Gill Junction Injury and Microbial Disorders Induced by Microcystin-Leucine Arginine in Lithobates catesbeianus Tadpoles
Abstract
:1. Introduction
2. Results
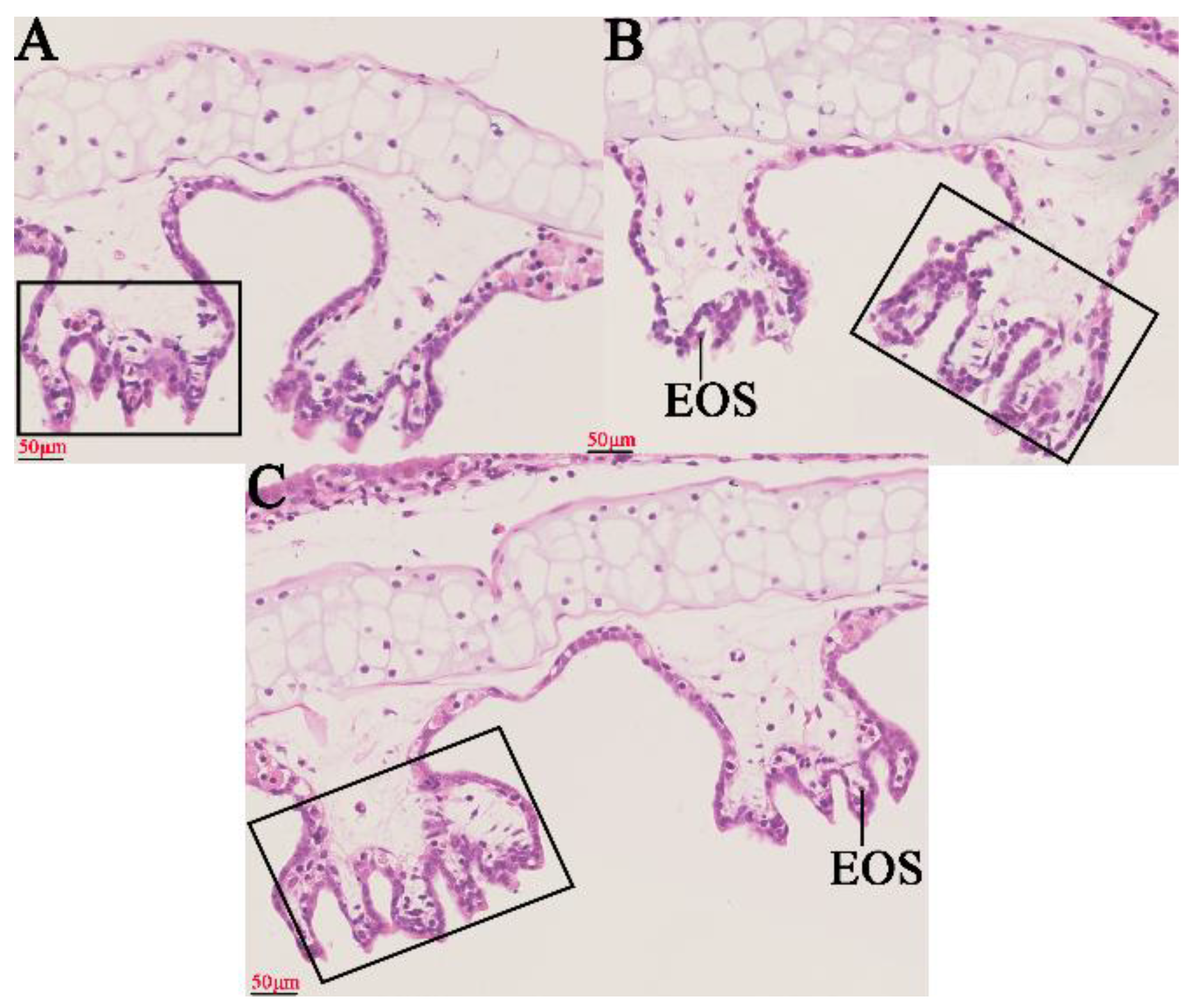
2.1. Histopathological Changes in the Gills
2.2. Transcriptome Analysis
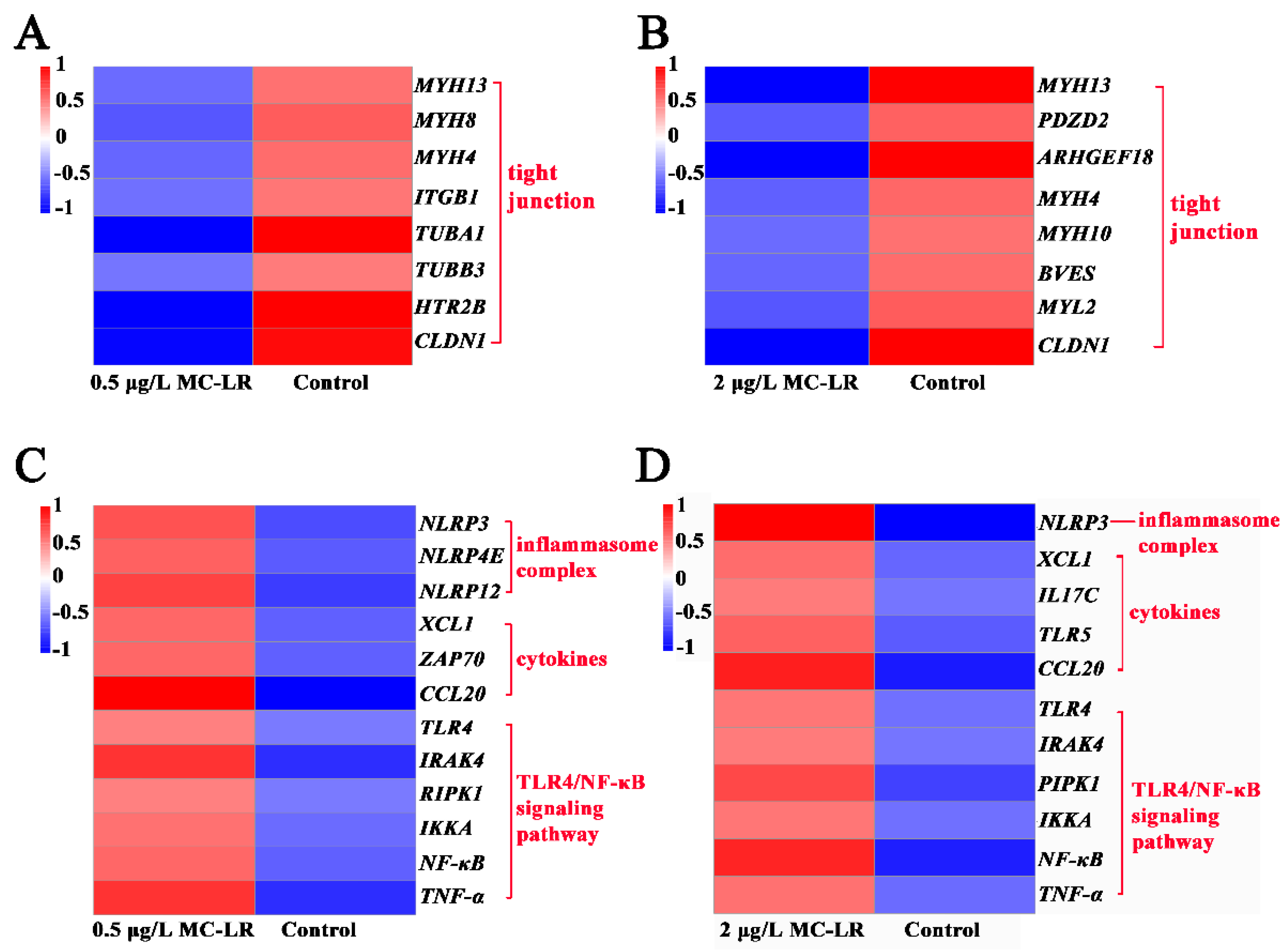
2.2.1. DEGs Associated with Gill Tight Junctions
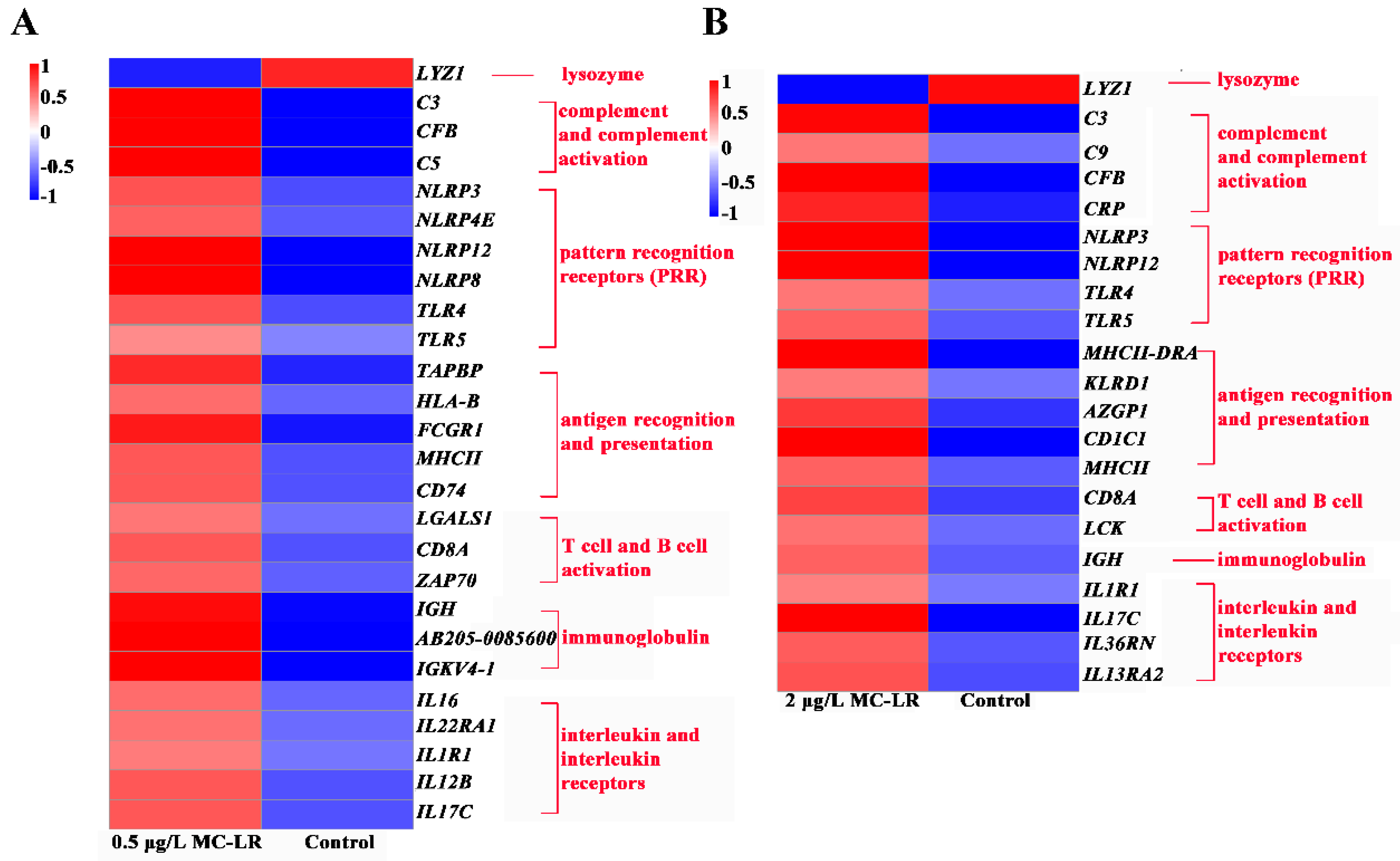
2.2.2. DEGs Associated with Inflammation
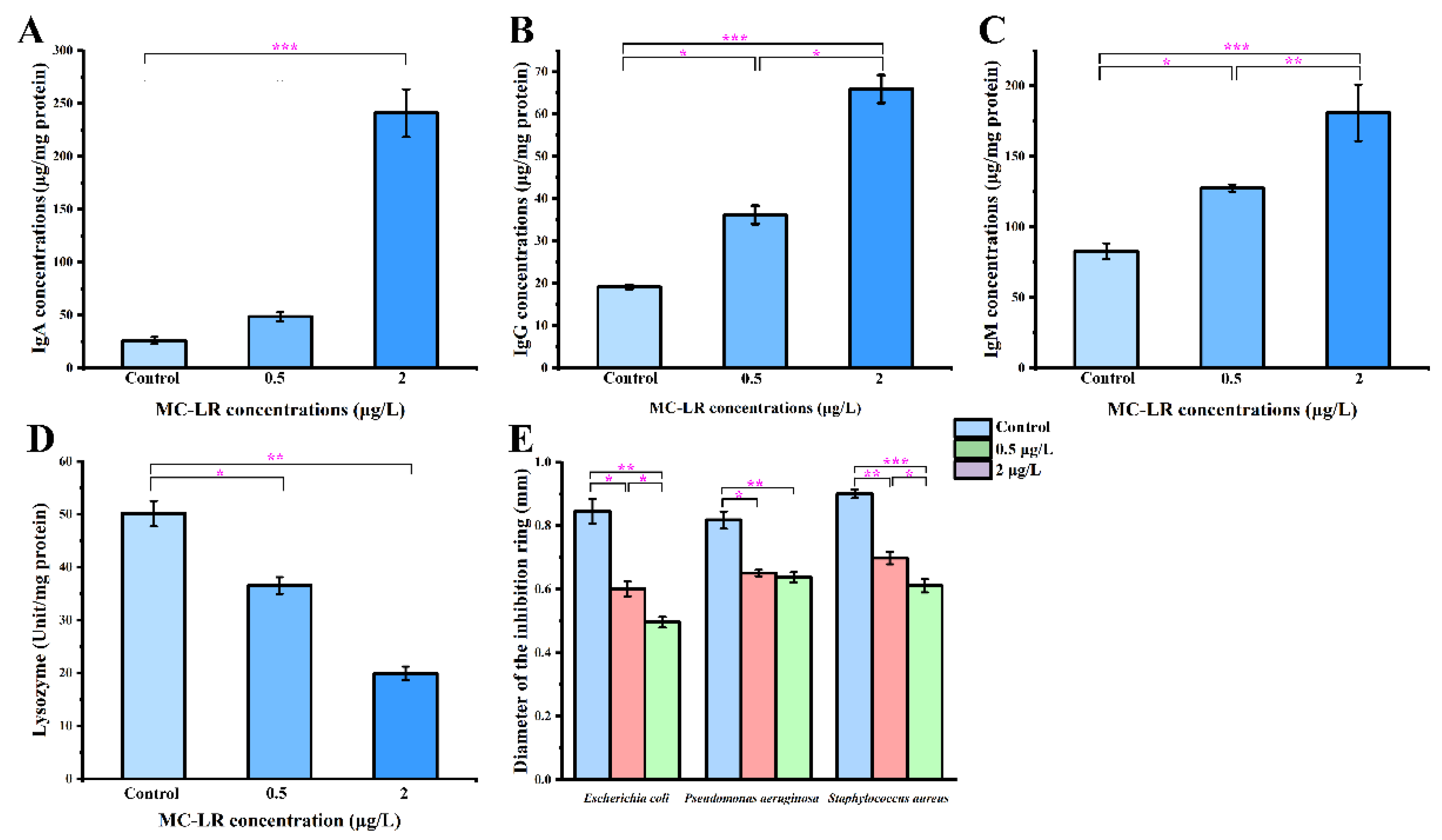
2.2.3. DEGs Associated with Immunity
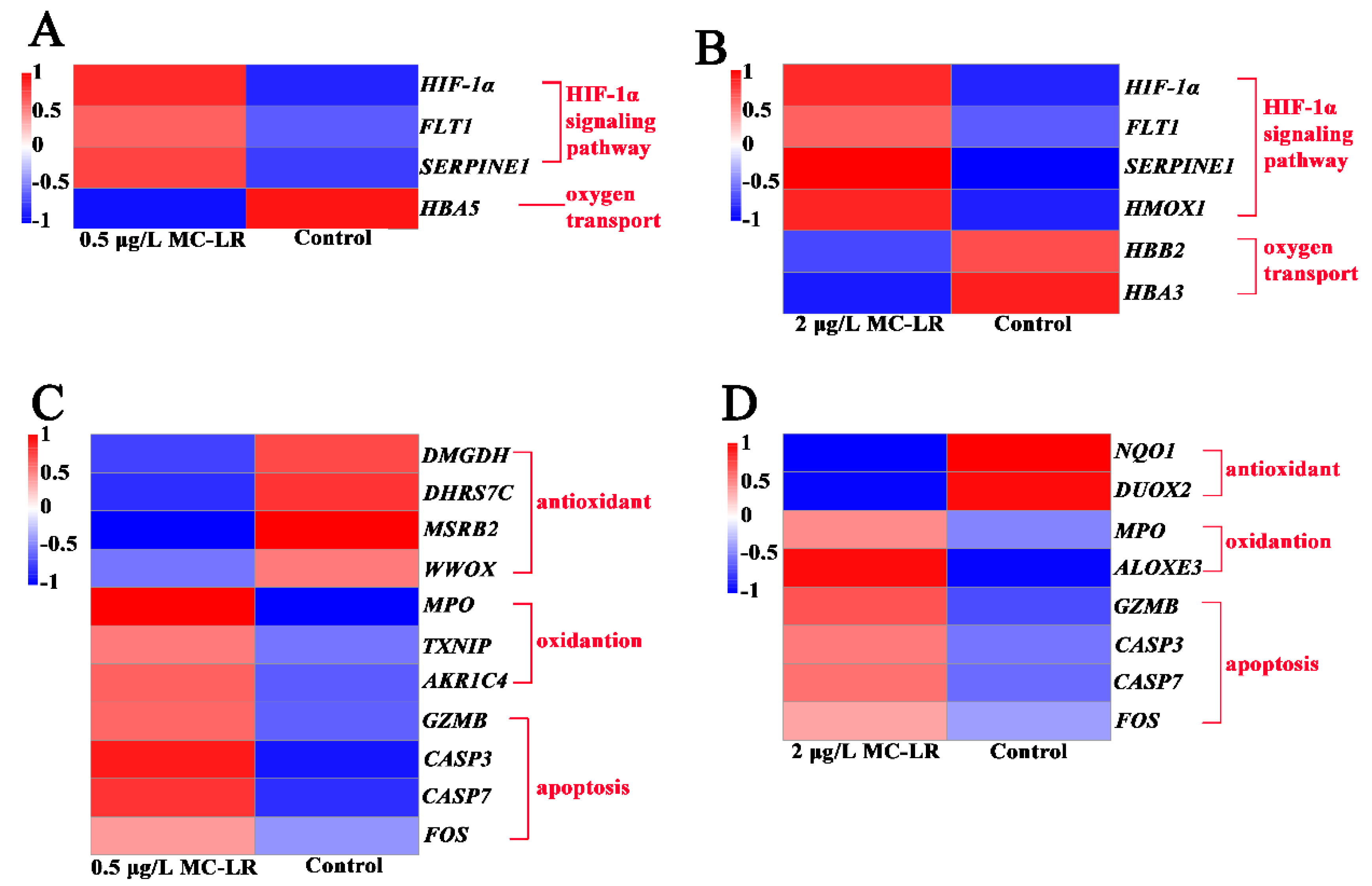
2.2.4. DEGs Associated with Hypoxic Stress and Oxygen Transport
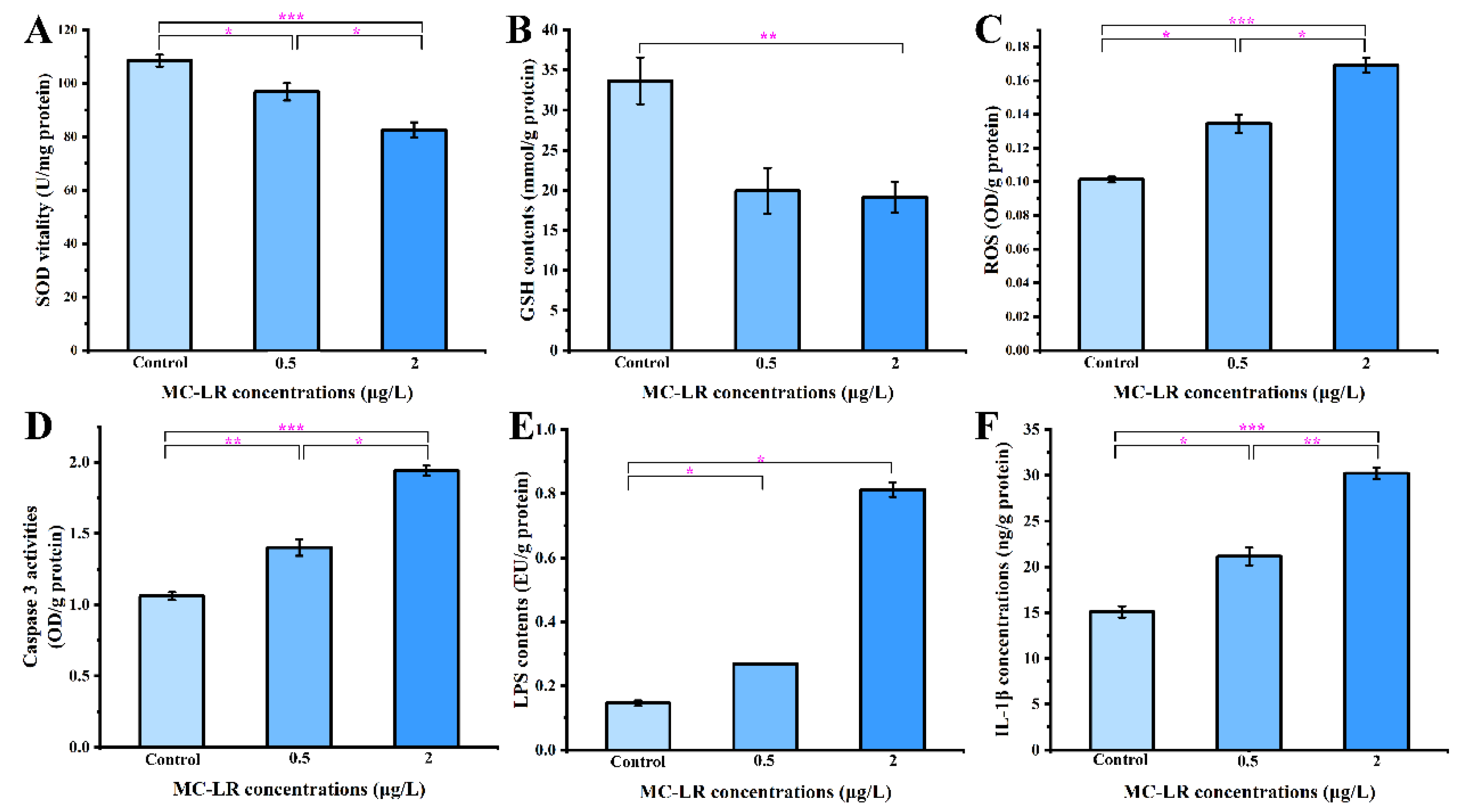
2.2.5. DEGs Related to Oxidative Stress and Apoptosis
2.3. Biochemical Indicators of Gills and Antibacterial Activity
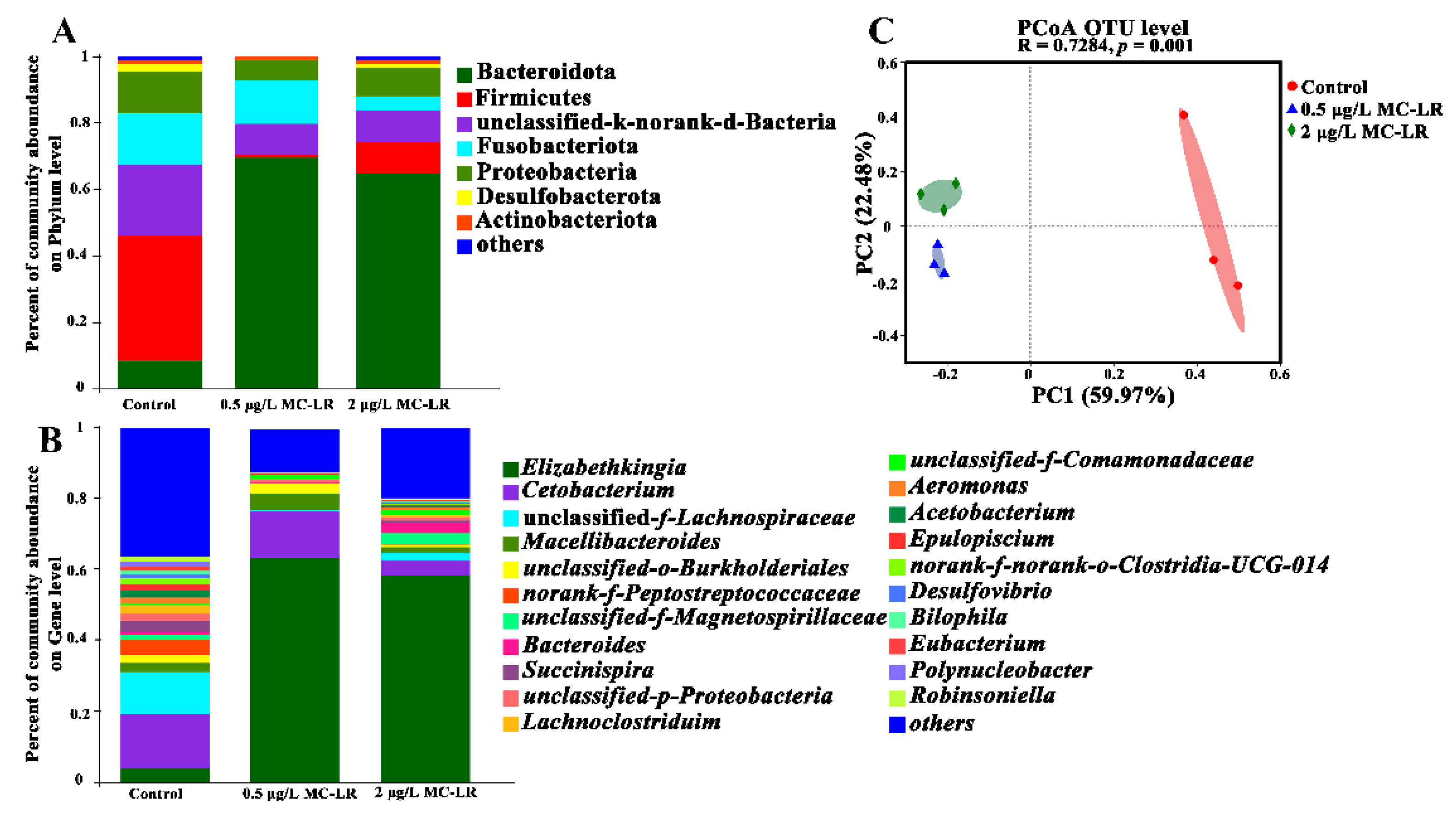
2.4. Dysbiosis of the Gill Microbiome
2.5. Correlation between Gill Microbiota and Inflammatory Events Triggered by LPS
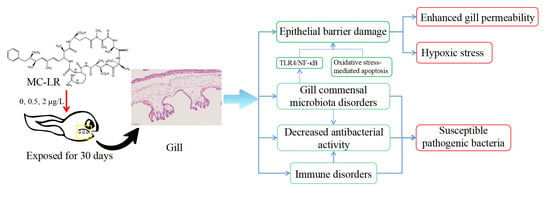
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Rearing of Animals and Sample Collection
5.3. Histopathology of Gill
5.4. Biochemical Parameters of Gill
5.5. Antibacterial Activity of the Gill
5.6. Transcriptome Sequencing and Bioinformatic Analyses
5.7. 16S rRNA Amplicon Sequencing and Bioinformatics
5.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NLRP12 | Nucleotide-binding and leucine-rich repeat receptor family pyrin domain containing 12 |
| IL17C | Interleukin 17C |
| C3 | Complement component 3 |
| HMOX1 | Heme oxygenase 1 |
| TLR5 | Toll like receptor 5 |
| NLRP3 | Nucleotide-binding and leucine-rich repeat receptor family pyrin domain containing 3 |
| GZMB | Granzyme B |
| FOS | Fos proto-oncogene, AP-1 transcription factor subunit |
| CASP7 | Caspase 7 |
| CLDN1 | Claudin 1 |
| LPS | lipopolysaccharide |
| TLR4 | Toll Like Receptor 4 |
| NF-κB | Nuclear Factor Kappa B |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor Necrosis Factor Alpha |
| HIF-1α | Hypoxia Inducible Factor 1 Subunit Alpha |
| FLT1 | Fms Related Receptor Tyrosine Kinase 1 |
| SERPINE1 | Serpin Family E Member 1 |
| GSH | Glutathione |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide Dismutase |
| LZM | Lysozyme |
| IgA | Immunoglobulin A |
| IgM | Immunoglobulin M |
| IgG | Immunoglobulin G |
| MYH13 | Myosin Heavy Chain 13 |
| MYH8 | Myosin Heavy Chain 8 |
| MYH4 | Myosin Heavy Chain 4 |
| ITGB1 | Integrin Subunit Beta 1 |
| TUBA1A | Tubulin Alpha 1a |
| PDZD2 | PDZ domain-containing protein 2 |
| ARHGEF18 | Rho/Rac Guanine Nucleotide Exchange Factor 18 |
| MYH10 | Myosin Heavy Chain 10 |
| BVES | Blood Vessel Epicardial Substance |
| MYL2 | Myosin Light Chain 2 |
| NLRP4E | Nucleotide-binding and leucine-rich repeat receptor Family Pyrin Domain Containing 4 |
| CCL20 | C-C Motif Chemokine Ligand 20 |
| XCL1 | X-C Motif Chemokine Ligand 1 |
| ZAP70 | Zeta Chain Of T Cell Receptor Associated Protein Kinase 70 |
| IRAK4 | Interleukin 1 Receptor Associated Kinase 4 |
| RIPK1 | Receptor Interacting Serine/Threonine Kinase 1 |
| IKKA | Component Of Inhibitor Of Nuclear Factor Kappa B Kinase Complex, CHUK |
| C5 | Complement component 5 |
| CFB | Complement Factor B |
| NLRP8 | Nucleotide-binding and leucine-rich repeat receptor Family Pyrin Domain Containing 8 |
| HLA-B | Major Histocompatibility Complex, Class I, B |
| CD8A | CD8a Molecule |
| IGKV4-1 | Immunoglobulin Kappa Variable 4-1 |
| C9 | Complement component 9 |
| CRP | C-Reactive Protein |
| MHCII-DRA | Major Histocompatibility Complex, Class II, DR Alpha |
| IGH | Immunoglobulin Heavy Locus |
| IL6C | Interleukin 6C |
| HBB2 | Hemoglobin Subunit Beta |
| HBA3 | Hemoglobin Subunit Alpha 3 Subunit |
| DMGDH | Dimethylglycine Dehydrogenase |
| DHRS7C | Dehydrogenase/Reductase 7C |
| MSRB2 | Methionine Sulfoxide Reductase B2 |
| WWOX | WW Domain Containing Oxidoreductase |
| TXNIP | Thioredoxin Interacting Protein |
| AKR1C4 | Aldo-Keto Reductase Family 1 Member C4 |
| MPO | Myeloperoxidase |
| NQO1 | NAD(P)H Quinone Dehydrogenase 1 |
| DUOX2 | Dual Oxidase 2 |
| ALOXE3 | Arachidonate Lipoxygenase 3 |
| CASP3 | Caspase 3 |
| PCoA | Principal Coordinate Analysis |
References
- Bicknell, B.; Liebert, A.; McLachlan, C.S.; Kiat, H. Microbiome changes in humans with Parkinson’s Disease after photobiomodulation therapy: A retrospective study. J. Pers. Med. 2022, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L. Respiratory Tract disorders in fishes. Vet. Clin. North Am. Exot. Anim. Pract. 2021, 24, 267–292. [Google Scholar] [CrossRef]
- Jin, Q.; Feng, C.; Xia, P.; Bai, Y. Hardness-Dependent Water Quality Criteria for Protection of Freshwater Aquatic Organisms for Silver in China. International journal of environmental research and public health 2022, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, I.; Brunelli, E.; Berg, C.; Bonacci, A.; Tripepi, S.J.A.T. Endosulfan acute toxicity in bufo gills: Ultrastructural changes and nitric oxide synthase localization. Aquat. Toxicol. 2008, 86, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Shi, Y.; Shan, Z.; Yang, L.; Wang, X.; Shi, L. Bioaccumulation, oxidative stress and HSP70 expression in Cyprinus carpio L. exposed to microcystin-LR under laboratory conditions. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2012, 155, 483–490. [Google Scholar] [CrossRef]
- Ray, A.; Gautam, A.; Das, S.; Pal, K.; Das, S.; Karmakar, P.; Ray, M.; Ray, S. Effects of copper oxide nanoparticle on gill filtration rate, respiration rate, hemocyte associated immune parameters and oxidative status of an Indian freshwater mussel. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2020, 237, 1–15. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. Excli. J. 2018, 17, 1101–1136. [Google Scholar] [PubMed]
- Miranda, D.A.; Peaslee, G.F.; Zachritz, A.M.; Lamberti, G.A. A worldwide evaluation of trophic magnification of per- and polyfluoroalkyl substances in aquatic ecosystems. Integr. Environ. Assess. Manag. 2022, 00, 1–13. [Google Scholar] [CrossRef]
- Lowrey, L.; Woodhams, D.C.; Tacchi, L.; Salinas, I. Topographical Mapping of the rainbow trout (Oncorhynchus mykiss) microbiome reveals a diverse bacterial community with antifungal properties in the skin. Appl. Environ. Microbiol. 2015, 81, 6915–6925. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Wang, Q.; Huang, Z.; Ding, L.; Xu, Z. Immunoglobulins, mucosal immunity and vaccination in teleost fish. Front. Immunol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Bloecher, N.; Powell, M.; Hytterød, S.; Gjessing, M.; Wiik-Nielsen, J.; Mohammad, S.N.; Johansen, J.; Hansen, H.; Floerl, O.; Gjevre, A.G. Effects of cnidarian biofouling on salmon gill health and development of amoebic gill disease. PLoS ONE 2018, 13, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, B.; Liu, Y.; Chuan, H.; Liu, Y.; Xie, P. Immunoassay technology: Research progress in microcystin-LR detection in water samples. J. Hazard. Mater. 2022, 424 Pt B, 127406. [Google Scholar] [CrossRef]
- Chen, J.; Xie, P.; Lin, J.; He, J.; Zeng, C.; Chen, J. Effects of microcystin-LR on gut microflora in different gut regions of mice. J. Toxicol. Sci. 2015, 40, 485–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Shu, Y.; Dai, Y.; Gao, Y.; Liu, S.; Wang, W.; Jiang, H.; Zhang, H.; Hong, P.; Wu, H. Microcystin-leucine arginine exposure induced intestinal lipid accumulation and MC-LR efflux disorder in Lithobates catesbeianus tadpoles. Toxicology 2022, 465, 153058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, L.; Zhang, P.; He, J.; Liu, Y.; Matsuura, H.; Watanabe, M.M. Grazing on toxic cyanobacterial blooms by tadpoles of edible frog Rana grylio. Phycol. Res. 2012, 60, 20–26. [Google Scholar] [CrossRef]
- Song, L.; Chen, W.; Peng, L.; Wan, N.; Gan, N.; Zhang, X. Distribution and bioaccumulation of microcystins in water columns: A systematic investigation into the environmental fate and the risks associated with microcystins in Meiliang Bay, Lake Taihu. Water Res. 2007, 41, 2853–2864. [Google Scholar] [CrossRef]
- Puddick, J.; Prinsep, M.R.; Wood, S.A.; Kaufononga, S.A.; Cary, S.C.; Hamilton, D.P. High levels of structural diversity observed in microcystins from Microcystis CAWBG11 and characterization of six new microcystin congeners. Mar. Drugs 2014, 12, 5372–5395. [Google Scholar] [CrossRef]
- Bittner, M.; Štern, A.; Smutná, M.; Hilscherová, K.; Žegura, B. Cytotoxic and genotoxic effects of cyanobacterial and algal extracts-microcystin and retinoic acid content. Toxins 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Kholodkevich, S.; Sharov, A.; Feng, Y.; Ren, N.; Sun, K. Microcystin-LR-induced changes of hepatopancreatic transcriptome, intestinal microbiota, and histopathology of freshwater crayfish (Procambarus clarkii). Sci. Total Environ. 2020, 711, 134549. [Google Scholar] [CrossRef]
- Panksep, K.; Tamm, M.; Mantzouki, E.; Rantala-Ylinen, A.; Laugaste, R.; Sivonen, K.; Tammeorg, O.; Kisand, V. Using microcystin gene copies to determine potentially-toxic blooms, example from a Shallow Eutrophic Lake Peipsi. Toxins 2020, 12, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Huang, Q.; Huang, X.; Zhao, H.; Guan, B.; Ban, K.; Zhu, X.; Ma, Z.; Tang, Y.; Su, Z. Genetic variant of PP2A subunit gene confers an increased risk of primary liver cancer in Chinese. Pharmgenomics Pers. Med. 2021, 14, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Niu, Y.; Xie, P.; Chen, J.; Ma, Z.; Tao, M.; Qi, M.; Wu, L.; Guo, L. Factors affecting temporal and spatial variations of microcystins in Gonghu Bay of Lake Taihu, with potential risk of microcystin contamination to human health. Sci. World J. 2010, 10, 1795–1809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tito, J.C.R.; Luna, L.M.G.; Noppe, W.N.; Hubert, I.A. First report on microcystin-LR occurrence in water reservoirs of eastern Cuba, and Environmental Trigger Factors. Toxins 2022, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Petrovan, S.O.; Schmidt, B.R. Volunteer conservation action data reveals large-scale and long-term negative population trends of a widespread Amphibian, the Common Toad (Bufo bufo). PLoS ONE 2016, 11, e0161943. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Chen, J.; Zhang, X.; Xie, P. A review of reproductive toxicity of microcystins. J. Hazard. Mater. 2016, 301, 381–399. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, H.; Xie, P.; He, J. A review of neurotoxicity of microcystins. Environ. Sci. Pollut. Res. Int. 2016, 23, 7211–7219. [Google Scholar] [CrossRef]
- Pavagadhi, S.; Natera, S.; Roessner, U.; Balasubramanian, R. Insights into lipidomic perturbations in zebrafish tissues upon exposure to microcystin-LR and microcystin-RR. Environ. Sci Technol. 2013, 47, 14376–14384. [Google Scholar] [CrossRef]
- Balch, G.C.; Vélez-Espino, L.A.; Sweet, C.; Alaee, M.; Metcalfe, C.D. Inhibition of metamorphosis in tadpoles of Xenopus laevis exposed to polybrominated diphenyl ethers (PBDEs). Chemosphere 2006, 64, 328–338. [Google Scholar] [CrossRef]
- Degitz, S.J.; Holcombe, G.W.; Flynn, K.M.; Kosian, P.A.; Korte, J.J.; Tietge, J.E. Progress towards development of an amphibian-based thyroid screening assay using Xenopus laevis. Organismal and thyroidal responses to the model compounds 6-propylthiouracil, methimazole, and thyroxine. Toxicol. Sci. 2005, 87, 353–364. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Jiang, H.; Yuen, C.N.T.; Wang, W.; He, J.; Zhang, H.; Liu, G.; Wei, L.; Chen, L.; Wu, H. Microcystin-leucine arginine induces skin barrier damage and reduces resistance to pathogenic bacteria in Lithobates catesbeianus tadpoles. Ecotoxicol. Environ. Saf. 2022, 238, 113584. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, C.; Wu, Y.; Ye, B.; Han, L.; Shou, X.; Wang, M.; Wang, J.; Jia, X. Toxic effects of microcystin-LR on the reproductive system of male Rana nigromaculata in vitro. Aquat. Toxicol. 2013, 126, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gorr, T.; Kleinschmidt, T.; Fricke, H. Close tetrapod relationships of the coelacanth Latimeria indicated by haemoglobin sequences. Nature 1991, 351, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Zhou, X.P.; Zhang, Q.; Zhu, F. The preventive effects of dexmedetomidine against intestinal ischemia-reperfusion injury in Wistar rats. Iran. J. Basic. Med. Sci. 2015, 18, 604–609. [Google Scholar] [PubMed]
- Martins, N.D.; Yunes, J.S.; Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Microcystin-LR leads to oxidative damage and alterations in antioxidant defense system in liver and gills of Brycon amazonicus (SPIX & AGASSIZ, 1829). Toxicon 2017, 139, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gosner, K.L.J.H. A simplified table for staging anuran embryos and larvae with notes on identification. JSTOR 1960, 16, 183–190. [Google Scholar]
- Da Costa, J.C.; de Souza, S.S.; Castro, J.D.S.; Amanajás, R.D.; Val, A.L. Climate change affects the parasitism rate and impairs the regulation of genes related to oxidative stress and ionoregulation of Colossoma macropomum. Sci. Rep. 2021, 11, 22350. [Google Scholar] [CrossRef]
- Ibrahem, M.D. Evolution of probiotics in aquatic world: Potential effects, the current status in Egypt and recent prospectives. J. Adv. Res. 2015, 6, 765–791. [Google Scholar] [CrossRef] [Green Version]
- Cosin-Roger, J.; Ortiz-Masià, M.D.; Barrachina, M.D. Macrophages as an emerging source of wnt ligands: Relevance in mucosal integrity. Front. Immunol. 2019, 10, 2297. [Google Scholar] [CrossRef]
- Heiss, W.D. The ischemic penumbra: How does tissue injury evolve? Ann. N. Y. Acad. Sci. 2012, 1268, 26–34. [Google Scholar] [CrossRef]
- Prince, L.R.; Graham, K.J.; Connolly, J.; Anwar, S.; Ridley, R.; Sabroe, I.; Foster, S.J.; Whyte, M.K. Staphylococcus aureus induces eosinophil cell death mediated by α-hemolysin. PLoS ONE 2012, 7, e31506. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.C.; Bonzon, C.; Green, D.R.J.P. The machinery of programmed cell death. Pharmacol. Ther. 2001, 92, 57–70. [Google Scholar] [CrossRef]
- Chen, L.; Xie, P. Mechanisms of Microcystin-induced Cytotoxicity and Apoptosis. Mini Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, S.; Guo, X.; Xie, P.; Chen, J. The role of GSH in microcystin-induced apoptosis in rat liver: Involvement of oxidative stress and NF-κB. Environ. Pollut. 2016, 31, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Cai, C.; Wang, J.; Gao, N.; Zhang, H. Endocrine-disrupting effects and reproductive toxicity of low dose MCLR on male frogs (Rana nigromaculata) in vivo. Aquat. Toxicol. 2014, 155, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ziková, A.; Lorenz, C.; Lutz, I.; Pflugmacher, S.; Kloas, W. Physiological responses of Xenopus laevis tadpoles exposed to cyanobacterial biomass containing microcystin-LR. Aquat. Toxicol. 2013, 128–129, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Guilloty, F.; Saeed, A.M.; Duffort, S.; Cano, M.; Ebrahimi, K.B.; Ballmick, A.; Tan, Y.; Wang, H.; Laird, J.M.; Salomon, R.G. T cells and macrophages responding to oxidative damage cooperate in pathogenesis of a mouse model of age-related macular degeneration. PLoS ONE 2014, 9, e88201. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, H.; Xu, X.; Zhang, Y. Comprehensive analysis of genomic and immunological profiles in Chinese and Western hepatocellular carcinoma populations. Aging 2021, 13, 11564–11594. [Google Scholar] [CrossRef]
- Hoang, H.H.; Wang, P.C.; Chen, S.C. Interleukin 34 Serves as a novel molecular adjuvant against nocardia seriolae infection in largemouth bass (Micropterus salmoides). Vaccines 2020, 8, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, A.J.; Bhat, N.; Karron, R.A.; O’Brien, K.L.; Murdoch, D.R. Disk diffusion bioassays for the detection of antibiotic activity in body fluids: Applications for the Pneumonia Etiology Research for Child Health project. Clin. Infect. Dis. 2012, 54 (Suppl. 2), S159–S164. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Zhang, M.; Ni, Y.; Shi, J.; Gao, R.; Wang, F.; Dong, Z.; Zhu, L.; Liu, Y.; Xu, H. Impact of low hemoglobin on the development of contrast-induced nephropathy: A retrospective cohort study. Exp. Ther. Med. 2016, 12, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Bledsoe, J.W.; Pietrak, M.R.; Burr, G.S.; Peterson, B.C.; Small, B.C. Functional feeds marginally alter immune expression and microbiota of Atlantic salmon (Salmo salar) gut, gill, and skin mucosa though evidence of tissue-specific signatures and host-microbe coadaptation remain. Anim. Microbiome 2022, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiil. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Kong, Q.; Zhan, L.; Qiu, Z.; Huang, Q.; Song, X. TIPE2 ameliorates lipopolysaccharide-induced apoptosis and inflammation in acute lung injury. Inflamm. Res. 2019, 68, 981–992. [Google Scholar] [CrossRef]
- Takeuchi, O.; Hoshino, K.; Kawai, T.; Sanjo, H.; Takada, H.; Ogawa, T.; Takeda, K.; Akira, S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999, 11, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhang, X.; Chen, J.; Zhang, X.; Fan, H.; Li, S.; Xie, P. NF-κB plays a key role in microcystin-RR-induced HeLa cell proliferation and apoptosis. Toxicon 2014, 87, 120–130. [Google Scholar] [CrossRef]
- Christen, V.; Meili, N.; Fent, K. Microcystin-LR induces endoplasmatic reticulum stress and leads to induction of NFκB, interferon-alpha, and tumor necrosis factor-alpha. Environ. Sci. Technol. 2013, 47, 3378–3385. [Google Scholar] [CrossRef]
- Adegoke, E.O.; Wang, C.; Machebe, N.S.; Wang, X.; Wang, H.; Adeniran, S.O.; Zhang, H.; Zheng, P.; Zhang, G. Microcystin-leucine arginine (MC-LR) induced inflammatory response in bovine sertoli cell via TLR4/NF-kB signaling pathway. Environ. Toxicol. Pharmacol. 2018, 63, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Bellocchi, C.; Fernández-Ochoa, Á.; Montanelli, G.; Vigone, B.; Santaniello, A.; Quirantes-Piné, R.; Borrás-Linares, I.; Gerosa, M.; Artusi, C.; Gualtierotti, R. Identification of a Shared Microbiomic and Metabolomic Profile in Systemic Autoimmune Diseases. J. Clin. Med. 2019, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Zhao, Y.; Jiang, X.; Li, R.; Xie, H.; Ge, L.; Xie, B.; Yang, X.; Zhang, L. Exposure to Formaldehyde Perturbs the Mouse Gut Microbiome. Genes 2018, 9, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano-Aguilar, G.I.; Jang, S.; Lakshman, S.; Gupta, R.; Beshah, E.; Sikaroodi, M.; Vinyard, B.; Molokin, A.; Gillevet, P.M.; Urban, J.F., Jr. The effect of dietary mushroom Agaricus bisporus on intestinal microbiota composition and host immunological function. Nutrients 2018, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Shelly, A.; Gupta, P.; Ahuja, R.; Srichandan, S.; Meena, J.; Majumdar, T. Impact of microbiota: A paradigm for evolving herd immunity against viral diseases. Viruses 2020, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Frega, G. Microbiota: Overview and Implication in Immunotherapy-Based Cancer Treatments. Int. J. Mol. Sci. 2019, 20, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, M.; Xie, Y.; Li, Y.; Zhou, W.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Ringø, E.; Ran, C.; Zhou, Z. Stabilized fermentation product of Cetobacterium somerae improves gut and liver health and antiviral immunity of zebrafish. Fish Shellfish. Immun. 2022, 120, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, D.; Gao, L.; Ouyang, Y.; Wen, Y.; Ai, C.; Chen, Y.; Zhao, C. Health benefits of Grifola frondosa polysaccharide on intestinal microbiota in type 2 diabetic mice. Food Sci. Hum. Well. 2022, 11, 68–73. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yang, L.; Yao, J. Changes in the profile of fecal microbiota and metabolites as well as serum metabolites and proteome after dietary inulin supplementation in dairy cows with subclinical mastitis. Front. Microbiol. 2022, 13, 809139. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; He, J.; Wang, H.; Zheng, L.; Wang, X.; Zhang, H.; Wu, H.; Shu, Y. Gill Junction Injury and Microbial Disorders Induced by Microcystin-Leucine Arginine in Lithobates catesbeianus Tadpoles. Toxins 2022, 14, 479. https://doi.org/10.3390/toxins14070479
Jiang H, He J, Wang H, Zheng L, Wang X, Zhang H, Wu H, Shu Y. Gill Junction Injury and Microbial Disorders Induced by Microcystin-Leucine Arginine in Lithobates catesbeianus Tadpoles. Toxins. 2022; 14(7):479. https://doi.org/10.3390/toxins14070479
Chicago/Turabian StyleJiang, Huiling, Jun He, Hui Wang, Lingling Zheng, Xiaoran Wang, Huijuan Zhang, Hailong Wu, and Yilin Shu. 2022. "Gill Junction Injury and Microbial Disorders Induced by Microcystin-Leucine Arginine in Lithobates catesbeianus Tadpoles" Toxins 14, no. 7: 479. https://doi.org/10.3390/toxins14070479
APA StyleJiang, H., He, J., Wang, H., Zheng, L., Wang, X., Zhang, H., Wu, H., & Shu, Y. (2022). Gill Junction Injury and Microbial Disorders Induced by Microcystin-Leucine Arginine in Lithobates catesbeianus Tadpoles. Toxins, 14(7), 479. https://doi.org/10.3390/toxins14070479