Characterization of Field-Evolved Resistance to Afidopyropen, a Novel Insecticidal Toxin Developed from Microbial Secondary Metabolites, in Bemisia tabaci
Abstract
:1. Introduction
2. Results
2.1. Reversion and Selection of Field-Collected Resistant HD Strain
2.2. Cross-Resistance Patterns
2.3. Synergism Tests
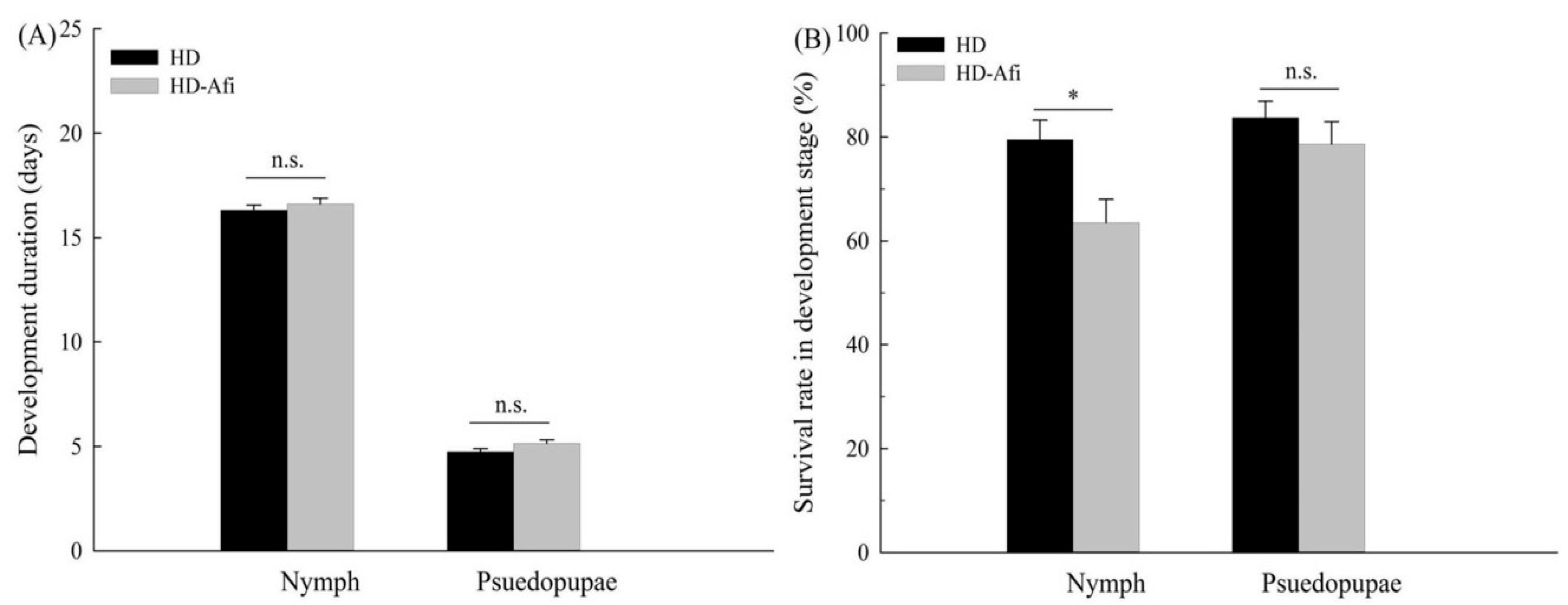
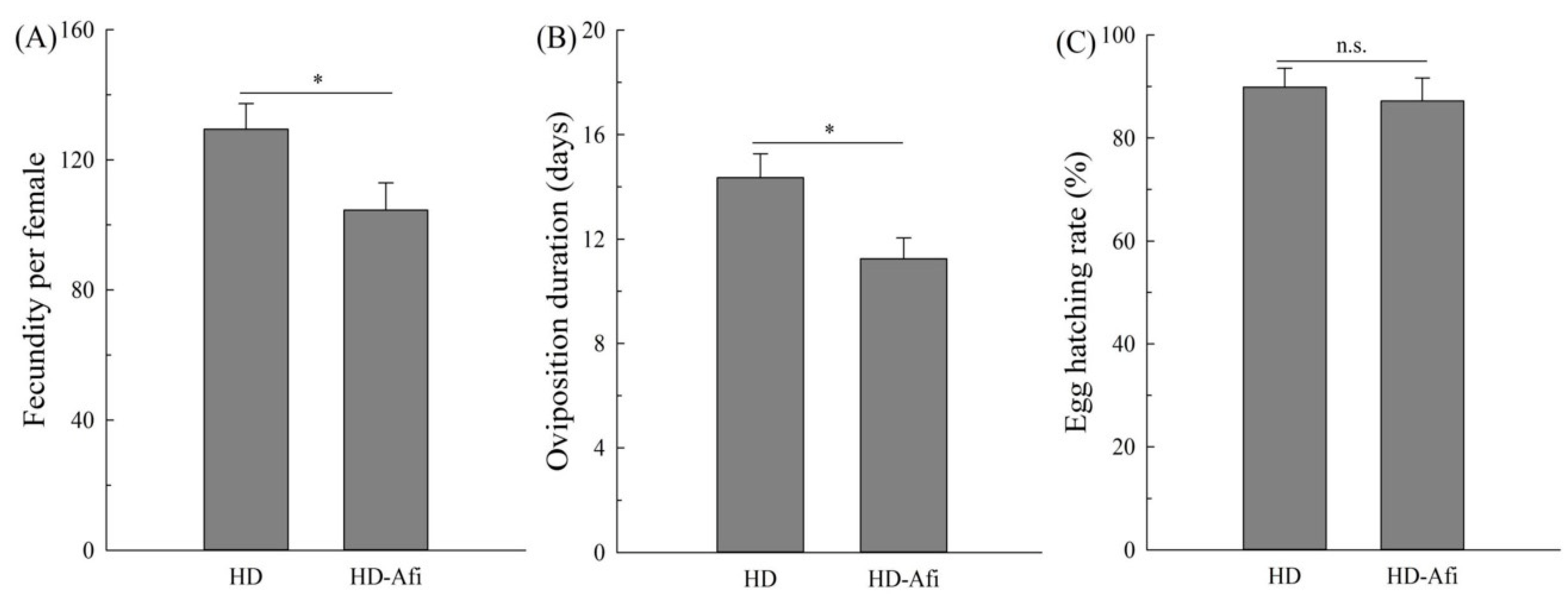
2.4. Fitness Comparisons
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Insecticide and Bioassays
4.3. Bioassays and Synergism Tests
4.4. Assessment of Fitness Costs
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horikoshi, G.R.; Mitomi, M.; Oyama, K.; Hirose, T.; Sunazuka, T.; Ōmura, S. Synthesis and insecticidal efficacy of pyripyropene derivatives. Part II-Invention of afidopyropen. J. Antibiot. 2019, 72, 661–681. [Google Scholar]
- Jeschke, P. Status and outlook for acaricide and insecticide discovery. Pest Manag. Sci. 2021, 77, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Ashfaq, M.; Stelinski, L.L. Susceptibility of Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), to the insecticide afidopyropen: A new and potent modulator of insect transient receptor potential channels. Appl. Entomol. Zool. 2018, 53, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Queiroz, O.D.S.; Nyoike, T.W.; Koch, R.L. Baseline susceptibility to afidopyropen of soybean aphid (Hemiptera: Aphididae) from the north central United States. Crop Prot. 2020, 129, 105020. [Google Scholar] [CrossRef]
- Slusher, E.K.; Cottrell, T.; Acebes-Doria, A.L. Effects of aphicides on pecan aphids and their parasitoids in pecan orchards. Insects 2021, 12, 241. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, H.J.; Xu, W.; Liu, J.T.; Geng, Z.Q.; Chu, D.; Guo, L. Pymetrozine-resistant whitefly Bemisia tabaci (Gennadius) populations in China remain susceptible to afidopyropen. Crop Prot. 2021, 149, 105757. [Google Scholar] [CrossRef]
- Leichter, C.A.; Thompson, N.; Johnson, B.R.; Scott, J.G. The high potency of ME-5343 to aphids is due to a unique mechanism of action. Pestic. Biochem. Physiol. 2013, 107, 169–176. [Google Scholar] [CrossRef]
- Koch, R.L.; da Silva Queiroz, O.; Aita, R.C.; Hodgson, E.W.; Potter, B.D.; Nyoike, T.; Ellers-Kirk, C.D. Efficacy of afidopyropen against soybean aphid (Hemiptera: Aphididae) and toxicity to natural enemies. Pest Manag. Sci. 2020, 76, 375–383. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Ammar, E.D.; Whitfield, A.E.; Redinbaugh, M.G. Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 2008, 46, 327–359. [Google Scholar] [CrossRef] [Green Version]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ghanim, M.; Roditakis, E.; Nauen, R.; Ishaaya, I. Insecticide resistance and its management in Bemisia tabaci species. J. Pest Sci. 2020, 93, 893–910. [Google Scholar] [CrossRef]
- Mota-Sanchez, D.; Wise, J.C. The Arthropod Pesticide Resistance Database. Michigan State University. 2017. Available online: http://www.pesticideresistance (accessed on 10 January 2022).
- Peng, Z.K.; Zheng, H.X.; Xie, W.; Wang, S.L.; Wu, Q.J.; Zhang, Y.J. Field resistance monitoring of the immature stages of the whitefly Bemisia tabaci to spirotetramat in China. Crop Prot. 2017, 98, 243–247. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.D.; Che, W.N.; Luo, C. First report of field resistance to cyantraniliprole, a new anthranilic diamide insecticide, on Bemisia tabaci MED in China. J. Integr. Agric. 2018, 17, 158–163. [Google Scholar] [CrossRef]
- Wang, R.; Fang, Y.; Mu, C.Q.; Qu, C.; Li, F.Q.; Wang, Z.Y.; Luo, C. Baseline susceptibility and cross-resistance of cycloxaprid, a novel cis-nitromethylene neonicotinoid insecticide, in Bemisia tabaci MED from China. Crop Prot. 2018, 110, 283–287. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.D.; Che, W.N.; Fang, Y.; Luo, C. Baseline susceptibility and biochemical mechanism of resistance to flupyradifurone in Bemisia tabaci. Crop Prot. 2020, 132, 105132. [Google Scholar] [CrossRef]
- Wang, R.; Gao, B.; Che, W.; Qu, C.; Zhou, X.; Luo, C. First report of field resistance to afidopyropen, the novel pyropene insecticide, on Bemisia tabaci Mediterranean (Q Biotype) from China. Agronomy 2022, 12, 724. [Google Scholar] [CrossRef]
- Basit, M. Status of insecticide resistance in Bemisia tabaci: Resistance, cross-resistance, stability of resistance, genetics and fitness costs. Phytoparasitica 2019, 47, 207–225. [Google Scholar] [CrossRef]
- Crowder, D.W.; Ellers-Kirk, C.; Tabashnik, B.E.; Carriere, Y. Lack of fitness costs associated with pyriproxyfen resistance in the B biotype of Bemisia tabaci. Pest Manag. Sci. 2009, 65, 235–240. [Google Scholar] [CrossRef]
- Feng, Y.T.; Wu, Q.J.; Xu, B.Y.; Wang, S.L.; Chang, X.L.; Xie, W.; Zhang, Y.J. Fitness costs and morphological change of laboratory-selected thiamethoxam resistance in the B-type Bemisia tabaci (Hemiptera: Aleyrodidae). J. Appl. Entomol. 2009, 133, 466–472. [Google Scholar] [CrossRef]
- Basit, M.; Sayyed, A.H.; Saeed, S.; Saleem, M.A. Lack of fitness costs associated with acetamiprid resistance in Bemisia tabaci (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2012, 105, 1401–1406. [Google Scholar] [CrossRef]
- Taquet, A.; Delatte, H.; Barrès, B.; Simiand, C.; Grondin, M.; Jourdan-Pineau, H. Insecticide resistance and fitness cost in Bemisia tabaci (Hemiptera: Aleyrodidae) invasive and resident species in La Réunion Island. Pest Manag. Sci. 2020, 76, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, J.S.; Che, W.N.; Wang, J.D.; Luo, C. Genetics and fitness costs of resistance to flupyradifurone in Bemisia tabaci from China. J. Integr. Agric. 2022, 21, 1436–1443. [Google Scholar] [CrossRef]
- Pu, X.; Yang, Y.H.; Wu, S.W.; Wu, Y.D. Characterisation of abamectin resistance in a field-evolved multiresistant population of Plutella xylostella. Pest Manag. Sci. 2010, 66, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Khakame, S.K.; Ye, C.; Yang, Y.H.; Wu, Y.D. Characterisation of field-evolved resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella, from China. Pest Manag. Sci. 2013, 69, 661–665. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.D.; Che, W.N.; Sun, Y.; Li, W.X.; Luo, C. Characterization of field-evolved resistance to cyantraniliprole in Bemisia tabaci MED from China. J. Integr. Agric. 2019, 18, 2571–2578. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.C.; Smith, L.B.; Silva, J.J.; Fan, Y.; Sun, H.; Scott, J.G. Fitness studies of insecticide resistant strains: Lessons learned and future directions. Pest Manag. Sci. 2021, 77, 3847–3856. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Afzal, M.B.; Shabbir, G.; Serrão, J.E.; Shad, S.A.; Muhammad, W. Laboratory selection of chlorpyrifos resistance in an invasive pest, Phenacoccus solenopsis (Homoptera: Pseudococcidae): Cross-resistance, stability and fitness cost. Pestic. Biochem. Physiol. 2017, 137, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Gong, Y.J.; Chen, J.C.; Su, X.C.; Cao, L.J.; Hoffmann, A.A.; Wei, S.J. Laboratory selection for resistance to sulfoxaflor and fitness costs in the green peach aphid Myzus persicae. J. Asia. Pac. Entomol. 2018, 21, 408–412. [Google Scholar] [CrossRef]
- Zhang, X.L.; Mao, K.K.; Liao, X.; He, B.Y.; Jin, R.H.; Tang, T.; Wan, H.; Li, J.H. Fitness cost of nitenpyram resistance in the brown planthopper Nilaparvata lugens. J. Pest Sci. 2018, 91, 1145–1151. [Google Scholar] [CrossRef]
- Liao, X.; Mao, K.; Ali, E.; Jin, R.H.; Li, Z.; Li, W.H.; Li, J.H.; Wan, H. Inheritance and fitness costs of sulfoxaflor resistance in Nilaparvata lugens (Stål). Pest Manag. Sci. 2019, 75, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.S.; Tang, Q.L.; Xia, J.; Lv, N.N.; Gao, X.W. Fitness costs of sulfoxaflor resistance in the cotton aphid, Aphis gossypii Glover. Pestic. Biochem. Physiol. 2019, 158, 40–46. [Google Scholar] [CrossRef]
- Liu, Z.W.; Han, Z.J. Fitness costs of laboratory-selected imidacloprid resistance in the brown planthopper, Nilaparvata lugens Stål. Pest Manag. Sci. 2006, 62, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.P.; Chu, D.; Ge, D.Q.; Wang, S.L.; Wu, Q.J.; Xie, W.; Jiao, X.G.; Liu, B.M.; Yang, X.; Yang, N.N.; et al. Further spread of and domination by Bemisia tabaci (Hemiptera: Aleyrodidae) biotype Q on field crops in China. J. Econ. Entomol. 2011, 104, 978–985. [Google Scholar] [CrossRef] [PubMed]
| HD Strain (without Selection) | HD-Afi Strain (Selected with Afidopyropen) | |||||
|---|---|---|---|---|---|---|
| G a | LC50 (95% CL) b (mg L−1) | Slope ± SE | RR c | LC50 (95% CL) b (mg L−1) | Slope ± SE | RR c |
| 1 | 256.320 (208.229–311.908) | 1.315 ± 0.140 | 36.5 | |||
| 2 | 189.807 (137.770–242.631) | 1.135 ± 0.139 | 27.0 | 299.889 (243.016–362.395) | 1.359 ± 0.141 | 42.7 |
| 3 | 140.582 (108.137–181.141) | 1.014 ± 0.133 | 20.0 | 380.671 (303.682–458.726) | 1.545 ± 0.153 | 54.2 |
| 4 | 77.052 (58.461–97.009) | 1.116 ± 0.137 | 11.0 | 476.515 (329.807–624.553) | 1.001 ± 0.135 | 67.8 |
| 5 | 48.763 (33.716–63.451) | 1.273 ± 0.153 | 6.9 | 534.401 (437.275–634.564) | 1.623 ± 0.154 | 76.0 |
| 6 | 37.446 (31.012–44.505) | 1.522 ± 0.144 | 5.3 | 560.945 (434.339–691.779) | 1.65 ± 0.11 | 79.8 |
| 7 | 21.139 (14.683–27.455) | 1.336 ± 0.152 | 3.0 | 740.959 (578.096–922.376) | 1.52 ± 0.11 | 105.4 |
| 8 | 25.320 (20.076–32.049) | 1.123 ± 0.137 | 3.6 | 715.198 (511.872–914.897) | 1.282 ± 0.148 | 101.7 |
| 9 | 22.536 (18.155–27.733) | 1.238 ± 0.137 | 3.2 | 762.536 (537.838–984.091) | 1.202 ± 0.144 | 108.5 |
| 10 | 20.470 (17.080–24.573) | 1.434 ± 0.139 | 2.9 | 733.063 (491.176–970.479) | 1.106 ± 0.143 | 104.3 |
| Insecticide | Strain | LC50 (mg L−1) (95% CL) a | Slope ± SE | RR1 b | RR2 c |
|---|---|---|---|---|---|
| Afidopyropen | MED-S | 7.104 (5.836–8.486) | 1.465 ± 0.144 | ||
| HD (F10) | 23.994 (18.802–30.420) | 1.076 ± 0.134 | 3.4 | ||
| HD-Afi (F10) | 750.818 (603.966–905.157) | 1.439 ± 0.145 | 105.7 | 31.3 | |
| Cyantraniliprole | MED-S | 1.040 (0.870–1.232) | 1.549 ± 0.146 | ||
| HD (F10) | 0.883 (0.724–1.069) | 1.345 ± 0.139 | 0.8 | ||
| HD-Afi (F10) | 1.341 (0.987–1.706) | 1.148 ± 0.142 | 1.3 | 1.5 | |
| Flupyradifurone | MED-S | 19.502 (14.602–25.191) | 0.989 ± 0.135 | ||
| HD (F10) | 25.232 (19.527–31.054) | 1.340 ± 0.145 | 1.3 | ||
| HD-Afi (F10) | 23.825 (19.811–28.979) | 1.405 ± 0.141 | 1.2 | 0.9 | |
| Imidacloprid | MED-S | 14.172 (11.067–17.590) | 1.193 ± 0.139 | ||
| HD (F10) | 13.542 (10.896–17.321) | 1.176 ± 0.137 | 1.0 | ||
| HD-Afi (F10) | 15.078 (11.115–18.976) | 1.328 ± 0.149 | 1.1 | 1.1 | |
| Sulfoxaflor | MED-S | 8.860 (6.569–11.148) | 1.256 ± 0.144 | ||
| HD (F10) | 11.033 (8.097–13.925) | 1.528 ± 0.143 | 1.2 | ||
| HD-Afi (F10) | 208.212 (174.280–243.020) | 1.875 ± 0.163 | 23.5 | 18.9 | |
| Thiamethoxam | MED-S | 11.152 (8.187–14.119) | 1.220 ± 0.145 | ||
| HD (F10) | 12.224 (9.812–15.214) | 1.192 ± 0.137 | 1.1 | ||
| HD-Afi (F10) | 14.291 (9.891–18.734) | 0.998 ± 0.136 | 1.3 | 1.2 |
| Strain | Insecticide/Synergist | LC50 (mg L−1) (95% CL) a | Slope ± SE | RR | SR b |
|---|---|---|---|---|---|
| MED-S | Afidopyropen | 7.736 (6.141–9.365) | 1.478 ± 0.151 | ||
| Afidopyropen + PBO | 7.051 (5.562–9.310) | 1.045 ± 0.133 | 1.1 | ||
| Afidopyropen + DEM | 7.324 (5.802–9.019) | 1.230 ± 0.140 | 1.1 | ||
| Afidopyropen + TPP | 7.127 (5.777–9.060) | 1.210 ± 0.138 | 1.1 | ||
| HD (F10) | Afidopyropen | 22.002 (16.921–28.872) | 0.970 ± 0.132 | 2.8 | |
| Afidopyropen + PBO | 24.731 (18.825–30.712) | 1.275 ± 0.142 | 3.2 | 0.9 | |
| Afidopyropen + DEM | 25.452 (19.301–33.635) | 0.928 ± 0.131 | 3.3 | 0.9 | |
| Afidopyropen + TPP | 27.871 (22.287–35.190) | 1.126 ± 0.134 | 3.6 | 0.8 | |
| HD-Afi (F10) | Afidopyropen | 732.112 (575.255–905.446) | 1.187 ± 0.135 | 94.6 | |
| Afidopyropen + PBO | 129.914 (101.106–167.823) | 1.016 ± 0.133 | 16.8 | 5.6 | |
| Afidopyropen + DEM | 754.248 (580.028–1039.142) | 0.928 ± 0.131 | 97.5 | 1.0 | |
| Afidopyropen + TPP | 695.300 (536.987–868.780) | 1.129 ± 0.134 | 89.9 | 1.1 |
| Fitness Component | HD | HD-Afi |
|---|---|---|
| Number of neonates for tests | 300 | 300 |
| Number of pseudopupae | 238 | 190 |
| Number of adults | 199 | 149 |
| Number of female adults | 91 | 70 |
| Mean eggs laid per female | 129.5 | 104.6 |
| Hatchability (%) | 89.9 | 87.2 |
| Predicted neonate number of next generation | 10594 | 6384 |
| Net reproductive rate (R0) | 35.3 | 21.3 |
| Relative fitness a | 1 | 0.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Zhang, Q.; Zhou, X.; Zhang, M.; Yang, Q.; Su, Q.; Luo, C. Characterization of Field-Evolved Resistance to Afidopyropen, a Novel Insecticidal Toxin Developed from Microbial Secondary Metabolites, in Bemisia tabaci. Toxins 2022, 14, 453. https://doi.org/10.3390/toxins14070453
Wang R, Zhang Q, Zhou X, Zhang M, Yang Q, Su Q, Luo C. Characterization of Field-Evolved Resistance to Afidopyropen, a Novel Insecticidal Toxin Developed from Microbial Secondary Metabolites, in Bemisia tabaci. Toxins. 2022; 14(7):453. https://doi.org/10.3390/toxins14070453
Chicago/Turabian StyleWang, Ran, Qinghe Zhang, Xuan Zhou, Mi Zhang, Qingyi Yang, Qi Su, and Chen Luo. 2022. "Characterization of Field-Evolved Resistance to Afidopyropen, a Novel Insecticidal Toxin Developed from Microbial Secondary Metabolites, in Bemisia tabaci" Toxins 14, no. 7: 453. https://doi.org/10.3390/toxins14070453
APA StyleWang, R., Zhang, Q., Zhou, X., Zhang, M., Yang, Q., Su, Q., & Luo, C. (2022). Characterization of Field-Evolved Resistance to Afidopyropen, a Novel Insecticidal Toxin Developed from Microbial Secondary Metabolites, in Bemisia tabaci. Toxins, 14(7), 453. https://doi.org/10.3390/toxins14070453






