Realizing the simultaneous liquid chromatography-tandem mass spectrometry based quantification of >1200 biotoxins, pesticides and veterinary drugs in complex feed. J. Chormatogr. A. 2020, 1629, 461502. [Google Scholar] [CrossRef] [PubMed]
- Shimshoni, J.A.; Cuneah, O.; Sulyok, M.; Krska, R.; Galon, N.; Sharir, B.; Shlosberg, A. Mycotoxins in corn and wheat silage in Israel. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2013, 30, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Nichea, M.J.; Palacios, S.A.; Chiacchiera, S.M.; Sulyok, M.; Krska, R.; Chulze, S.N.; Torres, A.M.; Ramirez, M.L. Presence of multiple mycotoxins and other fungal metabolites in native grasses from a wetland ecosystem in Argentina intended for grazing cattle. Toxins 2015, 7, 3309–3329. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Correlation. In Applied Statistics for the Behavioral Sciences; Houghton Mifflin College Division: Boston, MA, USA, 2003; pp. 485–528. [Google Scholar]
- Oldick, B.; Firkins, J.; St-Pierre, N. Estimation of microbial nitrogen flow to the duodenum of cattle based on dry matter intake and diet composition. J. Dairy Sci. 1999, 82, 1497–1511. [Google Scholar] [CrossRef]




| Dietary Related Factors | ||||
|---|---|---|---|---|
| Dietary Component | Farm Frequency of Inclusion (%) | Average ± SD | Range | |
| Grass silage (%DM) | 97.5 | 40.4 ± 16.3 | 10.4–86.7 | |
| Maize silage (%DM) | 82.8 | 22.4 ± 14.3 | 1.7–59.1 | |
| Hay (%DM) | 18.2 | 0.9 ± 3.2 | 0.6–29.8 | |
| Straw (%DM) | 62.1 | 1.8 ± 2.1 | 0.01–10.0 | |
| BSG (%DM) | 27.3 | 4.11 ± 2.4 | 0.34–13.5 | |
| Other silages (%DM) | 10.1 | 6.29 ± 5.67 | 0.47–23.6 | |
| Forage (%DM) | 100 | 65.9 ± 10.1 | 32.4–89 | |
| Chemical composition | ||||
| Dry matter (%) | 37.1 ± 4.7 | 25.7–54.6 | ||
| Crude protein (%DM) | 15.4 ± 2.0 | 9.9–21.2 | ||
| Ash (%DM) | 8.2 ± 2.5 | 4.8–18.5 | ||
| Crude fat (%DM) | 2.7 ± 0.5 | 1.2–4.6 | ||
| Neutral detergent fibre (% DM) | 50.4 ± 7.0 | 36.8–75.2 | ||
| Non-fibre carbohydrate (% DM) | 23.3 ± 7.3 | 0.8–41.3 | ||
| Particle size | ||||
| >19 mm (%) | 46.8 ± 19.8 | 2.3–96.0 | ||
| 8–19 mm (%) | 22.7 ± 11.2 | 2–53.6 | ||
| 1.18–8 mm (%) | 25.6 ± 9.3 | 1.6–49.0 | ||
| <1.18 mm (%) | 4.6 ± 2.9 | 0.3–13.7 | ||
| Hygienic status | Proper | Minor deficiency | Significant deficiency | Vast deficiency |
| Grass silage (%) | 54.9 | 27.5 | 9.8 | 7.8 |
| Maize silage (%) | 45.7 | 43.9 | 3.7 | 6.7 |
| Hay (%) | 91.7 | 5.6 | 2.8 | 0 |
| Straw (%) | 80.5 | 17.1 | 1.6 | 0.8 |
| BSG (%) | 55.6 | 37 | 1.9 | 5.6 |
| Concentrate (%) | 97 | 1 | 1 | 1 |
| Geo-climatic factors | ||||
| Average ± SD | Range | |||
| Altitude (m.a.s.l.) | 480.3 ± 162.1 | 262–1300 | ||
| Temperature (mean month of sampling) (°C) a | 15.47 ± 6.19 | −0.8–22.4 | ||
| Temperature (maize’s growing season) (°C) b | 18.7 ± 1.1 | 13–22 | ||
| Relative humidity (%) c | 70.1 ± 3.3 | 60.3–78 | ||
| Rainfall (mm) d | 294.5 ± 60.3 | 179–594 | ||
| Group | Metabolite | Positive Samples (%) 1 | Concentration (µg/kg DM) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Average ± SD | Median | Range | |||||||
| Alternaria | Alternariol 3 | 45.5 | 8.55 | ± | 13.8 | 5.65 | 1.09 | – | 118 |
| Alternariolmethylether 3 | 42.4 | 5.69 | ± | 3.6 | 5.50 | 1.07 | – | 20.0 | |
| Altenuisol | 1.0 | 15.3 | ± | 5.3 | 15.3 | 9.96 | – | 20.6 | |
| Altersetin | 47.0 | 34.3 | ± | 26.4 | 26.4 | 4.16 | – | 143 | |
| Infectopyrone | 78.3 | 348 | ± | 490 | 169 | 6.96 | – | 3810 | |
| Pyrenophorol | 2.5 | 8.31 | ± | 9.6 | 4.05 | 1.90 | – | 27.5 | |
| Radicinin | 1.0 | 4.44 | ± | 2.7 | 4.44 | 1.72 | – | 7.17 | |
| Tentoxin | 30.8 | 3.79 | ± | 2.2 | 3.41 | 1.15 | – | 12.1 | |
| Tenuazonic acid 3 | 78.8 | 178 | ± | 83.1 | 153 | 76.1 | – | 549 | |
| Zinndiol | 1.0 | 19.8 | ± | 1.9 | 19.8 | 17.9 | – | 21.7 | |
| Zinniol | 2.5 | 42.0 | ± | 23.7 | 36.4 | 22.4 | – | 87.6 | |
| Total 4 | 98.5 | 445 | ± | 491 | 304 | 2.62 | – | 3930 | |
| Aspergillus | Aflatoxin B1 5 | 0 | - | - | - | ||||
| Averufin | 7.6 | 2.69 | ± | 1.6 | 2.95 | 1.07 | – | 8.03 | |
| Bis(methylthio)gliotoxin | 4.0 | 12.8 | ± | 6.5 | 11.9 | 5.67 | – | 25.7 | |
| Deoxygerfelin | 2.0 | 9.37 | ± | 11.5 | 3.84 | 0.75 | – | 29.0 | |
| Deoxynortryptoquivalin | 1.5 | 3.20 | ± | 0.0 | 3.20 | 3.20 | – | 3.20 | |
| Flavoglaucin | 75.8 | 21.4 | ± | 54.2 | 5.94 | 0.65 | – | 368 | |
| Fumigaclavine | 1.0 | 6.08 | ± | 1.1 | 6.08 | 5.00 | – | 7.15 | |
| Fumigaclavine C | 2.0 | 28.4 | ± | 20.9 | 24.2 | 6.52 | – | 58.6 | |
| Fumiquinazolin D | 2.0 | 26.7 | ± | 11.5 | 25.8 | 14.3 | – | 40.9 | |
| Integracin A | 7.1 | 23.1 | ± | 69.8 | 1.95 | 1.11 | – | 275 | |
| Integracin B | 11.6 | 50.7 | ± | 219 | 2.87 | 1.05 | – | 1080 | |
| Kojic acid | 56.1 | 165 | ± | 62.2 | 145 | 132 | – | 516 | |
| Methylsulochrin | 1.0 | 18.2 | ± | 1.9 | 18.2 | 16.3 | – | 20.1 | |
| Mevinolin | 14.1 | 36.1 | ± | 35.2 | 23.8 | 12.0 | – | 150 | |
| Sterigmatocystin 3 | 17.2 | 3.60 | ± | 2.3 | 2.65 | 1.19 | – | 10.3 | |
| Trypacidin | 0.5 | - | - | 2.78 | |||||
| Versicolorin C | 2.5 | 5.80 | ± | 3.3 | 7.60 | 1.75 | – | 9.7 | |
| Total 4 | 88.4 | 141 | ± | 159 | 150 | 1.03 | – | 1680 | |
| Ergot alkaloids | Chanoclavine | 18.2 | 7.90 | ± | 12.0 | 3.23 | 0.95 | – | 55.8 |
| Festuclavine | 1.0 | 11.4 | ± | 8.6 | 11.4 | 2.75 | – | 20.0 | |
| Ergocornine | 9.1 | 6.43 | ± | 7.7 | 4.32 | 1.26 | – | 34.8 | |
| Ergocorninine | 5.6 | 5.53 | ± | 5.7 | 3.38 | 1.60 | – | 22.1 | |
| Ergocristine | 4.5 | 7.86 | ± | 3.4 | 6.90 | 1.90 | – | 13.5 | |
| Ergocristinine | 2.5 | 5.12 | ± | 2.8 | 4.14 | 1.35 | – | 8.53 | |
| Ergocryptine | 8.6 | 10.6 | ± | 11.0 | 7.21 | 0.95 | – | 43.2 | |
| Ergocryptinine | 2.0 | 8.94 | ± | 4.0 | 9.63 | 3.49 | – | 13.0 | |
| Ergometrine | 0.5 | - | - | 7.18 | |||||
| Ergosine | 9.1 | 6.76 | ± | 4.8 | 5.43 | 0.30 | – | 17.2 | |
| Ergosinine | 8.6 | 5.01 | ± | 6.1 | 2.90 | 0.30 | – | 24.5 | |
| Ergotamine | 6.6 | 9.64 | ± | 15.4 | 4.94 | 1.61 | – | 62.3 | |
| Ergotaminine | 6.1 | 9.19 | ± | 14.4 | 3.99 | 2.00 | – | 56.2 | |
| Total 4 | 32.3 | 19.5 | ± | 37.3 | 8.01 | 0.95 | – | 219 | |
| Fusarium | 15-Hydroxyculmorin 3,6 | 94.4 | 128 | ± | 156 | 87.3 | 10.6 | – | 1600 |
| Acuminatum B | 7.6 | 47.5 | ± | 18.7 | 38.8 | 23.2 | – | 80.7 | |
| Antibiotic Y | 40.4 | 35.1 | ± | 33.1 | 24.4 | 8.52 | – | 175 | |
| Apicidin 3 | 75.8 | 16.1 | ± | 15.0 | 12.2 | 0.75 | – | 105 | |
| Apicidin D2 | 8.1 | 14.7 | ± | 13.1 | 6.95 | 6.95 | – | 57.2 | |
| Aurofusarin 3 | 96.0 | 59.3 | ± | 42.3 | 46.9 | 6.79 | – | 349 | |
| Beauvericin 3 | 100 | 10.3 | ± | 9.1 | 7.38 | 0.98 | – | 71.7 | |
| Bikaverin 3 | 66.2 | 25.6 | ± | 24.6 | 18.4 | 3.83 | – | 161 | |
| Chrysogine | 8.6 | 32.0 | ± | 33.6 | 23.9 | 1.68 | – | 136 | |
| Culmorin 3 | 92.4 | 361 | ± | 324 | 272 | 35.3 | – | 2952 | |
| Deoxynivalenol (5000) 5 | 92.4 | 153 | ± | 230 | 104 | 14.8 | – | 2900 | |
| DON-3-glucoside 6 | 9.1 | 33.9 | ± | 41.0 | 19.0 | 19.0 | – | 195 | |
| Enniatin A 3 | 65.2 | 1.79 | ± | 3.2 | 1.07 | 0.20 | – | 31.1 | |
| Enniatin A1 3 | 99.5 | 6.92 | ± | 5.7 | 5.28 | 0.40 | – | 32.3 | |
| Enniatin B 3 | 100 | 40.2 | ± | 28.1 | 31.4 | 4.34 | – | 175 | |
| Enniatin B1 3 | 100 | 25.9 | ± | 18.7 | 21.2 | 2.42 | – | 126 | |
| Enniatin B2 3 | 69.2 | 1.34 | ± | 0.9 | 1.07 | 0.22 | – | 6.81 | |
| Epiequisetin 3 | 53.5 | 5.08 | ± | 8.2 | 3.07 | 1.07 | – | 63.4 | |
| Equisetin3 | 97.0 | 13.4 | ± | 22.3 | 7.73 | 1.60 | – | 224 | |
| Fumonisin A1 (precussor) | 1.0 | 3.97 | ± | 0.4 | 3.97 | 3.62 | – | 4.32 | |
| Fumonisin B1 5 | 70.7 | 120 | ± | 118 | 93.5 | 26.5 | – | 1120 | |
| Fumonisin B2 5 | 35.4 | 51.9 | ± | 32.9 | 45.3 | 17.0 | – | 243 | |
| Fumonisin B3 | 6.1 | 43.3 | ± | 29.4 | 26.5 | 19.9 | – | 129 | |
| Fumonisin B4 | 4.5 | 33.9 | ± | 24.9 | 18.0 | 18.0 | – | 96.9 | |
| Fusaproliferin | 4.5 | 184 | ± | 76.8 | 174 | 81.6 | – | 338 | |
| Fusapyron 3 | 2.0 | 10.9 | ± | 9.6 | 6.42 | 3.49 | – | 27.5 | |
| HT-2 glucoside 6 | 1.0 | 14.4 | ± | 8.4 | 14.4 | 6.00 | – | 22.7 | |
| HT-2 toxin 5 | 27.8 | 27.3 | ± | 28.2 | 20.5 | 9.27 | – | 217 | |
| Moniliformin 3 | 40.4 | 23.4 | ± | 22.4 | 16.1 | 4.61 | – | 148 | |
| Monoacetoxyscirpenol | 6.6 | 13.6 | ± | 7.6 | 11.0 | 5.52 | – | 29.5 | |
| Nivalenol | 8.6 | 311 | ± | 247 | 269 | 34.6 | – | 804 | |
| Siccanol 3 | 54.0 | 709 | ± | 805 | 494 | 106 | – | 7220 | |
| T-2 toxin 5 | 12.1 | 4.97 | ± | 2.5 | 4.25 | 2.13 | – | 14.6 | |
| W493 | 65.7 | 21.7 | ± | 69.9 | 5.64 | 1.00 | – | 671 | |
| Zearalenone (500) 5 | 77.8 | 25.2 | ± | 36.9 | 14.7 | 1.90 | – | 378 | |
| Sum of enniatins | 100 | 75.0 | ± | 50.4 | 61.1 | 7.36 | – | 324 | |
| Sum of T-2 and HT-2 toxins (250) 5 | 32.3 | 25.3 | ± | 27.4 | 20.4 | 2.13 | – | 217 | |
| Sum of fumonisins | 71.2 | 150 | ± | 169 | 106 | 26.5 | – | 1590 | |
| Sum of fumonisins B1 and B2 (50,000) 5 | 71.2 | 145 | ± | 149 | 102 | 26.5 | – | 1370 | |
| Sum of type A trichothecenes | 36.9 | 25.0 | ± | 29.8 | 19.0 | 2.13 | – | 246 | |
| Sum of type B trichothecenes | 92.9 | 184 | ± | 266 | 113 | 14.8 | – | 3070 | |
| Total 4 | 100 | 1390 | ± | 1510 | 1070 | 109 | – | 17,800 | |
| Penicillium | 7-Hydroxypestalotin | 3.0 | 4.39 | ± | 2.6 | 2.60 | 2.60 | – | 9.07 |
| Andrastin A | 16.7 | 25.8 | ± | 33.7 | 12.0 | 1.80 | – | 140 | |
| Andrastin B | 4.0 | 68.8 | ± | 66.6 | 48.4 | 16.5 | – | 238 | |
| Andrastin C | 3.5 | 270 | ± | 170 | 247 | 43.4 | – | 603 | |
| Barceloneic acid | 18.2 | 36.4 | ± | 29.9 | 24.7 | 7.84 | – | 133 | |
| Citreohybridinol | 1.0 | 3.77 | ± | 1.6 | 3.77 | 2.16 | – | 5.38 | |
| Citrinin | 1.0 | 20.7 | ± | 14.0 | 20.7 | 6.67 | – | 34.7 | |
| Curvularin | 6.1 | 49.7 | ± | 64.3 | 14.9 | 2.54 | – | 182 | |
| Dehydrocurvularin | 1.5 | 54.5 | ± | 35.2 | 32.8 | 26.5 | – | 104 | |
| Fellutanine A | 93.9 | 96.3 | ± | 62.5 | 78.9 | 27.7 | – | 466 | |
| Griseofulvin | 0.5 | - | - | 1.83 | |||||
| Hydroxyandrastin C | 3.0 | 10.8 | ± | 7.0 | 9.41 | 3.10 | – | 20.8 | |
| Marcfortine A | 23.2 | 9.49 | ± | 15.4 | 3.88 | 0.45 | – | 81.0 | |
| Marcfortine C | 6.1 | 3.08 | ± | 3.2 | 1.57 | 0.45 | – | 12.1 | |
| Mycophenolic acid 3 | 21.2 | 47.5 | ± | 104 | 15.8 | 1.52 | – | 661 | |
| Ochratoxin A (250) 5 | 1.0 | 7.50 | ± | 0.3 | 7.50 | 7.16 | – | 7.84 | |
| Pestalotin | 14.1 | 5.59 | ± | 2.8 | 3.30 | 1.88 | – | 11.3 | |
| Phenopyrrozin | 96.5 | 52.8 | ± | 36.8 | 42.7 | 10.8 | – | 352 | |
| Questiomycin A | 5.1 | 27.1 | ± | 14.1 | 20.8 | 11.1 | – | 59.5 | |
| Questiomycin Derivat | 18.7 | 58.4 | ± | 153 | 32.6 | 9.82 | – | 973 | |
| Questiomycine | 36.4 | 8.17 | ± | 9.3 | 5.23 | 1.50 | – | 49.2 | |
| Roquefortine C | 18.7 | 30.3 | ± | 64.7 | 14.5 | 3.56 | – | 387 | |
| Roquefortine D | 1.5 | 9.69 | ± | 7.7 | 4.25 | 4.25 | – | 20.6 | |
| Total 4 | 99.5 | 205 | ± | 176 | 166 | 2.71 | – | 1680 | |
| Lichen- associated fungi | Lecanoric acid | 6.1 | 4.71 | ± | 6.0 | 1.45 | 1.45 | – | 18.1 |
| Usnic acid | 11.6 | 3.83 | ± | 3.2 | 2.53 | 0.50 | – | 12.7 | |
| Total 4 | 16.2 | 4.57 | ± | 4.9 | 2.47 | 0.50 | – | 18.9 | |
| Other fungi | Alamethicine | 1.5 | 65.5 | ± | 40.1 | 61.2 | 18.8 | – | 117 |
| Ascochlorin | 9.6 | 3.35 | ± | 3.3 | 2.07 | 1.15 | – | 13.6 | |
| Ascofuranone | 0.5 | - | - | 3.57 | |||||
| Bassianolide | 2.0 | 8.25 | ± | 9.4 | 3.90 | 0.80 | – | 24.4 | |
| Calphostin C | 3.0 | 2.89 | ± | 2.5 | 1.98 | 1.09 | – | 8.34 | |
| Cytochalasin B | 13.1 | 48.3 | ± | 51.2 | 34.6 | 8.87 | – | 234 | |
| Cytochalasin C | 1.0 | 8.77 | ± | 0.9 | 8.77 | 7.90 | – | 9.6 | |
| Destruxin B | 27.3 | 5.66 | ± | 7.5 | 3.26 | 0.20 | – | 44.1 | |
| Emestrin | 3.5 | 16.2 | ± | 11.3 | 22.3 | 3.50 | – | 31.0 | |
| Epoxycytochalsin C | 7.6 | 3.59 | ± | 3.7 | 0.60 | 0.60 | – | 12.2 | |
| Ilicicolin A | 13.1 | 2.53 | ± | 2.8 | 1.42 | 0.50 | – | 10.1 | |
| Ilicicolin B | 38.9 | 4.79 | ± | 6.5 | 1.89 | 1.02 | – | 36.5 | |
| Ilicicolin E | 5.6 | 4.53 | ± | 3.0 | 3.93 | 0.50 | – | 10.2 | |
| Ilicicolin H | 22.2 | 16.1 | ± | 20.8 | 10.5 | 0.50 | – | 123 | |
| LL-Z 1272e | 1.5 | 10.4 | ± | 8.3 | 8.89 | 1.03 | – | 21.3 | |
| Monocerin | 33.3 | 68.1 | ± | 162 | 11.9 | 0.65 | – | 893 | |
| Myriocin | 1.0 | 41.4 | ± | 24.0 | 41.4 | 17.4 | – | 65.3 | |
| Rubellin D | 57.1 | 34.8 | ± | 54.2 | 15.5 | 0.85 | – | 301 | |
| Neoechinulin A | 35.9 | 27.6 | ± | 54.2 | 17.7 | 2.00 | – | 429 | |
| Sporidesmolide II | 51.5 | 65.8 | ± | 114 | 23.6 | 0.25 | – | 617 | |
| Ternatin | 1.5 | 7.59 | ± | 6.5 | 6.39 | 0.25 | – | 16.1 | |
| Total 4 | 89.9 | 115 | ± | 177 | 45.1 | 1.15 | – | 1060 | |
| Sum of fungal metabolites | 100 | 2260 | ± | 1690 | 1993 | 302 | – | 19,100 | |
| Phytoestrogens | Biochanin | 100 | 21,900 | ± | 15,800 | 23,000 | 226 | – | 52,050 |
| Coumestrol | 80.8 | 524 | ± | 1140 | 111 | 2.50 | – | 8290 | |
| Daidzein | 99.5 | 5780 | ± | 6670 | 3110 | 25.0 | – | 45,900 | |
| Daidzin | 89.9 | 4527 | ± | 4580 | 3300 | 3.38 | – | 23,900 | |
| Formonetin | 21.2 | 78,700 | ± | 67,900 | 58,400 | 13,800 | – | 289,000 | |
| Genistein | 100 | 9460 | ± | 8950 | 6730 | 179 | – | 52,600 | |
| Genistin | 93.4 | 6000 | ± | 6130 | 3980 | 33.0 | – | 36,500 | |
| Glycitein | 53.0 | 9430 | ± | 10,200 | 4530 | 138 | – | 48,100 | |
| Glycitin | 80.8 | 1205 | ± | 1160 | 930 | 12.5 | – | 7540 | |
| Ononin | 73.7 | 435 | ± | 1050 | 160 | 14.0 | – | 11,540 | |
| Total 4 | 100 | 70,200 | ± | 67,100 | 50,800 | 1080 | – | 411,000 | |
| Other plant metabolites | Abscisic acid | 89.4 | 785 | ± | 552 | 627 | 136 | – | 4315 |
| Chaconin | 11.6 | 31.4 | ± | 41.3 | 7.50 | 5.60 | – | 161 | |
| Colchicine | 3.5 | 71.2 | ± | 87.9 | 31.6 | 13.5 | – | 282 | |
| Linamarin | 47.0 | 2850 | ± | 2860 | 1520 | 82.5 | – | 14,200 | |
| Lotaustralin | 74.2 | 1300 | ± | 2160 | 558 | 18.1 | – | 13,700 | |
| Xanthotoxin | 62.6 | 37.4 | ± | 74.2 | 10.9 | 0.90 | – | 450 | |
| Total 4 | 98.0 | 3090 | ± | 4260 | 1522 | 5.37 | – | 24,400 | |
| Sum of plant metabolites | 100 | 73,500 | ± | 67,300 | 54,500 | 1204 | – | 413,000 | |
| Unspecific | 3-Nitropropionic acid | 8.1 | 43.4 | ± | 41.2 | 20.8 | 10.7 | – | 158 |
| Asperglaucide | 72.7 | 5.82 | ± | 12.5 | 3.23 | 0.60 | – | 136 | |
| Asperphenamate | 69.2 | 8.41 | ± | 24.1 | 2.64 | 0.50 | – | 216 | |
| Brevianamid F | 100 | 264 | ± | 147 | 256 | 17.0 | – | 899 | |
| Chrysophanol | 53.5 | 576 | ± | 1390 | 276 | 19.0 | – | 12,500 | |
| Citreorosein | 18.2 | 178 | ± | 197 | 108 | 28.3 | – | 954 | |
| cyclo(L-Pro-L-Tyr) | 100 | 4100 | ± | 2320 | 3720 | 569 | – | 15,400 | |
| cyclo(L-Pro-L-Val) | 100 | 13,700 | ± | 6390 | 12,780 | 2720 | – | 36,900 | |
| Emodin | 97.0 | 249 | ± | 355 | 92.4 | 4.26 | – | 1957 | |
| Endocrocin | 9.6 | 292 | ± | 255 | 215 | 40.5 | – | 1090 | |
| Iso-Rhodoptilometrin | 45.5 | 2.07 | ± | 2.1 | 1.22 | 0.40 | – | 9.01 | |
| N-Benzoyl-Phenylalanine | 5.6 | 28.9 | ± | 37.8 | 14.2 | 1.00 | – | 111 | |
| Norlichexanthone | 25.8 | 20.2 | ± | 103 | 0.55 | 0.55 | – | 745 | |
| Oxyskyrin | 0.5 | - | - | 6.36 | |||||
| Physcion | 20.2 | 844 | ± | 683 | 655 | 49.7 | – | 2560 | |
| Rugulusovine | 100 | 271 | ± | 132 | 257 | 19.6 | – | 817 | |
| Skyrin | 49.0 | 4.2 | ± | 4.1 | 2.92 | 0.15 | – | 28.8 | |
| Tryptophol | 77.8 | 1030 | ± | 1200 | 564 | 49.2 | – | 6380 | |
| Sum of unspecific metabolites | 100 | 20,000 | ± | 8870 | 18,600 | 3740 | – | 52,400 | |
| Sum of all detected metabolites | 100 | 95,400 | ± | 68,900 | 78,300 | 15,100 | – | 432,000 | |
| Concentration (Log-µg/kg) | n | Intercept | SE | p Value | Influencing Factors | Coefficients | SE | p Value | R2 | RMSE |
|---|---|---|---|---|---|---|---|---|---|---|
| Alternaria metabolites | 190 | 5.2607 | 0.0821 | <0.001 | Straw | +0.3851 | 0.0616 | <0.001 | 0.26 | 0.757 |
| Straw × Straw | −0.0282 | 0.0082 | <0.001 | |||||||
| Fusarium metabolites | 198 | 6.2526 | 0.5082 | <0.001 | MS | +0.0695 | 0.0147 | <0.001 | 0.52 | 0.579 |
| Sieve > 19 mm | −0.0158 | 0.0072 | 0.030 | |||||||
| Straw | −0.5902 | 0.1714 | <0.001 | |||||||
| NFC | −0.0413 | 0.0174 | 0.019 | |||||||
| MS × MS | −0.00082 | 0.0002 | <0.001 | |||||||
| MS × Straw | +0.00398 | 0.0019 | 0.042 | |||||||
| MS × Sieve > 19 mm | −0.00041 | 0.0002 | 0.016 | |||||||
| Straw × NFC | +0.02352 | 0.0069 | <0.001 | |||||||
| Straw × Sieve > 19 mm | +0.01151 | 0.0031 | <0.001 | |||||||
| NFC× Sieve > 19 mm | +0.00064 | 0.0003 | 0.047 | |||||||
| Straw × NFC × Sieve > 19 mm | −0.00047 | 0.0001 | <0.001 | |||||||
| Deoxynivalenol | 182 | 5.7616 | 0.8443 | <0.001 | MS | +0.09058 | 0.0247 | <0.001 | 0.22 | 0.677 |
| Rainfall | −0.01240 | 0.0049 | 0.013 | |||||||
| MS × Rainfall | −0.00017 | 0.0001 | 0.031 | |||||||
| MS × MS | −0.00057 | 0.0002 | 0.010 | |||||||
| Rainfall × Rainfall | +0.000022 | 0.0000 | 0.009 | |||||||
| Zearalenone | 154 | 0.9462 | 0.5023 | 0.057 | EE | +0.3897 | 0.1421 | 0.007 | 0.22 | 0.918 |
| Sieve > 19 mm | −0.0124 | 0.0041 | 0.003 | |||||||
| Hygiene GS | +0.2147 | 0.0745 | 0.004 | |||||||
| Fumonisins B1 and B2 | 125 | 4.6964 | 0.110 | <0.001 | Straw | −0.07163 | 0.0278 | 0.011 | 0.09 | 0.606 |
| Hygiene MS | +0.1470 | 0.0585 | 0.013 | |||||||
| Beauvericin | 198 | −1.3010 | 0.6717 | 0.054 | MS | +0.0152 | 0.0037 | <0.001 | 0.32 | 0.654 |
| Sieve 1.18–8 mm | +0.0198 | 0.0055 | <0.001 | |||||||
| Crop temperature | +0.1439 | 0.0374 | <0.001 | |||||||
| Culmorin | 183 | 4.4483 | 0.3138 | <0.001 | MS | +0.06254 | 0.0157 | <0.001 | 0.34 | 0.611 |
| Sieve > 19 mm | −0.00234 | 0.0046 | 0.611 | |||||||
| MS × MS | −0.00072 | 0.0002 | 0.001 | |||||||
| MS × Sieve > 19 mm | −0.00039 | 0.0002 | 0.025 | |||||||
| Enniatins | 198 | 3.5175 | 0.1333 | <0.001 | Brewery’s spent grains | +0.1111 | 0.0192 | <0.001 | 0.19 | 0.600 |
| Siccanol | 107 | 7.0348 | 0.4132 | <0.001 | MS | +0.0016 | 0.0067 | 0.808 | 0.30 | 0.627 |
| Sieve > 19 mm | −0.0098 | 0.0033 | 0.003 | |||||||
| Straw | −0.0487 | 0.0524 | 0.353 | |||||||
| MS × Straw | +0.0052 | 0.0021 | 0.016 | |||||||
| Penicillium metabolites | 187 | 3.9964 | 0.2731 | <0.001 | Temp sampling | +0.0726 | 0.0233 | 0.002 | 0.12 | 0.483 |
| Forage | +0.1135 | 0.0035 | 0.002 | |||||||
| Temp sampling × Temp sampling | −0.0024 | 0.0097 | 0.013 | |||||||
| Total fungal metabolites | 190 | 9.0404 | 0.7690 | <0.001 | MS | −0.0118 | 0.0012 | 0.345 | 0.44 | 0.408 |
| Straw | +0.0864 | 0.0509 | 0.091 | |||||||
| Ash | −0.3912 | 0.1364 | 0.005 | |||||||
| NFC | −0.0148 | 0.0055 | 0.008 | |||||||
| Sieve > 19 mm | +0.0003 | 0.0035 | 0.932 | |||||||
| Hygiene GS | −0.0633 | 0.0314 | 0.045 | |||||||
| Ash × Ash | +0.01395 | 0.0056 | 0.014 | |||||||
| Straw × Straw | −0.01337 | 0.0046 | 0.004 | |||||||
| Straw × Sieve > 19 mm | +0.00185 | 0.0008 | 0.019 | |||||||
| MS × Sieve > 19 mm | −0.00031 | 0.0001 | 0.011 | |||||||
| MS × Ash | +0.00421 | 0.0017 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penagos-Tabares, F.; Khiaosa-ard, R.; Schmidt, M.; Bartl, E.-M.; Kehrer, J.; Nagl, V.; Faas, J.; Sulyok, M.; Krska, R.; Zebeli, Q. Cocktails of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Diets of Dairy Cows in Austria: Inferences from Diet Composition and Geo-Climatic Factors. Toxins 2022, 14, 493. https://doi.org/10.3390/toxins14070493
Penagos-Tabares F, Khiaosa-ard R, Schmidt M, Bartl E-M, Kehrer J, Nagl V, Faas J, Sulyok M, Krska R, Zebeli Q. Cocktails of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Diets of Dairy Cows in Austria: Inferences from Diet Composition and Geo-Climatic Factors. Toxins. 2022; 14(7):493. https://doi.org/10.3390/toxins14070493
Chicago/Turabian StylePenagos-Tabares, Felipe, Ratchaneewan Khiaosa-ard, Marlene Schmidt, Eva-Maria Bartl, Johanna Kehrer, Veronika Nagl, Johannes Faas, Michael Sulyok, Rudolf Krska, and Qendrim Zebeli. 2022. "Cocktails of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Diets of Dairy Cows in Austria: Inferences from Diet Composition and Geo-Climatic Factors" Toxins 14, no. 7: 493. https://doi.org/10.3390/toxins14070493
APA StylePenagos-Tabares, F., Khiaosa-ard, R., Schmidt, M., Bartl, E.-M., Kehrer, J., Nagl, V., Faas, J., Sulyok, M., Krska, R., & Zebeli, Q. (2022). Cocktails of Mycotoxins, Phytoestrogens, and Other Secondary Metabolites in Diets of Dairy Cows in Austria: Inferences from Diet Composition and Geo-Climatic Factors. Toxins, 14(7), 493. https://doi.org/10.3390/toxins14070493





